Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
3D Printers Future Directions of 3D Printing Cardiac Care
Authors: Baby Chimata, Dr. IV Ramarao
DOI Link: https://doi.org/10.22214/ijraset.2024.65755
Certificate: View Certificate
Abstract
Three-dimensional (3D) printing is at the crossroads of printer and materials engineering, non-invasive diagnostic imaging, computer-aided design, and structural heart intervention. Cardiovascular applications of this technology development include the use of patient-specific 3D models for medical teaching, exploration of valve and vessel function, surgical and catheter-based procedural planning, and early work in designing and refining the latest innovations in percutaneous structural devices. In this review, we discuss the methods and materials being used for 3D printing today. We discuss the basic principles of clinical image segmentation, including coregistration of multiple imaging datasets to create an anatomic model of interest. With applications in congenital heart disease, coronary artery disease, and surgical and catheter-based structural disease, 3D printing is a new tool that is challenging how we image, plan, and carry out cardiovascular interventions.
Introduction
I. INTRODUCTION
Three-dimensional (3D) printing has become an increasingly used tool in medicine, with the literature documenting its applications from the education of medical students and healthcare professionals to assisting clinical decision-making such as pre-surgical planning and simulation or intraoperative guidance and enhancing doctor-to-patient communication [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Patient-specific 3D printed models based on imaging datasets, most commonly using computed tomography (CT) and magnetic resonance imaging (MRI), have been proven to replicate anatomy and pathology with high accuracy when compared to the original source data (Figure 1) [16,17,18,19,20]. With the generation of high-quality and highly accurate 3D printed models, the applications have been extended to various clinical domains, with 3D printed models serving as a valuable additional tool to enhance diagnosis, surgical planning, and treatment strategies, with eventual improvements in patient outcomes [15,16,17,18,19,20].
3D printed heart models showing normal anatomy and pathology. (a) Normal heart model created from cardiac CT and is partitioned into three pieces allowing visualization of interventricular septum. (b) Repaired tetralogy of Fallot (ToF) from an adult patient. The model was created from cardiac magnetic resonance imaging (MRI) and separated into two pieces allowing for clear visualization of overriding aorta and pulmonary infundibular stenosis. (c) Unrepaired ToF heart model from an infant. The model was created from 3D echocardiographic images and partitioned into two pieces showing the ventricular septal defect (VSD). (d) Unrepaired ToF heart model from an infant with superior and inferior portions showing VSD and the aortic overriding in relation to the VSD. Reprinted with permission under the open access from Loke et al. [20].

Three-dimensional (3D) printing is a fabrication technique used to transform digital objects into physical models. Also known as additive manufacturing, the technique builds structures of arbitrary geometry by depositing material in successive layers on the basis of a specific digital design. Several different methods exist to accomplish this type of fabrication and many have recently been used to create specific cardiac structural pathologies. Although the use of 3D printing technology in cardiovascular medicine is still a relatively new development, advancement within this discipline is occurring at such a rapid rate that a contemporary review is warranted. In this review, we address the 3D printing technologies that are relevant to cardiovascular medicine and discuss the principles of clinical image segmentation. We also present several recently reported applications of 3D printing and discuss the unresolved issues and future directions of this emerging technology. (Three-dimensional (3D) printing is a manufacturing process that transforms digital designs into physical objects. Also known as additive manufacturing, this method builds complex structures layer by layer based on a digital blueprint. Different techniques are available for 3D printing, each suited to particular applications, including recent uses in creating specific models for cardiac health studies. Although still a relatively new field, the use of 3D printing in cardiovascular medicine is advancing rapidly. This review highlights the relevance of 3D printing for cardiovascular applications, explains the fundamental principles behind various 3D printing technologies used in clinical imaging, and discusses recent applications along with future research directions.)
II. PRINTING TECHNOLOGY
The first 3D printing technology was introduced by Charles Hull in 1986 (1), and the industry has grown to now encompass many different manufacturing technologies. There are several 3D printing technologies with promising applications in medicine. Stereolithography fabricates a solid object from a photo polymeric resin using digitally guided ultraviolet laser light (some new versions use visible light) to harden the surface layer of the polymer liquid. Fused deposition modelling creates a 3D structure by extruding melted thermoplastic filaments layer by layer along with a physical support material that is later dissolved away. Selective laser melting creates strong parts of fused metal or ceramic powder using a high-power laser beam and is also preferred for building functional prototypes or medical implants, such as facial bone or sternal bone replacements 2, 3. PolyJet technology creates 3D prints through a process of jetting thin layers of liquid photopolymers that are instantly hardened using ultraviolet light and can incorporate multiple materials and colours simultaneously. Poyet is capable of producing highly complex models with smooth surfaces and thin walls (down to a resolution of 0.016 mm) (4) and is a commonly used technique for the fabrication of flexible, patient-specific anatomical models that combine several different materials. For the creation of cardiovascular models, the 3D printing method of choice depends upon the required complexity, durability, and desired surface quality of the model. (The first 3D printing technology was developed by Charles Hull in 1986, and since then, the field has evolved to include various manufacturing techniques. Some of these technologies hold promising potential for medical applications. For instance, stereolithography constructs objects from a liquid resin that hardens layer by layer when exposed to guided ultraviolet light. In contrast, fused deposition modelling (FDM) creates objects by extruding melted thermoplastic filaments layer by layer, with support structures that can be removed afterward. Selective laser melting (SLM) builds durable components from powdered metal or ceramics using a high-powered laser, often used for making functional prototypes and medical implants, among other applications.)
The creation of a patient-specific 3D model begins with clinical imaging. An imaging dataset must be volumetric, which limits the modalities to electrocardiography-gated computer tomography (CT), volumetric 3D echocardiography, and cardiac magnetic resonance (CMR). Volumetric 3D echocardiography is an attractive data source because it is abundantly available, relatively low-cost, and lacks ionizing radiation. For models of clearly imaged cardiac structures, such as ventricular chambers and valve leaflets, a 3D transoesophageal echocardiography (TEE) data source may be sufficient to create a 3D patient-specific model. However, ultrasound-based imaging is subject to artifact and unique limitations, such as anatomic data loss within an ultrasound “shadow.” To date, CT has been the principle imaging modality for 3D printing, because CT imaging can provide submillimetre tissue resolution, can clearly identify bone and pathologic calcium deposition, and is a commonly acquired imaging method before surgical or other structural interventions. In addition to excellent spatial resolution, CT is able to image patients with pacemakers, pacemaker wires, and metal implants that are not compatible with CMR scanning. In contrast, CMR can acquire high-resolution images without ionizing radiation and distinguish tissue composition without iodinated contrast media. CMR images have been used for 3D print modelling of congenital heart chambers and vasculature and for the reconstructive modelling of intracardiac tumours. However, the spatial resolution of CMR is generally lower than CT, which limits its use for the evaluation of coronary arteries or the small morphological features within heart valve complexes.( 3D Imaging Techniques in Cardiovascular Medicine
Volumetric 3D echocardiography and cardiac magnetic resonance imaging (CMR) are invaluable tools for visualizing cardiac structures. 3D echocardiography provides real-time, detailed images of heart components like ventricular chambers and valve leaflets. This imaging method is useful in creating patient-specific 3D models; however, challenges remain, such as imaging artifacts and resolution limitations, which may impact model accuracy.
