Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Triazoles as Antifungal: A Comprehensive Exploration of their Mechanisms, Resistance and Advancements
Authors: Manisha Joshi, Anita Singh, Priya Gupta, Hansika Bansal
DOI Link: https://doi.org/10.22214/ijraset.2024.60355
Certificate: View Certificate
Abstract
Fungal infections have been a serious problem since long time. However, development of new antifungals has improved the situation and helped a lot in overcoming the problems caused due to invasive fungal infections. It is very crucial to understand the mechanism behind fungal infections and generation of resistance. Extensive use of azole antifungals has caused their resistance leading to medication failure. Focus on antimicrobials got a boom after Covid 19 outbreak but still fungal infections are least focused. Fluconazole a widely used antifungal have no action against Candida albicans. Due to excessive use resistance have been created this reason warns us that there is a great need to introduce new and better antifungals in the market. Beside this new antifungal like Echinocandin and second generation of triazole antifungals are clinically important. On the top of this Posaconazole (triazole second generation) is the azole active against Zygomycete fungi. Combination therapy is an additional method for treating invasive fungal infections. However, in the end, there is a mismatch between the supply and demand for antifungals.an additional method for treating invasive fungal infections. Hence need to introduce novel antifungals have become very important. The main aim of this article is to focus on the emerging fungal infections and mechanism of resistance along with study of fluconazole as antifungal agents.
Introduction
I. INTRODUCTION
Recent advancement in antimicrobial therapy is a very important topic to focus on. The increased use of antimicrobials since last few years have caused a sudden increase in drug resistance. However antifungal resistance study has lagged than study of antibacterials as fungal infection is not thought to be important to give attention on. For about 30 years Amphotericin B was a very important drug to treat and control severe fungal infection beside the fact it causes significant nephrotoxicity. In the ending of 1980s and starting of 1990s Imidazole’s and Triazole’s were approved for safe and effective treatment of local and systemic infections. Fluconazole a triazole derivative is a highly safe drug for treatment of fungal infections because of which it is used extensively. It was used to treat 16 million patients which includes more than 300,000 AIDS cases. This data is only for US after the launch of the drug. Due to such extensive use drug resistance is created in the microorganisms[1].
Fungal infection has become an emerging problem since few years. The resistance to various fungal species is the major problem. Resistance develops when microbe generate some specific mechanism to defeat the drug. About 7% of blood samples tested at CDC of candida species are fluconazole resistant [2].
Mostly prescribed antifungal for candida species is of azole category i.e., Fluconazole. This drug works by inhibition of ergosterol synthesis these are 14 alpha demethylase inhibitors [3].
Drug resistance is a globally emerging serious problems it has caused a great limitation in treatment options. People who have compromised immune systems are more vulnerable to generate serious antifungal resistance. Some strains of fungi have become more resistant to drugs and are called superbugs [4].
Antimicrobial drugs are always prone to drug resistance due to adaptive nature of microorganisms [5].
There is great gap between drug demand and supply because of limited antifungals with less toxicity and side effects. Clinically reported mortality rate of different species varies but most common 3 species reported for major infection are 50-90 percent for Aspergillus fumigatus, 20-70 percent for Cryptococcus neoformans, and 20-40 percent for Candida albicans. Treating this fungal infection caused by several species is challenging without harming the host [6].
Azole is one of major class of antifungal drug used for treatment of various fungal infections. The antifungals of this category used clinically contain either two or three nitrogen in its azole ring which further classified as imidazole (ketoconazole, clotrimazole) and triazole ( fluconazole, voriconazole or itraconazole) [7].
Triazole category drugs are important pharmacophore groups they interact with biological target with high affinity due to their ability to form hydrogen bonds, stiffness, solubility, and dipole characteristic.
Every single derivative like voriconazole, posaconazole, ravuconazole, isavuconazole, albaconazole, efinaconazole are derived from fluconazole or itraconazole. These derivatives are designed to overcome the limitations found in their parent drugs [8].
II. WHY DO WE STUDY ABOUT FUNGI?
In the whole environment fungi are multicellular, eukaryotic organisms that make approximately 0.1% of the gut microbiome. Fungi can be beneficial as well as infectious. When utilized as food like yeast, mushroom etc and in pharmaceuticals a great example is penicillin. However, fungi are opportunistic in nature and can cause infections which can range in severity from minor (e.g., ringworm, athletes’ foot) to fatal (e.g., aspergillosis).
WHO and the US Centre for Disease Control and Prevention are two examples of organisations which have found a number of invasive disease-causing fungi as emerging threats. A highly significant clinical pathogen Candida auris causes candidiasis specially in immunocompromised individuals and is resistance to antifungal drug fluconazole and amphotericin B. Candida auris has a 39% mortality rate CDC has categorised as a “urgent threat”.
Fungi belonging to the genus aspergillus are another major source of concern. Fumigates is a mould that can lead to aspergillosis by infecting the respiratory system and lungs. A. fumigates has demonstrated a rise in azole resistance since 1990, which has led to treatment failures [9].
