Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Review on Genotoxic Impurities in Pharmaceutical Dosage Forms and Strategies for Identification
Authors: S. H. Kalyankar, B. P. Patil, P. K. Chakor, R. C. Jagdale, K. S. Deshmukh
DOI Link: https://doi.org/10.22214/ijraset.2024.64926
Certificate: View Certificate
Abstract
Genotoxic pollutants can be broadly defined as pollutants that, regardless of the mechanism, cause significant changes in genetic material. People all over the world suffer from various health problems due to genotoxic pollutants. Recent recommendations from drug regulatory agencies in Europe and the United States (USA) require the monitoring of genotoxic and potentially genotoxic contamination in drug formulations at the ppm level. Therefore, this review evaluates several regulatory agency guidelines for monitoring genotoxic contamination, impurity levels, and toxicity in pharmaceutical products. The review also discusses analytical considerations for measuring genotoxic pollutants at trace levels and concludes with several examples of measuring several important classes of genotoxic pollutants. We will also explain our results. The article provides detailed information on genotoxicity studies of waste solutions, metal wastes, and isolated pollutants.
Introduction
I. INTRODUCTION
The pharmacy and pharmaceutical industry have grown rapidly over the past century, from small pharmacies and compounding pharmacies to multi-billion dollar global corporations. In addition to research and development of new drugs, drug safety is also important. Recent decades have seen many drug scandals, ranging from dangerous chemicals and wrong dosage forms to drugs that are intentionally fortified and adulterated. Patient safety and comfort are major concerns in medical care today. This requires quality pots and pans, as well as detailed crafting instructions and tricks. Such efforts by health care agencies are certainly important. For example, changing the term GMP from Good Manufacturing Practice to cGMP ("c" stands for "current") indicates a continued commitment to GMP requirements [1]. Similarly, in 2004, the United States Food and Drug Administration (USFDA) announced the transition from quality-by-design (QbD) to (QbT) testing. The International Council for Harmonization (ICH) has successfully adopted this approach, leading to the introduction of several quality standards. The main goal of QbD is to develop large-scale manufacturing processes based on the idea of ??producing high-quality products with minimal changes. In chemistry, the term "impurity" refers to a chemical substance that differs from the chemical composition of a certain chemical phase [2].
Three basic requirements must be met to determine compounds with purity properties [3]. First, pure chemistry must be thermodynamic at least in one chemical phase and characterized by a one-component phase diagram. Second, pure chemicals must demonstrate that their properties are truly homogeneous (that is, their properties do not change after passing through the steps of analytical chemistry). Only attempts to isolate and purify and attempts to obtain pure chemicals have failed. Finally, according to the standard chemical definition, there should be no traces of other chemical species. In reality, no chemical is 100% pure, as there are varying degrees of contamination. As detection limits in analytical chemistry decrease, the number of pollutants detected increases [4].
Impurities are considered a problem in chemical synthesis, but are not a concern as long as their identity is known and their quantity is controlled. Chemists can obtain compounds with a purity of 97.5% or less as long as these reagents do their job [5]. From a medical point of view, this is very complicated. Here, chemical synthesis products are designed and manufactured to address specific medical indications, not just chemicals. Contamination is very important here, as contamination can be accidentally transmitted to the patient [6].
A. Genotoxic impurities
Genotoxic pollutants are compounds that damage the genetic material (DNA, RNA) of cells when exposed to any level that affects cell integrity.
Genotoxic contamination in pharmaceuticals has attracted the attention of the industry due to its serious adverse effects on patient health. The presence of trace amounts of these pollutants can cause genetic mutations, carcinogenesis and gene disruption, leading to neoplastic diseases [7]. Genotoxicity leads to aneuploidy, inhibition of topoisomerase, overexpression of defense mechanisms, interaction with the mitotic spindle machine leading to carcinogenesis, mutagenesis, teratogenicity, and cytotoxicity. Therefore, the monitoring and control of genotoxic contaminants is an important task for analysts in the development and production of new drugs. Genotoxic pollutants can be produced from raw materials, solvents, catalysts, and reagents used in drug synthesis. It causes mutations and increases the risk of cancer [8].