CMR, another widely-used imaging technique, has become essential for examining soft tissues with high resolution. Unlike other imaging methods, CMR provides precise images without radiation exposure, making it suitable for assessing conditions before and after surgical procedures. Its advanced tissue differentiation capabilities make CMR particularly useful in evaluating structural and functional abnormalities, such as myocardial fibrosis and scarring.
CT imaging is also commonly applied in 3D printing for cardiac applications. Due to its ability to capture high-resolution, cross-sectional images, CT can create accurate 3D models of vascular and anatomical structures. This feature is especially advantageous when imaging calcified or metallic materials, such as stents and artificial valves, though CT often requires contrast agents to enhance soft-tissue detail.
Each of these imaging modalities offers unique benefits for 3D printing in cardiovascular applications. For instance, while CT excels in capturing fine morphological details of arteries, CMR is often preferred for soft-tissue evaluation. Ultimately, selecting the most appropriate imaging method depends on the clinical goal, whether it’s to visualize coronary arteries or analyse small anatomical structures.)
Image segmentation is the process of converting the 3D anatomical information obtained by CT, CMR, or 3D echocardiography volumetric imaging datasets into a 3D patient-specific digital model of the target anatomic structures. Increasing interest in anatomical modelling and the growing need for personalized structural heart interventions has encouraged the evolution of segmentation techniques. Initially, segmentation was on the basis of CT images only 5, 6, 7, 8, 9; however, more recently, CMR images have been utilized to replicate congenital heart and systemic vasculature disorders 10, 11, 12, 13, 14. The feasibility of reconstructing the mitral leaflets and annulus from 3D TEE images has been demonstrated by multiple investigators 1, 15, 16, 17, 18, 19, 20, and efforts to combine echocardiographic data acquired from multiple views or echocardiographic data combined with CT data have been reported 19, 21.
Segmentation involves several steps, as illustrated in Figure 1. Prior to segmentation, the acquired imaging dataset is exported into a Digital Imaging and Communication in Medicine (DICOM) format (3D TEE images are converted into Cartesian DICOM format). From the DICOM dataset, the target anatomic geometry is identified and segmented on the basis of the threshold intensity of pixels in the grey-scale 2-dimensional (2D) image projections (axial, sagittal, and coronal). Segmentation masks are created such that pixels with the same intensity range are grouped and assigned to be printed using a single material (step 2). Segmentation masks are converted into 3D digital models (step 3) using rendering techniques, and these patient-specific 3D digital models are saved as a stereolithography file. Frequently, this 3D digital model may be further modified within computer-aided design (CAD) software, where adjustments can be made to reflect the purpose of the 3D-printed model (e.g., colour coding a region of interest, texturing blended materials, or adding coupling components for evaluation of the 3D-printed model within a flow loop) (19). In general, the spatial resolution afforded by TEE is adequate for many 3D modelling purposes; however, the anatomic resolution can be further improved by combining ultrasound datasets acquired from different imaging perspectives. For example, a deep trans gastric TEE image window of the mitral valve (MV) apparatus including the papillary muscles can be digitally combined with data from a mycetophagid view of the mitral leaflets to create a more complete dataset of the entire MV complex (19). In addition, segmentation can be enhanced by the digital nonregistration of DICOM data from complementary imaging modalities (e.g., TEE visualization of the chordae tendineae combined with CT delineation of the mitral annular calcification). The nonregistration is on the basis of discreet anatomy or Patho anatomy that is present in both DICOM datasets, such as focal calcification or prosthetic material. In short, the segmentation step describes the identification of the region of interest, and may include the addition of anatomic data from more than 1 imaging source. When segmentation is complete, the final digital model is saved as a stereolithography file within the CAD-based software and is exported for 3D printing
Figure 1. 3D-Printed Modelling of Patient-Specific Anatomy
Step 1: Computed tomography (CT) imaging dataset used for image processing. Step 2: Segmentation process and creation of segmentation mask. Step 3: Converting segmentation mask into 3-dimensional (3D) digital patient-specific model. Step 4: Adjusted digital 3D patient-specific model suitable for implantation in flow loop and medical imaging acquisition. Step 5: 3D-printed, MultiMate rial, patient-specific model.
There are several commercial software packages, as well as open-source freeware platforms, that can be used for the patient-specific image segmentation 22, 23, 24, 25. The desired features of the model and the type of clinical imaging data used will generally dictate the choice of segmentation software. The source of the DICOM data (CT, CMR, or echocardiography), desired complexity of the patient-specific model, and extent of operator experience with the software may greatly influence the time required for image segmentation. For example, a relatively simple segmentation of a uniform vascular structure, such as a segment of the aorta, might be completed within 20 min. However, the complex segmentation of multiple anatomic elements to be printed with a combination of different colours and materials might take up to 12 h, even by an experienced operator.
III. CARDIOVASCULAR APPLICATIONS
3D-printed patient-specific models can be created for a number of different applications including creation of anatomic teaching tools, development of functional models to investigate intracardiac flow, and creation of deformable blended-material models for complex procedural planning, and increasingly, patient-specific models are being deployed to assist efforts to create or refine intra-cardiac devices.
A. Teaching Tools
An early application of 3D print modelling was to create models for anatomic teaching or demonstration. Like the plastic heart models familiar to most health care professionals, a 3D-printed model can rapidly convey a complex anatomic arrangement, but has the added value of also depicting patient-specific anatomic pathology. Such models can be instructional, both to teach medical professionals about normal and abnormal structural relationships and even to help the lay public better understand certain structural heart conditions. Patient-specific models of congenital heart defects have been used for critical-care training of residents and nurses, and have shown the potential to enhance the communication between cardiologist and patients 26, 27. Examples include instructional models depicting congenital heart defects, valve stenosis, and catheter-based valve implantation or repair procedures. Increasingly, these 3D models can be constructed with particular colours, variable material hardness, and even layered texturing if needed to convey sophisticated or unusual cardiovascular pathology (Figure 2).
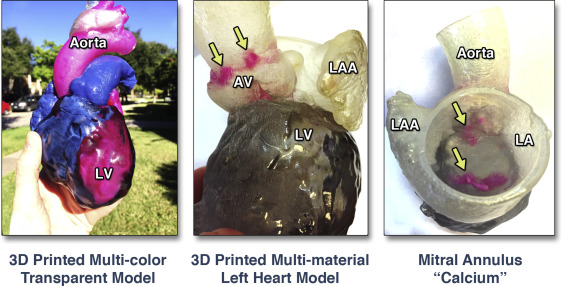
Figure 2. MultiMate rial and Multi-coloured, Patient-Specific, 3D-Printed Heart for Educational Purposes and Communication With Patients
A whole heart model color-coded to emphasize blood circulation (left). A left heart model 3D printed with different colours to indicate calcium deposition (arrows) within the aortic root complex (canter) and the mitral valve annulus (right). LA = left atrium; LAA = left atrial appendage; LV = left ventricle; other abbreviation as in Figure 1.