Causes of antifungal resistance:
- Poor sanitation
- Excessive use of fungicides
- Limited discovery of advance antifungal
- Not following prescription correctly
- Mutation of fungi sequences
III. ANTIFUNGAL THERAPIES
There are currently several antifungal agents available for therapeutic use. Fungal drugs are divided into four categories and can be used alone or in combination help address a range of fungal illnesses. The combination therapy and hybrids are being used very commonly and have great results. Some common 'frontline' antifungals are listed below:
- Polyenes, such as amphotericin B, disrupt cell structure by causing fungal cell membranes to rupture.
- 5-fluorouracil (5-FC), for example, inhibits nucleic acid synthesis. Cryptococcus species are resistant fungi.
- Micafungin and other echinocandins inhibit fungal cell wall synthesis. Candida species are resistant fungi.
- Fluconazole and other azoles inhibit metabolism by preventing ergosterol synthesis. Candida species are resistant fungi.
IV. FUNGAL INFECTION AN EMERGING PROBLEM
Fungal infections have been added in some of the major problems. Existence of fungi is in 2 basic form moulds and yeasts. Single, small, oval cells are called yeast and fungi consisting of filaments called hyphae are moulds [10].
Fungal infections can re-emerge after some time some of very common fungal infections such as candidiasis, aspergillosis, cryptococcosis are very common. Candida is responsible for causing blood stream infections whose mortality rate is more than 30% similarly aspergillus is responsible to infect more than 45% susceptible host. Rate of deaths due to invasive fungal infections in patients have been increased to 67% [11].
People with poor circulation, weak immune system or who are suffering from diabetes are at high risk for fungal infections. Generally, anyone can be attacked by fungal infection. Parts like skin, nails or parts of body that generally traps moisture are highly prone to fungal infection. Patients who are undergoing cancer treatment or are on immunosuppressant medications are also at high risk of being caught by fungal infections [12].
Types Of Fungal Infection
Fungal infections have been divided according to the way they affect the body part these infections can be on the surface of skin or under the skin or inside the body organs.

- Superficial Fungal Infection: Means it will cause infection on the surface of skin like on skin mucous membranes, nails. Some of major examples are Candidiasis, Ringworm, Onychomycosis, Tinea versicolor.
- Subcutaneous Fungal Infection: Fungal infection under the surface of skin. Caused when fungus gets the way to move into cut or wound or any other type of injury. Examples are Eumycetoma, Chromoblastomycosis, Sporotrichosis.
- Deep Fungal Infection: Fungal infections which affect body organs like blood, UT, brain or lungs. Some of these infections are opportunistic and usually cause infection in people with weakened immune system. Examples are Aspergillosis, Blastomycosis, Histoplasmosis, Invasive candidiasis, Pneumocystis pneumonia.
Table:1Types of fungal infections and causative agents
|
S.no. |
Superficial fungal infection |
Subcutaneous fungal infection |
Deep fungal infection |
|
1. |
Ringworm: this group of fungi live on skin, hair or nails. Tinea Pedis/ athletes foot |
Sporotrichosis: rose gardener disease, can also occur in lings and other body parts. |
Blastomycosis: it causes infection in bones |
|
2. |
Onychomycosis: these causes infection in finger and toenails. |
Chromoblastomycosis: cause long lasting infection of skin. |
Aspergillosis: cause lung infection, pulmonary aspergillosis or can form fungal balls. |
|
3. |
Candidiasis: candida species mainly albicans cause skin and mucosal infection. |
Eumycetoma: it affects feet. |
Candida UTI: generally, bacteria cause UTI. |
|
4. |
Tinea versicolor: cause skin discoloration fungus responsible is Malassezia |
|
Can affect the heart, blood, brain, eyes, or bones: invasive candidiasis. |
Systemic fungal infection is further divided in 2 types: Opportunistic and Endemic.
a. Opportunistic systemic fungal infection: These types of infections are life threatening; they mainly occur when self defence mechanism is weak or not dominant due to any reason. As cases of immunosuppression are increasing due to autoimmune disease hence, we can notice a rise in systemic fungal infection. People who undergo bone marrow transplantation must take immunosuppressant’s so that rejection or failure of therapy can be minimised. Approx 20-50% patients of haematological cancer die with the evidence that they developed invasive fungal infection while they were on immunosuppressant treatment.
b. Endemic Mycoses: Endemic fungal infections are specific to any areas. Occur in certain geographical conditions. These infections are widely spread due to increased cases of HIV and increase in traveling from one country to another. One of most common examples is of COVID-19 which was widely spread all over the world. From endemic infection due to traveling and direct contact with infected person it became a pandemic problem [10].
Classification of Antifungals
The main classes of antifungals are echinocandins, polyenes, azoles, and allylamines. Many compounds of polyenes were developed after 1950, but Amphotericin B is the medication of choice in every case. 5-Flucytosine and antifungals are utilised in combination therapy. For a variety of fungal infections, the triazole derivatives fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole are frequently used.

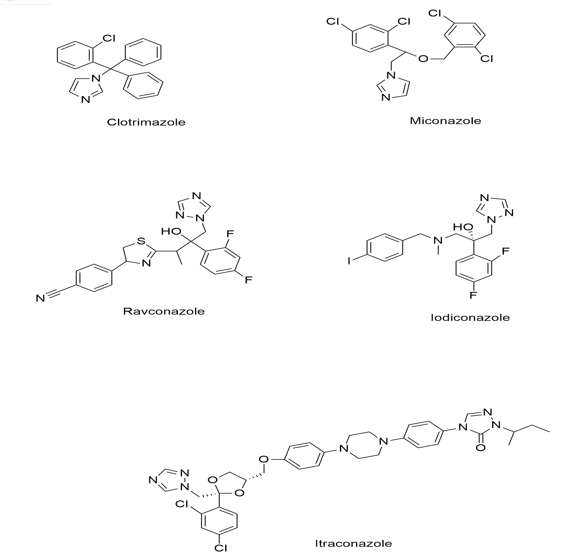
Triazoles, one of these kinds of broad spectrum antifungal medicines, work by preventing ergosterol from being biosynthesised, which is a necessary step in the manufacture of fungal membranes. Triazole antifungals have fewer negative effects and are frequently used [13].
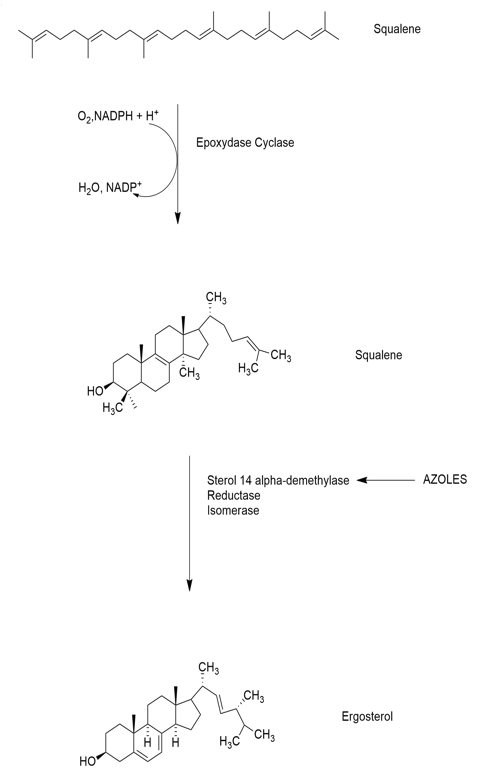
Triazole antifungals were derivatized from imidazole’s like miconazole. Triazoles inhibits ergosterol synthesis by inhibiting cytochrome P450 enzyme 14 alpha-demethylase. The transformation of lanosterol into ergosterol is carried out by this enzyme. Ergosterol is an important component of fungal cell membrane, triazole hence inhibit ergosterol synthesis and increase cell permeability. As triazoles are derived from imidazole like miconazole but these work only on superficial infections with poor oral bioavailability and high lipophilicity [14].
Thus, in search of a new and more beneficial agent whose bioavailability is high at the same time can be taken orally and intravenously, in mid of 1980 and 1990 fluconazole a triazole antifungal came in existence. This derivative brought a wide change in antifungal therapy as it has less side effect and toxic effect then previous existing antifungals. Fungal cytochrome P450 have an iron centre fluconazole tightly binds with iron centre. But strains that are triazole resistant have emerged which led to discovery of two new agents Voriconazole and Posaconazole [14].
V. TRIAZOLES AS ANTIFUNGAL
As discussed above triazoles are widely used antifungals with least side effects. Let as take fluconazole as a common drug from this category to understand its mechanism of action. Fluconazole is an FDA approved drug for treatment of various fungal infection like urinary tract infection, vaginal candidiasis, oesophageal candidiasis, systemic infections candidemia, pneumonia, cryptococcal meningitis. Non FDA approved treatment in blastomycosis, coccidioidomycosis and histoplasmosis.
VI. MECHANISM OF ACTION
Fluconazole beside being a highly effective antifungal agent have some limited activity to yeasts and against endemic fungi. Apart from this, fluconazole has highly effect against C. krusei and is quite active against candida and cryptococcus, whereas it is less active against C. glabrata.
Dugs under triazole category interacts with 14-demethylasewhich is helps in conversion of lanosterol to ergosterol. It also inhibits endogenous respiration and yeast growth. It is noticed that fungistatic activity of fluconazole is primarily caused by the loss of sterol [15].
Lanosterol 14-demethylase, an enzyme dependent on cytochrome P450 in fungi, is very specifically inhibited by fluconazole. Lanosterol must be converted to ergosterol by this enzyme in order to create the fungal cell walls. The single iron atom in the heme group of lanosterol 14-demethylase combines with the free nitrogen atom on the azole ring of fluconazole to form a stable connection. Consequently, the process of ergosterol production is halted by the inhibition of lanosterol demethylation. This stops oxygen activation. Then, it is discovered that methylated sterols are accumulating in the fungal cellular membrane, which results in the arrest of fungal growth. These accumulated sterols have a detrimental effect on the plasma membrane of the fungus cell.
A change in the amount or nature of the target enzyme may result in fluconazole resistance [16].
Other antifungals work by different mechanism of action some blocks the synthesis of lanosterol, some inhibit protein synthesis, some work by inhibiting ergosterol synthesis, whereas some drugs like amphotericin B makes pores in cell membrane and causes leakage in cell membrane.