B. Functional flow models
Patient-specific 3D models of aortic valve dysfunction can be readily created by combining the technologies of high-spatial resolution CT, CAD software, and MultiMate rial 3D printing. Aortic valve dysfunction is a spectrum of conditions that have recently been replicated using 3D printing and coupled to a flow phantom. Because severe aortic valve stenosis (AS) represents a relatively static valve configuration, a CT dataset can acquire the patient-specific anatomic detail of the aortic root, including the valve orifice area and regional calcium deposition. A recent report described the creation of 8 patient-specific MultiMate rial 3D models of severe AS and the performance of a functional assessment of each model under different in vitro flow conditions. Each model replicated well the specific anatomic geometry of degenerative severe AS, with a faithful reproduction of calcium deposition, cusp thickening, and valve orifice shape. The functional evaluation of each model by Doppler and catheter-based methods also replicated the patient-specific AS severity (Figure 3, Online Video 1) and suggested that the calculated aortic valve area may not always be a fixed value. For some patients, the valve orifice area of the functional model varied with increasing flow volume. Such patient-specific functional models may provide a controlled and reproducible testing environment with quantitation of flow under pre-specified conditions, with potential applications including the examination of low-flow, low-gradient AS conditions, or the validation of 4-dimensional cardiac CMR methods to quantify transvalvular flow volume.
Figure 3. Functional Modelling of Patient-Specific AS
When coupled to a flow loop, the echocardiographic image and hemodynamic profile of severe stenosis are replicated. Focal calcification within the echocardiographic image of the patient (yellow arrow) and the patient’s model (blue arrow) are indicated.
Patient-specific modelling of the aortic valve in diastole has also been used to replicate aortic valve regurgitation and compared with clinical Doppler measures of aortic regurgitation severity replicated in vitro (28). More recently, patient-specific models of the aortic valve and aortic root complex have been utilized effectively for the performance of in vitro or bench-top transcatheter aortic valve implantation (TAVR). These constructs allow for exploration of the patient-specific features that influence the performance of transcatheter-deployed prosthetic heart valves. Such models may be especially useful for evaluating clinically challenging situations, such as the non-invasive quantification of paravalvular regurgitation severity under controlled flow conditions or for the planned deployment of endovascular stents (Figure 4) (9).
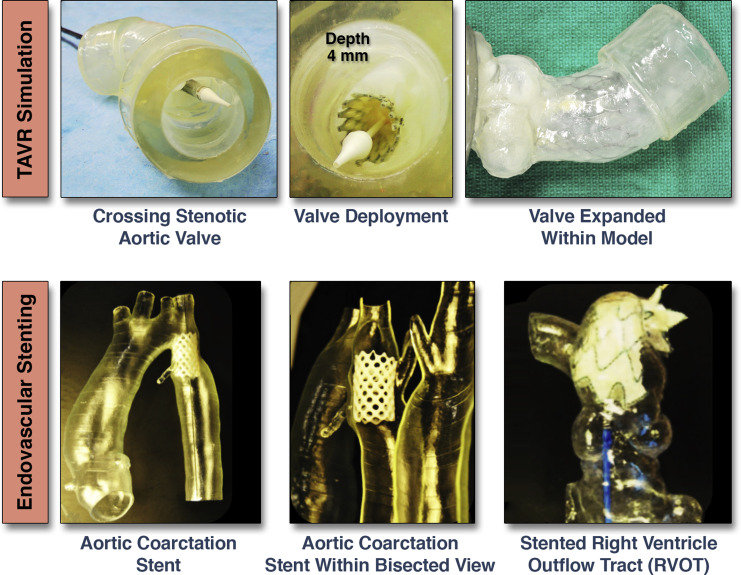
Figure 4. Transcatheter Valve and Stent Implantations Within Patient-Specific Models
Bench-top transcatheter aortic valve replacement (TAVR) performed within a model of aortic valve stenosis (top). Endovascular stenting within models of aortic coarctation and a pulmonary artery (bottom) Images courtesy of Dr. Giovanni Big lino.In addition to functional valve replication, the modelling of the coronary artery bed for several different applications has been recently reported. Javan et al. (29) demonstrated that various 3D print models were ideal for coronary visualization (Figure 5). 3D printing of coronary structures can enable visualization of stenotic regions, which may serve as a bench top tool to prepare and/or practice interventional procedures within a pulsatile flow loop environment. The coronary artery tree can be clearly defined by gated-CT methods, and when 3D printed in the diastolic phase, these models can be coupled to a flow loop to replicate epicardial coronary perfusion (30). Such models can provide a reference standard for testing of novel diagnostic measures (e.g., CT-derived fractional flow reserve, FFR) against a controlled, in vitro, forward-flow gold standard (31). Using 3D-printed coronary-like vessels, which were fabricated using a clear rigid material (Vero clear, Stratasys, Eden Prairie, Minnesota), Kolli et al. (31) showed that FFR decreased as a function of aortic pressure. This relationship was established using a set of idealized 3D-printed vessels with systematically placed stenotic regions that varied from 30% to 70%, resulting in absolute decreases in FFR of 0.03 to 0.20, respectively. This work demonstrates how 3D printing can recapitulate aspects of coronary flow in a quantitative and systematic manner, allowing standard diagnostic measures to be evaluated.
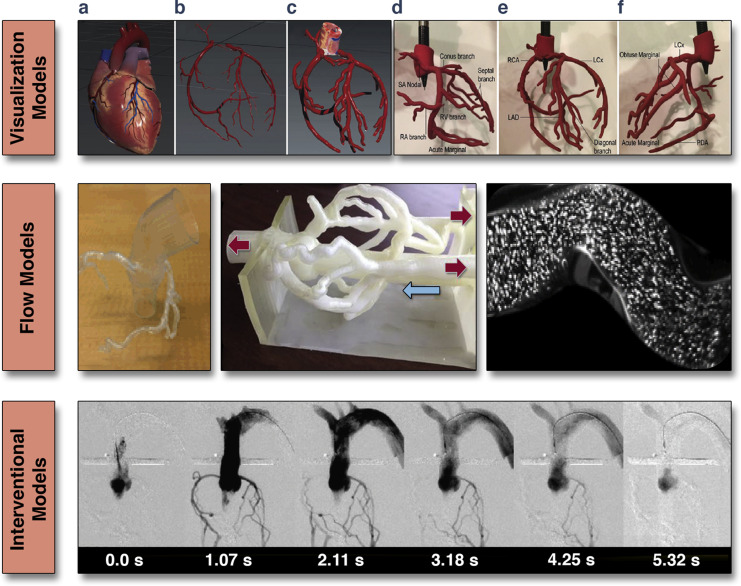
Figure 5. 3D-Printed Coronary Models
(Top) Visualization models: rigid and solid structures segmented from clinical data used to visualize paths and geometry of epicardial coronary arteries; (a) computationally designed 3D mesh; (b) extracted coronary arterial system; (c) increased vessel diameter to meet minimum wall thickness; (d to f) multiple views of a 3D printed coronary artery model. Adapted with permission from Javan et al. (29). (Middle) Flow models: hollow structures are used to allow flow of liquids and fluorescent particles to quantitatively assess hemodynamic of coronary vessels. (Bottom) Interventional models: hollow structures facilitating interventional procedures (e.g., angiography). Adapted with permission from Russ et al. (32). RA = right artery; RCA = right coronary artery; LAD = left anterior descending branch; LLCs = left circumflex artery; PDA = posterior descending artery; other abbreviation as in Figure 1.
Furthermore, when models are printed from optically transparent materials, techniques such as particle image velocimetry allow for direct visualization of complex flow dynamics (Figure 5). In addition to flow studies, these functional models can be used to simulate interventional procedures (32). Figure 5 shows a simulation angiogram. Beyond this, these models also have the potential to simulate devices such as coronary stents (e.g., drug-eluting or bioresorbable), which can be readily evaluated within this patient-specific coronary bed (Figure 5).
C. Procedural Planning
Congenital cardiovascular diseases are often associated with complex and unique geometry that can be very difficult to fully appreciate from 2D CT, CMR, or echocardiographic images (1). As such, 3D-printed modelling may play a key role to provide a more comprehensive understanding and functional evaluation of various congenital heart conditions. Recent applications of 3D-printed congenital heart models have included interventional pre-operative planning and simulations 33, 34, 35, 36, 37, 38; use of sterilized models during surgical procedures for enhanced structural orientation 11, 34, 39, 40; functional, patient-specific hemodynamic evaluations; and testing of novel procedural pathways 10, 41. A broad range of complex congenital heart anatomies have been reconstructed and 3D printed to enhance surgical planning, including the double-outlet right ventricle 26, 42, atrial septal defect (ASD) and ventricular septal defect 18, 38, 43, 44, tetralogy of Fallot 35, 45, as well as hypoplastic left heart syndrome 12, 27, 36. Moreover, it has been shown that 3D-printed models may assist with the accuracy of ventricular assist device cannula placement in patients with congenital heart disease (42). Detailed reviews of cardiac congenital 3D-printed modelling have recently been published 1, 37, 46. A patient-specific 3D-printed model used for pre-procedural planning of a catheter-based ASD closure is shown in Figure 6. In this instance, cone-beam CT images were acquired to assist in pre-procedural planning (Online Video 2). In this instance, such extensive pre-procedural planning was required to ensure that an ASD occlude device would not interfere with the function of previously implanted bioprosthetic aortic and mitral valves. In another example, a previously stented aortic coarctation with an abnormal subclavian artery originating from the stent site was 3D printed prior to a repeat intervention (Figure 4) (36).
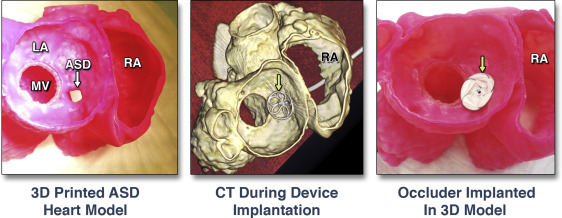
Figure 6. Pre-Procedural Planning of Catheter-Based Closure of an ASD
3D-printed model of an atrial septal defect (ASD) imaged by CT (left). 3D-printed model with bench-top implanted septal occlude device (yellow arrows) (middle).
In addition to the advanced imaging used for characterization of intracardiac neoplasm (e.g., echocardiography, CMR, and CT), it has been shown that patient-specific 3D-printed models are beneficial in the pre-operative and intraoperative surgical management of cardiac tumour excisions 47, 48, 49. Because cardiac tumours may extend into myocardial walls or valve structures, their radical and complete resection is rarely possible. Several reports have shown that knowledge gained by 3D-printed modelling positively influenced the surgical strategy for intracardiac tumour management 48, 49. This added value of 3D-printed models was on the basis of the ability to clearly depict tumour interaction with surrounding tissue, and multicolour and MultiMate rial models established clear tissue boundaries in ways that were difficult (or impossible) to appreciate using 2D or 3D imaging displays alone 34, 47. 3D models that replicate specific static anatomy have been developed to replicate both the form and function of select cardiovascular conditions. Valverde et al. (50) showed that most surgeons found the use of 3D models helpful in surgical planning (4 of 5) and would recommend the use of the technology to others (5 of 5).
Patient-specific MV models with leaflets have generally been 3D printed from only rigid materials, for the purpose of replicating static leaflet and annular geometry. However, some groups have developed patient-specific functional models of the MV with deformable leaflets. These models have recently been used to plan first-in-man structural heart procedures to repair MV function deploying both a Mitra Clip device (Abbott Vascular, Abbott Park, Illinois) as well as an occlude device to treat severe MV regurgitation (Figure 7) (51). The percutaneous Mitra Clip procedure is a well-established method to achieve an edge-to-edge MV repair, but as percutaneous MV replacement devices become available, the best treatment option for a specific patient may not be clear. Clinically, our ability to predict intraprocedural challenges (i.e., difficulty in grasping mitral leaflets) has been hampered by a lack of patient-specific MV models. 3D anatomic modelling may provide a solution to determine the ‘‘best-fit’’ amongst the possible catheter-based therapies available. In addition, such modelling may be valuable for predicting, and potentially avoiding, significant complications such as paravalvular regurgitation and device failure due to local calcification, or even the potentially devastating complication of left ventricular outflow tract (LVOT) obstruction by the device or displaced native valve tissue. Our recent clinical experience has taught us that fused-material 3D modelling can be invaluable for pre-procedural device selection when there are patient-specific concerns about valvular calcification and its effect on the device-landing zone on either surface of MV leaflets (51). As catheter-based structural heart interventions become increasingly complex, the ability to effectively model patient-specific geometry, as well as the interaction of an implanted device within that geometry, will become even more valuable. Such 3D models are a cheap arena to prevent mistakes and promote innovation, and in some canters, a 3D modelling program is already changing how the cardiovascular health professionals practice and prepare for specific structural heart interventions 8, 34, 36, 37, 51.
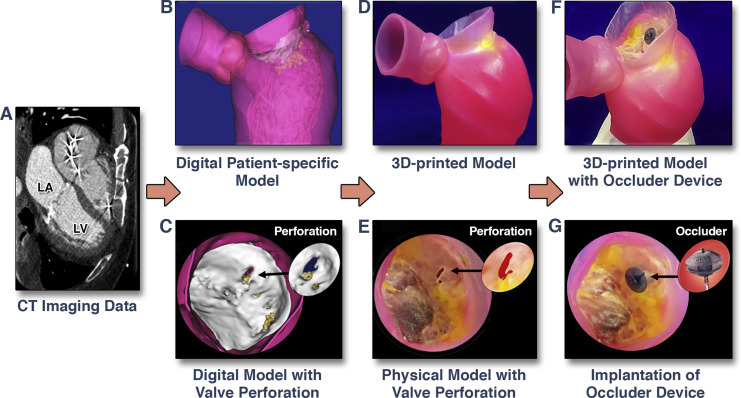
Figure 7. 3D-Printed Modelling for Patient-Specific Mitral Valve Repair With a Clip and a Plug
CT images (A) are used to create a digital model (B) of the mitral valve with a perforation (C). A MultiMate rial patient-specific 3D model (D) was printed to replicate the mitral valve geometry, regional calcium deposition, and pathology (E). 3D-printed model with implanted occlude device (F). Zoomed image of leaflet perforation repaired using an occlude device (G). Images adapted with permission from Little et al. (51). LA = left atrium; LV = left ventricle; other abbreviations as in Figure 1.
D. Device Innovation
The ability to test new or revised structural heart repair devices within a range of cardiac pathologies is particularly appealing. Although other modelling options have been relied upon for many years, device development using cadaveric models cannot be used for specific-patient procedure planning. Likewise, animal models invariably lack either the correct size or the pathological element (e.g., calcification) of human cardiovascular conditions. The ability to review a clinical cohort of patients with a specific treatment target (e.g., severe degenerative mitral regurgitation with prohibitive surgical risk), perform volumetric clinical imaging, and convert that digital data into a focused 3D model of blended material properties is currently available. An example of this application is the delivery of transcatheter mitral valve (TMVR) replacement devices.