All these mechanisms are explained below with the help of a diagram. In each step it is being explained how each drug functions differently on different enzymes.
VII. STRUCTURAL REPRESENTATION OF MECHANISM
As mentioned above these work by blocking P-450 that is important to catalyse 14-α -demethylation of lanosterol to ergosterol this causes release of 14-methylated sterols which causes permeability disturbance [17].


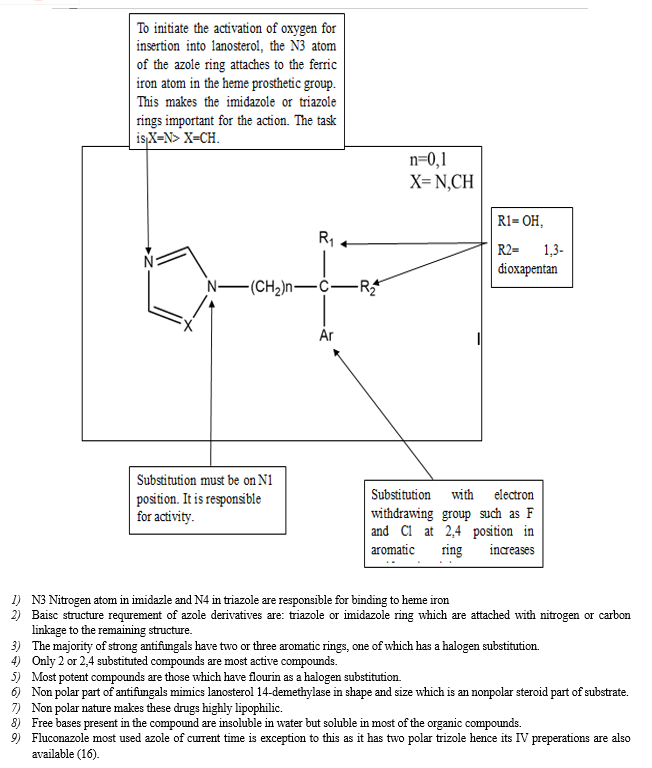
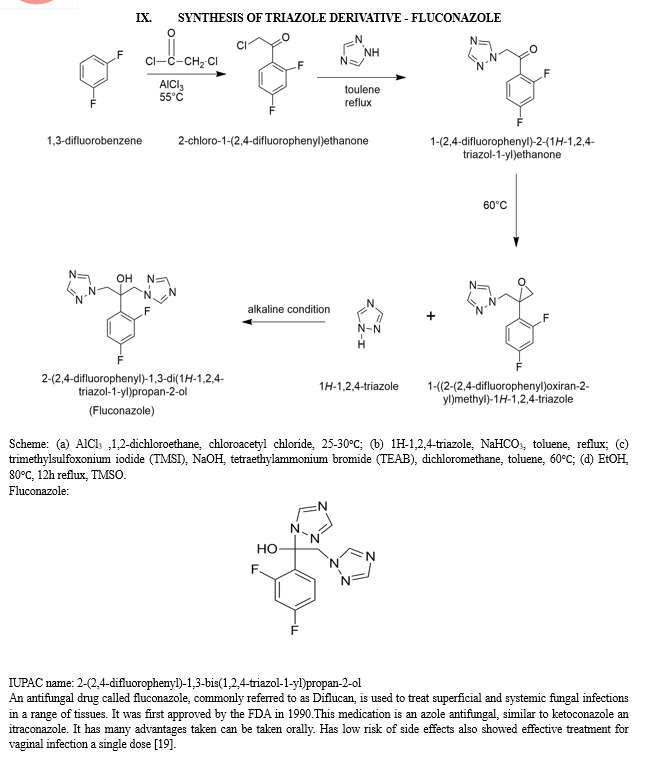
Fluconazole is a helpful treatment for fungal infections, such as Candida albicans, a kind of yeast since it has been demonstrated to have fungistatic action against the majority of strains of the following microorganisms. Examples of Candida species are Candida glabrata, Candida tropicalis, Candida parapsilosis, and Cryptococcus neoformans. This is accomplished by interfering with cell wall synthesis, growth, and adhesion in fungal cells, thereby treating fungal infections and their symptoms [20].
Additionally, in both normal and immunocompromised animal models with systemic and intracranial fungal infections brought on by Cryptococcus neoformans and systemic infections brought on by Candida albicans, fluconazole has been demonstrated to exhibit fungistatic action. The fact that fluconazole-resistant organisms have been shown to withstand different strains of organisms is noteworthy. This demonstrates that before considering fluconazole as an antifungal treatment, susceptibility testing is necessary [21].
The pharmacokinetic characteristics of oral and intravenous (IV) fluconazole administration are similar. In healthy individuals, a dose taken orally has a bioavailability of over 90%. Fluconazole absorbed through food has no effect on oral absorption, but it may take longer to reach the optimum concentration [16].
Fluconazole is effective, well tolerated has predictable pharmacokinetics hence can be used in majority of patients with candida infections including children, the elderly and immune suppressed persons. It also help in reducing the risk of infection in patients receiving cytotoxic chemotherapy [22].
With a half-life of roughly thirty hours and strong tissue penetration, fluconazole offers a broad therapeutic range and minimal toxicity. Due to these benefits, it is widely used which cause rising incidence of reduced susceptibility of some strains towards fluconazole. Since the main cause of resistance generation is lacking hence it should be prescribed in a limit with caution and strict indications in order to reduce the development of resistance [23].
Antifungals intake have been increased in past few years from 2008 to 2018. Countries having high economy are seen to have more consumption of antifungals. Fluconazole with 0.23 DDD/1000 inhabitants /day remains in 3rd number for the use in the country with middle to high income [24].
As fluconazole can penetration in CSF hence used to cure coccidioidal meningitis. During a study this azole derivative cured 99% of patients suffering from vaginal candidiasis by giving 150 mg orally for 3 days in 372 patients. This regimen was followed according to the clotrimazole regimen [25].
X. VORICONAZOLE
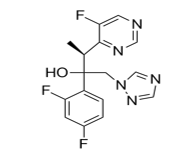
IUPAC name: (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1,2,4-triazol-1-yl) butan-2-ol
Aspergillus, Candida, Cryptococcus neoformans, and a few uncommon moulds are among the many clinically significant fungal infections that may be effectively treated with the initial second-generation triazole. When clinically appropriate, oral and intravenous versions of voriconazole can be switched since it is easily absorbed after oral administration and has an oral bioavailability of above 90%. Because of capacity-limited clearance, voriconazole has nonlinear pharmacokinetics and is therefore dose-dependent. As the dosage of voriconazole increases, the area under the plasma concentration-time curve (AUC) rises supra proportionally. The pharmacokinetics of voriconazole in youngsters (up to 12 years old) seem to be linear [26].
Like all azole medications, voriconazole works by blocking the cytochrome P450 (CYP450)-dependent demethylation of 14a-lanosterol, a crucial stage in the synthesis of ergosterol in fungal cell membranes. Similar to other azoles, voriconazole seems to have fungistatic properties against yeasts. Conversely, azoles belonging to the second generation, such as voriconazole, have fungicidal properties against certain filamentous species [27].
Because CYP450 inducers like carbamazepine, rifampin, and long-acting barbiturates diminish voriconazole concentrations, it is best to avoid using them in combination with voriconazole. It is not advised to take these two medications at the same time since doing so lowers voriconazole levels and increases rifabutin blood concentrations to fatal levels. Voriconazole and phenytoin, a CYP2C9 substrate and powerful CYP450 inducer, have a similar 2-way interaction. Phenytoin lowers voriconazole levels; if the two medications are administered concurrently, the oral voriconazole dosage should be increased. Voriconazole, on the other hand, raises phenytoin levels by fighting for the CYP2C9 enzyme that metabolizes phenytoin. When the two drugs are taken together, phenytoin levels must be closely checked [27].
All though voriconazole is a well-tolerated and has very less side effects but some of the majorly reported side effects are whether dose related or problems like reversible vision disturbance, blurred vision or photophobia [28].
XI. POSACONAZOLE
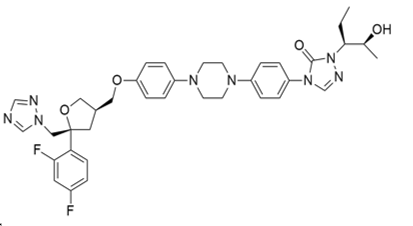
IUPAC name:
4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl) oxolan-3-yl] methoxy] phenyl] piperazin-1-yl] phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
Posaconazole is a triazole antifungal with a wide range of efficacy. Oral suspension is one way that it can be given. This triazole is of the second generation. Its structure is comparable to that of itraconazole when compared to other antifungal medications. The side chain of posaconazole help to bind with CYP51 tightly. Its binding is better than fluconazole and voriconazole [29].
Posaconazole is particularly effective against Candida species in vitro. Itraconazole and fluconazole are less effective against all Candida species and Cryptococcus neoformans. It has fungistatic and fungicidal action against most Candida species isolates in vitro and in vivo, inhibiting 97% of Candida species. Posaconazole has not yet been studied in an intravenous formulation; this may be an advantage as compared to other broad-spectrum antifungal medicines with intravenous formulations.
The dose of posaconazole is given four times up to 7 days at a dose of 200 mg orally called loading dose and the 400 mg twice a day called maintenance dose [30]. Posaconazole oral absorption is limited and is easily released from suspension and as the solubility is low in acid and basic conditions hence its absorption depends on food intake. The limited metabolism of posaconazole is largely facilitated by phase 2 biotransformation’s through the routes of uridine diphosphate glucuronosyltransferase enzymes. Therefore, inducers or inhibitors of different clearance pathways may have an impact on posaconazole plasma concentrations [31].
Within six month 43% adverse effects were reported. Most common side effect was found to be gastrointestinal problem and headache [29].