Unlike TAVR or Mitra Clip technologies, the development of TMVR devices and delivery techniques has been slower and more challenging (52). Specific anatomic considerations are fundamental for the successful implementation of such devices; yet, the principle anatomic features can vary significantly between prospective patients. The mitral annular area, anterior mitral leaflet length, aortic-mitral angle, LVOT area, and specific sub valvular and annular calcium depositions are just some of the considerations. Although many of these anatomic features can now be clearly delineated and evaluated with increasingly sophisticated 3D visualization software (e.g., 3mensio [Pie Medical Imaging BV, AJ Maastricht, the Netherlands]), in general, such tools fail to provide insight about how the device will deform or alter the native anatomy in a physical model (or within the patient). Therefore, the creation of an anatomically accurate, yet deformable patient-specific 3D model has all of the attributes of a detailed digital model, but also provides for bench-top evaluation of the deformation of the critical anatomic relations influenced by the implanted TMVR device (e.g., the magnitude of anterior leaflet displacement into the LVOT) (Central Illustration). In fact, if 3D patient-specific models are created with material properties representing diseased human tissue, then 2 central features of a structural intervention may be assessed a priori: 1) the effect of the device on anatomic configuration of native structures; and 2) the effect of native structures (especially calcium) on the deployed configuration of the implanted device (e.g., failed expansion of a TAVR device within a heavily calcified aortic root). Applications for 3D printed modelling in cardiovascular diseases are summarized in Table 1.
Central Illustration. Creation of a Patient-Specific MultiMate rial 3D Model of the Mitral Valve Apparatus
Utilization of the 3-dimensional (3D) printed model to perform a bench-top transcatheter mitral valve replacement (TMVR) procedure to evaluate for possible left ventricular outflow tract (LVOT) obstruction. See Online Video 3. AL = anterior leaflet; CT = computed tomography; LA = left atrium; LV = left ventricle; PL = posterior leaflet; 2D = 2-dimensional.
Table 1. Applications of 3D-Printed Modelling in Cardiovascular Diseases
|
First Author (Ref. #) |
Clinical Condition |
Printing Method |
Findings |
|
Computed Tomography |
|||
|
Sogdian et al. (11) |
Aortic arch pseudoaneurysm |
Stereolithography; rigid models |
Creation of custom-made occluder for aortic pseudo-aneurysm |
|
Jacobs et al. (47) |
LV with aneurysm; RV tumor |
Binder jetting; multicolor plaster-based material |
3D model facilitated surgical resection |
|
Schmauss et al. (48) |
RV tumor |
Stereolithography; multicolor rigid material |
3D model facilitated surgical resection |
|
Severe AS |
PolyJet; rigid and flexible materials |
Evaluation of AS models under patient-specific flow conditions |
|
|
Yang et al. (55) |
Hypertrophic cardiomyopathy |
PolyJet; multiple materials |
Septal myomectomy guidance and procedural planning |
|
Russ et al. (32) |
Circle of Willis, cardiac arteries and femoral artery |
PolyJet; TangoPlus flexible material |
Pre-interventional procedural practice in flow loop |
|
Little et al. (51) |
Severe MR |
PolyJet; multiple materials |
MV model used for selection of repair devices in catheter-based clip and plug repair procedure |
|
Chukwu et al. (38) |
ASD |
Not specified; rigid model |
Pre-procedural planning of transcatheter occlusion of ASD |
|
Al Jabbari et al. (49) |
LA and RA tumour |
PolyJet; multiple materials |
3D model facilitated surgical resection |
|
Echocardiography |
|||
|
Binder et al. (15) |
Normal and dysfunctional MV |
Stereolithography; rigid models |
Demonstrated feasibility of MV models on the basis of 3D TEE |
|
Kapur et al. (20) |
MV annulus |
Fused deposition molding; rigid plastic |
Generated MV annulus models to facilitate MV repair |
|
Olivieri et al. (18) |
VSD; paravalvular regurgitation |
PolyJet; rigid material |
Demonstrated feasibility and accuracy of 3D TEE-based models |
|
Mahmood et al. (16) |
Normal and dysfunctional MV |
PolyJet; rigid annulus, flexible leaflets |
Demonstrated feasibility of 3D TEE-based MV models creation |
|
Vukic Vic et al. (19) |
LV and calcified MV apparatus |
PolyJet; multiple materials, differentiated tissue stiffness |
3D TEE and CT datasets combined to model for pre-procedural planning |
|
Witschey et al. (17) |
Normal and dysfunctional MV |
Fused deposition molding; plastic |
MV models created using automated image segmentation |
|
CMR |
|||
|
Markl et al. (14) |
Thoracic aortic vasculature |
PolyJet; Tango Plus material |
Conversion of patient CMR data into physical vessels replica |
|
Schiano et al. (10) |
Dysfunctional PV |
PolyJet; rigid material |
Demonstrated accuracy of CMR-based models for percutaneous valve implantation |
|
Greil et al. (13) |
Normal and congenital heart |
Laser sintering; rigid material |
Demonstrated accuracy of CMR-based models |
|
Big lino et al. (12) |
Hypoplastic aortic arch and RVOT |
PolyJet; flexible, Tango Plus |
Evaluation of Tango Plus materials for arterial vessels replication |
|
Valverde et al. (50) |
Aortic coarctation |
Fused polymer filament; rigid and flexible |
Demonstrated utility of models for interventional planning |
|
Costello et al. (43) |
VSD |
PolyJet; rigid materials |
Demonstrated utility of heart models for medical student teaching |
AS = aortic stenosis; ASD = atrial septal defect; AV = aortic valve; CMR = cardiac magnetic resonance; CT = computed tomography; LA = left atrium; LV = left ventricle; MR = mitral regurgitation; MV = mitral valve; PV = pulmonary valve; RA = right atrium; RV = right ventricle; RVOT = right ventricle outflow tract; TEE = transoesophageal echocardiography; VSD = ventricular septal defect.
IV. DEVELOPMENT OF PRINTING MATERIALS
The first 3D-printed models were fabricated from only a limited selection of rigid materials 5, 15, 53, but currently, the choice of available 3D print materials is considerably more varied. A relatively simple model can still be constructed using a single rigid material. Such models are generally more cost effective when the purpose is to simply visualize the anatomic relationship of specific structures 16, 17, 54, as shown in Figure 2. Commonly used rigid 3D print materials are from the Vero Plus family of polymers (Stratasys, Eden Prairie, Minnesota), which are available in a large variety of colours, colour blends, and opaque or transparent print formulations. However, an accurate replication of cardiac tissue mechanics requires that patient-specific models be fabricated from more flexible materials. Fortunately, there have been remarkable advances in material engineering that have created increasingly complex material blends that can approximate the mechanical properties of some cardiac tissues. For example, the elastomeric properties of the Tango Plus family of materials (Stratasys) are used to create deformable cardiac models within a broad range of stiffness and compliance specifications 9, 33, 41, 51, 55.
Among the available 3D printing technologies, the PolyJet technology is commonly used to fabricate patient-specific models because they allow for the replication of very complex anatomical structures by combining multiple colours and materials simultaneously. Poyet machines (Object 500 Connex 3, Stratasys) print 3D objects by adding high-resolution layers (down to 16 microns resolution) and the selection of materials to approximate specific tissue properties, ranging from very soft (Tango Plus) to hard (Vero Plus) materials.
The use of material blends are often necessary for an accurate representation of abnormal features within heart valves, such as the calcific structures within the aortic valve and MV (Figure 7) (8), or to replicate a complex of anatomical elements with different tissue characteristics (Central Illustration) 19, 51. The choice of materials is particularly important for fabrication of functional models for experiments in pressurized flow loops with tailored hemodynamic conditions 8, 9, 33, 41. For instance, Mara Giannis et al. (9) were able to fabricate a series of fully functional aortic stenosis models implantable in flow loop, replicating the entire aortic valve complex using flexible Tango Plus material, while the calcified aggregates within the valve cusps were 3D printed using a hard Vero Plus material. They also demonstrated the compatibility of Tango Plus materials for echocardiographic imaging acquisition. Recent advancements in multilateral 3D-printed modelling was extended to the complex MV apparatus with hard mitral annulus,
Despite the almost infinite possibilities of creating digital material blends, current 3D print materials can only replicate the mechanical properties of cardiovascular tissue to a certain extent 12, 19, 56. Mechanical testing of a range of Tango Plus materials and comparison to cardiac tissue samples (MV leaflet) showed that Tango Plus materials mimic the cardiac tissue mechanics only under a small range of leaflet deformations. As such, the accurate comparative analysis of human cardiac tissue and 3D print materials is problematic due to several issues, including: 1) significant variation in some tissue properties that occurs with aging; 2) substantial difference in mechanical properties of cardiac structures during their functional and static states; and 3) the paucity of published data regarding the mechanical properties of the anatomy being targeted for 3D print replication.
This area of material exploration is new and rapidly progressing. Recent investigations have demonstrated that creating a patterned mixture of materials 56, 57 or layered material composites within valve leaflets (19) could approximate the physiological behavior of those anatomical elements. Big lino et al. (12) examined distensible phantoms fabricated of Tango Plus materials and showed that this print material is suitable for manufacturing arterial vessels, although its mechanical characteristic may not be appropriate for modelling more compliant systemic vessels. Accordingly, the ability to model the physiological behavior of certain cardiovascular elements largely depends upon the local flow dynamics as well as the specific anatomic complexity being modelled. For example, coronary artery flow evaluation would require that the coronary vessel wall be fabricated with a compliant 3D print material (or material blend if focal atherosclerotic plaque were also being modelled), whereas the visualization of a ventricular septal defect for catheter-based repair may not necessitate replication of the septal wall compliance.
V. FUTURE DIRECTIONS
By combining the technologies of high-spatial resolution cardiac imaging, image processing software, and fused dual-material 3D printing, several hospital canters have recently demonstrated that patient-specific models of various cardiovascular pathologies may offer an important additional perspective on the condition 34, 36, 37, 42, 51. Patient-specific 3D MV models may directly affect our ability to select appropriate patients for structural heart therapy and anticipate procedural complications, and may potentially revise and improve the flood of intracardiac devices that are rapidly becoming available for therapeutic use.
Before these technologies can have an even broader effect, there are several issues that still need to be resolved. The accuracy of replication of the cardiac structural geometry must be validated across a wide range of source imaging modalities, 3D print methods, and cardiovascular modelling scenarios. The negative consequence of any modelling error may be very different if the model is created to teach or convey anatomy, compared with a model created for the detailed planning and device sizing of a catheter-based repair procedure. For example, the potential effect of variable loading conditions has yet to be determined. Efforts to replicate cardiac material properties with 3D print materials must show continued progress. Within a limited range of physiological performance, the native cardiac elements (e.g., vessel wall, chamber wall, and valve leaflet) can be modelled from a wide spectrum of 3D print material blends. The material properties of both normal and pathological native cardiac structures must be considered (if known) before 3D printing can even begin to approximate a similar static performance. Progress must continue toward the development of implantable, 3D-printed, patient-specific cardiac prostheses. Like the 3D-printed titanium devices now in use for maxillofacial and other orthopaedic repair procedures 58, 59, 60, 61, the near-term future for cardiac 3D printing may include custom manufacturing of repair devices, conduits, or occludes. This notion of a personalized 3D-printed cardiovascular prosthesis is not here today, but is now clearly visible on the horizon.
With applications in congenital heart disease, coronary artery disease, and surgical and catheter-based structural disease, 3D printing is a new tool that is challenging how we image, plan, and carry out cardiovascular interventions.
VII. LIMITATIONS, BARRIERS AND FUTURE DIRECTIONS
Over the last decades, there have been significant advancements in the use of 3D printing technology in cardiovascular disease, with reports showing great potential in clinical applications, as well as medical education and other areas. However, there exist some limitations and barriers that need to be considered when promoting 3D printing applications in cardiovascular disease. First, despite increasing reports in the literature, robust studies are still lacking, with the majority of the current studies based on case series or relatively small sample sizes (Table 1, Table 3 and Table 4). Further, follow-up studies of the mid to long-term outcomes of how 3D printed models contribute to education and clinical practice are scarce. Second, technological improvements have enabled the printing of realistic heart and vascular models with high accuracy in replicating anatomy and pathology (including complex conditions such as CHD); however, most of the current 3D printed models are static ones, and they do not really represent real cardiovascular circulatory physiology. Future research will need to address this limitation by developing more realistic 3D printed models, with 3D printed models connected to a fluid pump with the simulation of cardiac pulse sequences, as shown in Markkanen and other studies [60,87]. Third, the use of appropriate printing materials plays an important role, especially in the simulation of cardiac or interventional procedures, such as the operators need to experience a similar feeling when performing the simulation on 3D printed models so that clinicians and students can gain confidence and skills that are required to operate on real patients. This is already achievable with the current printing materials [25,63,70,88].
The main barriers to implementing 3D printing technology in routine cardiovascular practice are the relatively high costs associated with 3D printing (including image post-processing and segmentation) and the slow turnaround time. The first barrier will be addressed by using artificial intelligence, such as machine learning (ML) or deep learning (DL), to enhance the image segmentation process [91,92,93]. With printers available at clinical sites, the use of 3D printing technology in daily practice will become possible, and clinicians can incorporate 3D printed models into their diagnosis and decision-making process.