XII. CAUSES OF RESISTANCE
Anti-microbial resistance (AMR) will be the leading cause of death by 2050 according to WHO. AMR is great threat and will cause a lot of problem if no major steps are taken. Whenever it comes about AMR bacterial resistance is most discussed topic while antifungal resistance remains undiscussed and un-recognized. According to an estimation approximate more than 2 million people die per year due to fungal infection. Since so long the old classes of antifungal are used for the treatment due to which fungal infections are now resistance to approx. Every class of drugs hence it has become very important to develop some new antifungals in order to overcome serious drug resistance [9].
Fungal strains are more susceptible to mutation as they have small genome compared to plant and animal genome. Also, it is important to understand that a few types of antifungals can be used as fungi are eukaryotes and these may have capability to overlap with hosts cellular machinery. Anti-fungal resistance may be caused by various reasons some are [32]:

XIII. MECHANISM OF RESISTANCE
There are 3 main mechanisms by which antifungal resistance generates especially for triazole are: expression of unregulated 14-α-demethylase, some type of changes or alteration on binding site of triazole, multidrug efflux transporters up regulation. It is not necessary that only one type of mechanism will be followed by strain or drug resistance one strain can have more than one mechanism [3].
The most widely used class of antifungals is azoles. Hence most strains have generated resistance for azole derivative. One of the most common resistant strain Candida Albicans is resistant to fluconazole. This resistance case was 1st observed in AIDS patients who were suffering from recurring oropharyngeal candidiasis. These patients were treated with fluconazole for the long time. When research was performed on this treatment failure researcher noticed 3 genetic:
- In Erg11p there was a point mutation in coding sequence. It caused decreased in affinity for azoles.
- Gain of function (GOF) mutation in transcription regulator because of intrinsic upregulation of Erg 11p
- Upregulation of multidrug of multidrug efflux transporters Cdr1 and Cdr2 because of GOF mutation in transcription factor Tac1 and Mrr1.
All these steps may be followed in a sequence by sequential C albicans isolates may start with upregulation of transmembrane transporters which causes a decrease in intracellular accumulation of fluconazole. Also change in the coding sequence causes reduced fluconazole binding affinity [33].
XIV. ADVERSE EFFECTS
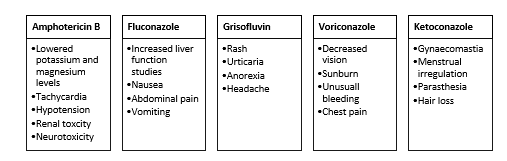
Fluconazole and itraconazole were already available on the market in 2004. In 2002, voriconazole was released. Isavuconazole and posaconazole received approval in 2006 and 2015, respectively. Voriconazole has been used often in adults and children over two years of age, however there is no proof that it should be administered to neonates or young children younger than two years old. There is no evidence to support the use of isavuconazole in paediatric patients under the age of 18, as its safety and efficacy have not been established. Posaconazole isavuconazole, voriconazole and fluconazole antifungals have been linked to dryness, drug rash, hair loss, diarrhoea, lethargy, nausea, and vomiting, as well as a prolonged QT interval [34].
Although isavuconazole has a similar mechanism of action and belongs to the same triazole family as the other four medications, it induces the opposite side effect, such as a shortening of the QT interval.14 Furthermore, itraconazole's boxed warning mentions medication interactions, cardiac consequences, and congestive heart failure (CHF) [35].
The key aspect of the clinical presentation is neurotoxicity, which is an uncommon and dangerous adverse event. Triazoles have been demonstrated in the past to raise plasma levels of vinca alkaloids and produce neurotoxicity due to their inhibitory impact on CYP3A4. It is not apparent how triazole antifungals produce neurotoxicity [35].
XV. RECENT ADVANCEMENT
Triazole hybrids
Triazole exist in 2 forms 1,2,3-triazole and 1,2,4-triazole. Both two forms have their own action.1,2,4-triazole is widely used for antifungal activity as N4 is responsible for binding to heme protein of the fungal cell. But due to increased amount of resistance and presence of superbugs has caused failure of already existing drugs. To overcome this problem triazole hybrids are designed. These are combination of triazole along with other rings which in combination gives better result than the non-substituted ring. Invasive fungal infection has caused many deaths hence introduction of new 1,2,4-triazole is necessary. 1,2,4-triazole have recently evaluated for development of new antifungals as well as antibacterials [36].
Some of recent study on hybrid of triazole:

Therefore, it is necessary to synthesise and develop novel antifungal drugs. 1,2,4-triazole is one of the most significant pharmacophore systems between five-membered heterocycles. The structure-activity relationship (SAR) of this nitrogen-containing heterocyclic molecule indicated possible antifungal action. A number of antifungal medications include the 1,2,4-triazole core in their nucleus. Triazoles' most potent and broad activity has confirmed them as pharmacologically significant moieties [36].
Triazoles have made it easier to treat a variety of fungal diseases, such as aspergillosis, candidiasis, and cryptococcal meningitis. However, the death rate from these infections is still too high even with antifungal therapy. Thus, scientists are putting a lot of effort into developing cutting-edge antifungal medications that specifically target unique fungus structures or activities. Rapid advances in molecular mycology have resulted in a focused hunt for additional target antifungals. Although we are entering a new age of antifungal medicine and will continue to face systemic fungal illnesses, our therapeutic choices will have substantially broadened [36].