References
[1] Giannopoulos A.A., Steigner M.L., George E., Barile M., Hunsaker A.R., Rybicki F.J., Mitsouras D. Cardiothoracic applications of 3-dimensional printing. J. Thorac. Imaging. 2016; 31:253–272. Doi: 10.1097/RTI. [2] Witowski J., Wake N., Grochowska A., Sun Z., Budzynski A., Major P., Popiela T.J., Pedziwiatr M. Investigating accuracy of 3d printed liver models with computed tomography. Quant. Imaging Med. Surg. 2019; 9:43–52. doi: 10.21037/qims.2018.09.16. [3] Perica E., Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant. Imaging Med. Surg. 2017; 7:668–677. doi: 10.21037/qims.2017.11.02. [4] Costello J., Olivieri L., Krieger A., Thabit O., Marshall M.B., Yoo S.J., Kim P.C., Jonas R.A., Nath D.S. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World. J. Pediatr. Congenit. Heart. Surg. 2014; 5:421–426. Doi: 10.1177/2150135114528721. [5] Costello J.P., Olivieri L.J., Su L., Krieger A., Alfares F., Thabit O., Marshall M.B., Yoo S.J., Kim P.C., Jonas R.A., et al. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit. Heart. Dis. 2015; 10:185–190. doi: 10.1111/chd.12238. [6] Javan R., Zeman M. A prototype educational model for hepatobiliary interventions: Unveiling the role of graphic designers in medical 3D printing. J. Digit. Imaging. 2018; 31:133–143. doi: 10.1007/s10278-017-0012-4. [7] Sun Z., Lau I., Wong Y.H., Yeong C.H. Personalized three-dimensional printed models in congenital heart disease. J. Clin. Med. 2019; 8:522. Doi: 10.3390/jcm8040522. [8] Sun Z., Lee S. A systematic review of 3D printing in cardiovascular and cerebrovascular diseases. Anatol. J. Cardiol. 2017; 17:423–435. Doi: 10.14744/AnatolJCardiol.2017.7464. [9] Lupulescu C., Sun Z. A systematic review of the clinical value and applications of three-dimensional printing in renal surgery. J. Clin. Med. 2019; 8:990. Doi: 10.3390/jcm8070990. [10] Sun Z., Liu D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant. Imaging Med. Surg. 2018; 8:311–325. Doi: 10.21037/qims.2018.03.09. [11] Lau I., Sun Z. Three-dimensional printing in congenital heart disease: A systematic review. J. Med. Radiat. Sci. 2018; 65:226–236. Doi: 10.1002/jmrs.268. [12] K.M. Farooqi, P.P. Sengupta Echocardiography and three-dimensional printing: sound ideas to touch a heart J Am Soc Echocardiogr, 28 (2015), pp. 398-403 [13] T. Sumida, N. Otawa, Y.U. Kamata, et al. Custom-made titanium devices as membranes for bone augmentation in implant treatment: clinical application and the comparison with conventional titanium mesh J Craniomaxillofac Surg, 43 (2015), pp. 2183-2188 [14] J.L. Aranda, M.F. Jimenez, M. Rodriguez, G. Varela Tridimensional titanium-printed custom-made prosthesis for sternocostal reconstruction Eur J Cardiothorac Surg, 48 (2015), pp. e92-e94 [15] D. Ibrahim, T.L. Broilo, C. Heitz, et al. Dimensional error of selective laser sintering, three-dimensional printing and PolyJet models in the reproduction of mandibular anatomy J Craniomaxillofacial Surg, 37 (2009), pp. 167-173 [16] K. Kato, T. Ishiguchi, K. Maruyama, S. Naganawa, T. Ishigaki Accuracy of plastic replica of aortic aneurysm using 3D-CT data for transluminal stent-grafting: experimental and clinical evaluation J Comput Assist Tomogr, 25 (2001), pp. 300-304 [17] K. Knox, C.W. Kerber, S.A. Singel, M.J. Bailey, S.G. Imbesi Rapid prototyping to create vascular replicas from CT scan data: making tools to teach, rehearse, and choose treatment strategies Catheter Cardiovasc Interv, 65 (2005), pp. 47-53 [18] M.S. Kim, A.R. Hansgen, O. Wink, R.A. Quaife, J.D. Carroll Rapid prototyping: a new tool in understanding and treating structural heart disease Circulation, 117 (2008), pp. 2388-2394 [19] D. Maragiannis, M.S. Jackson, S.R. Igo, S.M. Chang, W.A. Zoghbi, S.H. Little Functional 3D printed patient-specific modeling of severe aortic stenosis J Am Coll Cardiol, 64 (2014), pp. 1066-1068 [20] D. Maragiannis, M.S. Jackson, S.R. Igo, et al. Replicating patient-specific severe aortic valve stenosis with functional 3D modelling Circ Cardiovasc Imaging, 8 (2015), p. e003626 [21] S. Schievano, F. Migliavacca, L. Coats, et al. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data Radiology, 242 (2007), pp. 490-497 [22] R. Sodian, S. Weber, M. Markert, et al. Stereolithographic models for surgical planning in congenital heart surgery Ann Thorac Surg, 83 (2007), pp. 1854-1857 [23] G. Biglino, P. Verschueren, R. Zegels, A.M. Taylor, S. Schievano Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing J Cardiovasc Magn Reson, 15 (2013), p. 2 [24] G.F. Greil, I. Wolf, A. Kuettner, et al. Stereolithographic reproduction of complex cardiac morphology based on high spatial resolution imaging Clin Res Cardiol, 96 (2007), pp. 176-18 [25] M. Markl, R. Schumacher, J. Kuffer, T.A. Bley, J. Hennig Rapid vessel prototyping: vascular modeling using 3T magnetic resonance angiography and rapid prototyping technology MAGMA, 18 (2005), pp. 288-292 [26] T.M. Binder, D. Moertl, G. Mundigler, et al. Stereolithographic biomodelling to create tangible hard copies of cardiac structures from echocardiographic data: in vitro and in vivo validation J Am Coll Cardiol, 35 (2000), pp. 230-237 [27] F. Mahmood, K. Owais, C. Taylor, et al. Three-dimensional printing of mitral valve using echocardiographic data J Am Coll Cardiol Img, 8 (2015), pp. 227-229 [28] W.R. Witschey, A.M. Pouch, J.R. McGarvey, et al. Three-dimensional ultrasound-derived physical mitral valve modelling Ann Thorac Surg, 98 (2014), pp. 691-69 [29] L.J. Olivieri, A. Krieger, Y.H. Loke, D.S. Nath, P.C. Kim, C.A. Sable Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy J Am Soc Echocardiogr, 28 (2015), pp. 392-397 [30] M. Vukicevic, D.S. Puperi, K. Jane Grande-Allen, S.H. Little 3D printed modeling of the mitral valve for catheter-based structural interventions Ann Biomed Eng (2016 Jun 20) [E-pub ahead of print] Erratum in: Ann Biomed Eng 2016; 44:3432 [31] K.K. Kapur, N. Garg Echocardiography derived three-dimensional printing of normal and abnormal mitral annuli Ann Card Anaesth, 17 (2014), pp. 283-284 [32] J. Gosnell, T. Pietila, B.P. Samuel, H.K. Kurup, M.P. Haw, J.J. Vettukattil Integration of computed tomography and three-dimensional echocardiography for hybrid three-dimensional printing in congenital heart disease J Digit Imaging, 29 (2016), pp. 665-669 [33] R. Oyama, M. Jakab, A. Kikuchi, T. Sugiyama, R. Kikinis, S. Pujol Towards improved ultrasound-based analysis and 3D visualization of the fetal brain using the 3D Slice [34] T. Bauch, P. Vijayaraman, G. Dandamudi, K. Ellenbogen Three-dimensional printing for in vivo visualization of his bundle pacing leads Am J Cardiol, 116 (2015), pp. 485-486 View PDFView articleView in ScopusGoogle Scholar [35] A. Firouzian, R. Manniesging, Z.H. Flach, R. Risselada, F. Kooten, M. Sturkenboom Intracranial aneurysm segmentation in 3D CT angiography: method and quantitative validation with and without prior noise filtering Ultrasound Obstet Gynecol, 42 (2013), pp. 609-610 Eur J Radiol, 79 (2016), pp. 299-304 [36] N. Byrne, F.M. Velasco, A. Tandon, I. Valverde, T. Hussain A systematic review of image segmentation methodology, used in the additive manufacture of patient-specific 3D printed models of the cardiovascular system JRSM Cardiovasc Dis, 5 (2016) 2048004016645467 [37] S. Garekar, A. Bharati, M. Chokhandre, et al. Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect World J Pediatr Congenit Heart Surg, 7 (2016), pp. 344-350 [38] L. Kiraly, M. Tofeig, N.K. Jha, H. Talo Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair Interact Cardiovasc Thorac Surg, 22 (2016), pp. 238-240 [39] M. Vukicevic, D. Maragiannis, M. Jackson, S.H. Little Functional evaluation of a patient-specific 3D printed model of aortic regurgitation [abstr] Circulation, 132 (2015), p. A18647 [40] R. Javan, D. Herrin, A. Tangestanipoor Understanding spatially complex segmental and branch anatomy using 3D printing: liver, lung, prostate, coronary arteries, and circle of Willis Acad Radiol, 23 (2016), pp. 1183-1189 [41] G. Xiong, K. Kolli, H.A. Soohoo, J.K. Min In-vitro assessment of coronary hemodynamic in 3D printed patient-specific geometry [abstr] Circulation, 132 (2015), p. 19898 [42] K.K. Kolli, J.K. Min, S. Ha, H. Soohoo, G. Xiong Effect of varying hemodynamic and vascular conditions on fractional flow reserve: an in vitro study J Am Heart Assoc, 5 (2016), p. e003634 [43] M. Russ, R. O\'Hara, S.V. Setlur Nagesh, et al. Treatment planning for image-guided neuro-vascular interventions using patient-specific 3D printed phantoms Proc SPIE Int Soc opt Eng, 9417 (2015) [44] G. Biglino, C. Capelli, A. Binazzi, et al. Virtual and real bench testing of a new percutaneous valve device: a case study Euro Intervention, 8 (2012), pp. 120-128 [45] D. Schmauss, S. Haeberle, C. Hagl, R. Sodian Three-dimensional printing in cardiac surgery and interventional cardiology: a single-centre experience Eur J Cardiothoracic Surg, 47 (2015), pp. 1044-105 [46] J.R. Ryan, T.G. Moe, R. Richardson, D.H. Frakes, J.J. Nigro, S. Pophal A novel approach to neonatal management of tetralogy of Fallot, with pulmonary atresia, and multiple aortopulmonary collaterals J Am Coll Cardiol Img, 8 (2015), pp. 103-104 [47] G. Biglino, C. Capelli, A.M. Taylor, S. Schivano 3D printing cardiovascular anatomy: a single-center experience I.V. Shishkovsky (Ed.), New Trends in 3D Printing, IN-Tech Publishing House, Rijeka, Croatia (2016) [48] K.M. Farooqi, O. Saeed, A. Zaidi, et al. 3D printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure J Am Coll Cardiol HF, 4 (2016), pp. 301-311 [49] Y. Chaowu, L. Hua, S. Xin Three-dimensional printing as an aid in transcatheter closure of secundum atrial septal defect with rim deficiency: in vitro trial occlusion based on a personalized heart model Circulation, 133 (2016), pp. e608-e610 [50] A.M. Noecker, J.F. Chen, Q. Zhou, et al. Development of patient-specific three-dimensional pediatric cardiac models ASAIO J, 52 (2006), pp. 349-353 [51] M. Vranicar, W. Gregory, W.I. Douglas, S.P. Di, T.G. Di Sessa The use of stereolithographic hand held models for evaluation of congenital anomalies of the great arteries Stud Health Technol Inform, 132 (2008), pp. 538-543 [52] M. Vukicevic, T. Conover, M. Jaeggli, et al. Control of respiration-driven retrograde flow in the subdiaphragmatic venous return of the Fontan circulation ASAIO J, 60 (2014), pp. 391-399 [53] K.M. Farooqi, J.C. Nielsen, S.C. Uppu, et al. Use of 3-dimensional printing to demonstrate complex intracardiac relationships in double-outlet right ventricle for surgical planning Circ Cardiovasc Imaging, 8 (2015), p. e003043 [54] J.P. Costello, L.J. Olivieri, L. Su, et al. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians Congenit Heart Dis, 10 (2015), pp. 185-190 [55] S. Anwar, G.K. Singh, J. Varughese, et al. 3D printing in complex congenital heart disease: across a spectrum of age, pathology, and imaging techniques J Am Coll Cardiol Img (2016 Jul 14) [E-pub ahead of print] [56] S. Deferm, B. Meyns, D. Vlasselaers, W. Budts 3D-printing in congenital cardiology: from flatland to spaceland J Clin Imaging Sci, 6 (2016), p. 8 [57] M. Cantinotti, R. Giordano, G. Volpicelli, et al. Lung ultrasound in adult and paediatric cardiac surgery: is it time for routine use? [58] S. Jacobs, R. Grunert, F.W. Mohr, V. Falk 3D-imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study Interact Cardiovasc Thorac Surg, 7 (2008), pp. 6-9 [59] D. Schmauss, N. Gerber, R. Sodian Three-dimensional printing of models for surgical planning in patients with primary cardiac tumors J Thorac Cardiovasc Surg, 145 (2013), pp. 1407-1408 [60] O. Al Jabbari, W.K. Abu Saleh, A.P. Patel, S.R. Igo, M.J. Reardon Use of three-dimensional models to assist in the resection of malignant cardiac tumors J Card Surg, 31 (2016), pp. 581-583 [61] I. Valverde, G. Gomez, C. Suarez-Mejias, et al. 3D printed cardiovascular models for surgical planning in complex congenital heart diseases J CardiovascMagnReson, 17 (2015), p. 19S.H. Little, M. Vukicevic, E. Avenatti, M. Ramchandani, C.M. Barker 3D printed modelling for patient-specific mitral valve intervention: repair with a clip and a plug J Am Coll Cardiol Intv, 9 (2016), pp. 973-975 [62] A. Kheradvar, E.M. Groves, C.A. Simmons, et al.Emerging trends in heart valve engineering: Part III. Novel technologies for mitral valve repair and replacement Ann Biomed Eng, 43 (2015), pp. 858-870 [63] H. Kim, J. Lu, M.S. Sacks, K.B. Chandran Dynamic simulation of bioprosthetic heart valves using a stress resultant shell model Ann Biomed Eng, 36 (2008), pp. 262-275 [64] F. Mahmood, K. Owais, M. Montealegre-Gallegos, et al. Echocardiography derived three-dimensional printing of normal and abnormal mitral annuli Ann Card Anaesth, 17 (2014), pp. 279-283 [65] D.H. Yang, J.W. Kang, N. Kim, J.K. Song, J.W. Lee, T.H. Lim Myocardial 3-dimensional printing for septal myectomy guidance in a patient with obstructive hypertrophic cardiomyopathy Circulation, 132 (2015), pp. 300-301 [66] K. Wang, Y. Zhao, Y. Chang, et al. Controlling the mechanical behavior of dual-material 3D print edmeta-materials for patient-specific tissue-mimicking phantomsMaterials & Design, 90 (2016), pp. 704-712 [67] K. Wang, C. Wu, Z. Qian, C. Zhang, B. Wandal-material 3D printed metamaterials with turntables mechanical properties for patient-specific tissue-mimicking phantoms Additive Manufacturing, 12 (2016), pp. 31-37 [68] F.A. Shah, A. Snis, A. Matic, P. Thomsen, A. Palmquist 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface Acta Biomatter, 30 (2016), pp. 357-367
Copyright
Copyright © 2024 Baby Chimata, Dr. IV Ramarao. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65755
Publish Date : 2024-12-05
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