Conclusion
Although antimicrobial treatment is getting advance due to which now it is easy to manage the outbreak of any infection. But fungal infection needs much more attention, there is a lot to discover about antifungal therapy. Due to generation of resistance for fluconazole to candida albicans, it is now much more important to introduce new antifungals which can be administered by various routes. Improper medication and excessive use of antifungals need to be controlled to slow down the speed of resistance generation. Moreover, studies on hybrids are going on for effective treatment of fungal infections. Acknowledgement I would like to express my deepest thank to my college Department of Pharmaceutical Sciences, Sir J.C. Bose Technical Campus, Bhimtal, Kumaun University, Nainital
References
[1] M. A. Ghannoum and L. B. Rice, “Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance,” Clin Microbiol Rev, vol. 12, no. 4, pp. 501–517, Oct. 1999. [2] “Antimicrobial Resistance in Candida | Fungal Diseases | CDC.” Accessed: Sep. 29, 2023. [Online]. Available: https://www.cdc.gov/fungal/diseases/candidiasis/antifungal-resistant.html [3] S. G. Whaley, E. L. Berkow, J. M. Rybak, A. T. Nishimoto, K. S. Barker, and P. D. Rogers, “Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species,” Frontiers in Microbiology, vol. 7, 2017, Accessed: Sep. 29, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fmicb.2016.02173 [4] “Antifungal Resistance: What it is, Causes, Treatment & Prevention,” Cleveland Clinic. Accessed: Sep. 30, 2023. [Online]. Available: https://my.clevelandclinic.org/health/articles/21557-antifungal-resistance [5] C. Guerrero-Perilla, F. A. Bernal, and E. D. Coy-Barrera, “Molecular docking study of naturallyoccurring compounds as inhibitors of N-myristoyl transferase towards antifungal agents discovery,” Revista Colombiana de Ciencias Químico - Farmacéuticas, vol. 44, no. 2, pp. 162–178, May 2015, doi: 10.15446/rcciquifa.v44n2.56291. [6] P. S. Bora, A. Kumar, A. K. Singh, H. Singh, B. Narasimhan, and P. Kumar, “Molecular Docking and QSAR Studies of Indole Derivatives as Antifungal Agents,” Current Chinese Chemistry, vol. 3, no. 1, pp. 1–12. [7] D. J. Sheehan, C. A. Hitchcock, and C. M. Sibley, “Current and Emerging Azole Antifungal Agents,” Clin Microbiol Rev, vol. 12, no. 1, pp. 40–79, Jan. 1999. [8] “New triazole molecular hybrids as antifungal agents | Frontiers Research Topic.” Accessed: Oct. 16, 2023. [Online]. Available: https://www.frontiersin.org/research-topics/51854/new-triazole-molecular-hybrids-as-antifungal-agents [9] “Combatting Antifungal Resistance,” ASM.org. Accessed: Oct. 22, 2023. [Online]. Available: https://asm.org:443/Articles/2022/November/Combatting-Antifungal-Resistance [10] G. Garber, “An Overview of Fungal Infections:,” Drugs, vol. 61, no. Supplement 1, pp. 1–12, 2001, doi: 10.2165/00003495-200161001-00001. [11] F. C. Odds, A. J. P. Brown, and N. A. R. Gow, “Antifungal agents: mechanisms of action,” Trends in Microbiology, vol. 11, no. 6, pp. 272–279, Jun. 2003, doi: 10.1016/S0966-842X(03)00117-3 [12] “Fungal Infection (Mycosis): Types, Causes & Treatments,” Cleveland Clinic. Accessed: Oct. 09, 2023. [Online]. Available: https://my.clevelandclinic.org/health/diseases/24401-fungal-infections-mycosi [13] “Triazole Antifungal Agents,” 2011. [14] “Triazole Antifungal Agent - an overview | ScienceDirect Topics.” Accessed: Oct. 12, 2023. [Online]. Available: https://www.sciencedirect.com/topics/chemistry/triazole-antifungal-agent [15] A. Govindarajan, K. G. Bistas, C. J. Ingold, and A. Aboeed, “Fluconazole,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2023. Accessed: Oct. 13, 2023. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK537158/ [16] “Fluconazole.” Accessed: Oct. 13, 2023. [Online]. Available: https://go.drugbank.com/drugs/DB00196 [17] A. K. Kabi et al., “An Introduction on Evolution of Azole Derivatives in Medicinal Chemistry,” in Nanostructured Biomaterials: Basic Structures and Applications, B. P. Swain, Ed., in Materials Horizons: From Nature to Nanomaterials. , Singapore: Springer, 2022, pp. 79–99. doi: 10.1007/978-981-16-8399-2_4. [18] A. Ahmadi, E. Mohammadnejadi, P. Karami, and N. Razzaghi-Asl, “Current Status and Structure Activity Relationship of Privileged Azoles as Antifungal Agents (2016–2020),” International Journal of Antimicrobial Agents, vol. 59, no. 3, p. 106518, Mar. 2022, doi: 10.1016/j.ijantimicag.2022.106518. [19] P. Vandeputte, S. Ferrari, and A. T. Coste, “Antifungal Resistance and New Strategies to Control Fungal Infections,” International Journal of Microbiology, vol. 2012, Dec. 2011, doi: 10.1155/2012/713687. [20] F. Bongomin, S. Gago, R. O. Oladele, and D. W. Denning, “Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision,” Journal of Fungi, vol. 3, no. 4, Art. no. 4, Dec. 2017, doi: 10.3390/jof3040057. [21] B. J. Kullberg and M. C. Arendrup, “Invasive Candidiasis,” New England Journal of Medicine, vol. 373, no. 15, pp. 1445–1456, Oct. 2015, doi: 10.1056/NEJMra1315399. [22] M. V. Martin, “The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a review,” Journal of Antimicrobial Chemotherapy, vol. 44, no. 4, pp. 429–437, Oct. 1999, doi: 10.1093/jac/44.4.429. [23] S. Natsch, M. H. M. Steeghs, Y. A. Hekster, J. F. G. M. Meis, J. W. M. van der Meer, and B. J. Kullberg, “Use of fluconazole in daily practice: still room for improvement,” Journal of Antimicrobial Chemotherapy, vol. 48, no. 2, pp. 303–310, Aug. 2001, doi: 10.1093/jac/48.2.303. [24] S. Pathadka et al., “Global Consumption Trend of Antifungal Agents in Humans From 2008 to 2018: Data From 65 Middle- and High-Income Countries,” Drugs, vol. 82, no. 11, pp. 1193–1205, Jul. 2022, doi: 10.1007/s40265-022-01751-x. [25] E. M. Bailey, D. J. Krakovsky, and M. J. Rybak, “The Triazole Antifungal Agents: A Review of Itraconazole and Fluconazole,” Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, vol. 10, no. 2, pp. 146–153, 1990, doi: 10.1002/j.1875-9114.1990.tb02561.x. [26] U. Theuretzbacher, F. Ihle, and H. Derendorf, “Pharmacokinetic/Pharmacodynamic Profile of Voriconazole,” Clin Pharmacokinet, vol. 45, no. 7, pp. 649–663, Jul. 2006, doi: 10.2165/00003088-200645070-00002. [27] L. D. Saravolatz, L. B. Johnson, and C. A. Kauffman, “Voriconazole: A New Triazole Antifungal Agent,” Clinical Infectious Diseases, vol. 36, no. 5, pp. 630–637, Mar. 2003, doi: 10.1086/367933. [28] R. C. Rathbun and H. L. Hoffman, “Review of the safety and efficacy of voriconazole,” Expert Opinion on Investigational Drugs, vol. 11, no. 3, pp. 409–429, Mar. 2002, doi: 10.1517/13543784.11.3.409. [29] G. M. Keating, “Posaconazole,” Drugs, vol. 65, no. 11, pp. 1553–1567, Aug. 2005, doi: 10.2165/00003495-200565110-00007. [30] H. A. Torres, R. Y. Hachem, R. F. Chemaly, D. P. Kontoyiannis, and I. I. Raad, “Posaconazole: a broad-spectrum triazole antifungal,” The Lancet Infectious Diseases, vol. 5, no. 12, pp. 775–785, Dec. 2005, doi: 10.1016/S1473-3099(05)70297-8. [31] Y. Li, U. Theuretzbacher, C. J. Clancy, M. H. Nguyen, and H. Derendorf, “Pharmacokinetic/Pharmacodynamic Profile of Posaconazole,” Clin Pharmacokinet, vol. 49, no. 6, pp. 379–396, Jun. 2010, doi: 10.2165/11319340-000000000-00000. [32] G. Aperis and E. Mylonakis, “Newer triazole antifungal agents: pharmacology, spectrum, clinical efficacy and limitations,” Expert Opinion on Investigational Drugs, vol. 15, no. 6, pp. 579–602, Jun. 2006, doi: 10.1517/13543784.15.6.579. [33] R. Ben-Ami and D. P. Kontoyiannis, “Resistance to Antifungal Drugs,” Infectious Disease Clinics, vol. 35, no. 2, pp. 279–311, Jun. 2021, doi: 10.1016/j.idc.2021.03.003. [34] L. L. Benitez and P. L. Carver, “Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents,” Drugs, vol. 79, no. 8, pp. 833–853, Jun. 2019, doi: 10.1007/s40265-019-01127-8. [35] S. Chai, J.-L. Zhan, L.-M. Zhao, and X.-D. Liu, “Safety of triazole antifungals: a pharmacovigilance study from 2004 to 2021 based on FAERS,” Therapeutic Advances in Drug Safety, vol. 13, 2022, doi: 10.1177/20420986221143266. [36] Z. Kazeminejad, M. Marzi, A. Shiroudi, S. A. Kouhpayeh, M. Farjam, and E. Zarenezhad, “Novel 1, 2, 4-Triazoles as Antifungal Agents,” Biomed Res Int, vol. 2022, p. 4584846, Mar. 2022, doi: 10.1155/2022/4584846.
Copyright
Copyright © 2024 Manisha Joshi, Anita Singh, Priya Gupta, Hansika Bansal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET60355
Publish Date : 2024-04-15
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