Table 1: Genotoxic compounds in drug substances
|
Category / Stage |
Compounds |
|
Solvents |
Benzene, 1,2-dichloroethane |
|
Reagent |
Formaldehyde, epoxides, esters of phosphate & sulphonates |
|
Starting material |
Hydrazine, nitroso, acrylonitrile compounds |
|
Catalyst |
Toxic heavy metals, metal phosphates |
|
Intermediate |
Benzaldehyde, nitro compounds |
|
By-product |
Sulphonate esters, phosgene |
|
Degradation product |
N-oxides, aldehydes |
B. Classification of Genotoxic Impurities:
Chemical carcinogens have a significant impact on health in many countries, and international organizations such as the World Health Organization (WHO) have developed regulations to control them. According to the M7 recommendations of the International Conference on GTI Standardization and Evaluation in Pharmacy, they are divided into five types [1,9].
- Class 1: These pollutants are genotoxic and carcinogenic and present significant harm or risk.
- Class 2: This pollutant is known to be genotoxic, but unlikely to cause cancer. Finally, the "threshold toxicity (TTC) approach" should be used to control these pollutants to some extent.
- Class 3: These impurities have an unusual structure different from the drug formulation and unknown genotoxic potential. Impurities are defined in this group by structure-function relationships.
- Class 4: These compounds are structurally similar to medicinal compounds, have signaling functions and are not genotoxic.
- Class 5: There are no structural warnings for this pollutant. These pollutants are considered non-mutagenic.
Genotoxic contaminants (GTI) are regulated because they cause cancer in humans, and these active contaminants cause concern about high toxicity in pharmaceutical APIs. To ensure public safety, we need to know that GTI is in our medicines and control it in small amounts. The purpose of this paper is to review analytical methods and problems used in pharmaceuticals to access, monitor and control GTI [10–12].
C. Sources of Genotoxic impurities:
Impurities defined by the International Council for Harmonization (ICH), Food and Drug Administration (FDA), and USP include drug-related impurities, process-related impurities (PRI), solvent residues, and bimodal actives. Medicines are distributed component. Associated impurities are classified as the first type of API-related impurities due to specific reactions such as oxidation, dehydration, and decarbonization. Other types are caused by interactions between the API and the receptor, container, or impurities in the receptor, reagent, or solvent. Impurities associated with APIs may pose genotoxic, mutagenic, and carcinogenic risks due to their structure-activity relationships [13].
Genotoxic contamination can be introduced by various sources, mainly as raw materials and genotoxic intermediates used in the synthesis of drugs in the synthetic process. In addition, synthetic substances such as solvents, catalysts, and reagents used in drug synthesis act as genotoxic contaminants in pharmaceuticals. Deterioration of drugs by storage, exposure to light, atmospheric oxidation, or hydrolysis results in drug contamination. Stereoselective drug synthesis can involve stereoisomers of raw materials and intermediates, which help to remove impurities in the drug raw materials. Figure 1 shows the composition of impurities at different stages of drug synthesis [14,15].
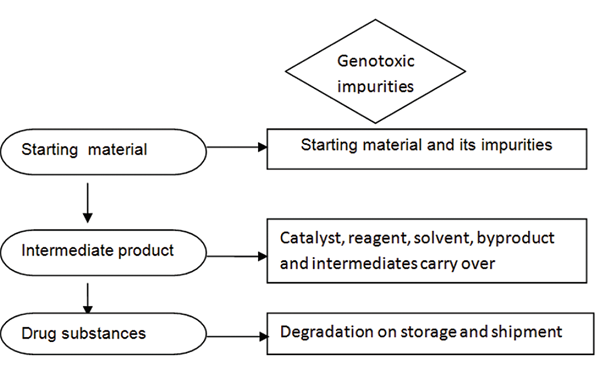
Figure 1: Sources of genotoxic impurities [15]
II. REGULATORY ASPECTS
There are many regulations and articles aimed at controlling the amount of PGI in pharmaceutical products using certain threshold values. Various industries and regulatory agencies have developed specific guidelines for disposing of genotoxic contaminants. Drug regulatory bodies such as USFDA, ICH and EMA have raised concerns about the presence of PGI in APIs and issued guidelines to limit PGI in APIs. To solve the problem, R&D researchers must actively identify PGI during development, develop appropriate analytical methods, and demonstrate control strategies for synthetic processes leading to PGI [15].
A. PHARMA Approach
The Pharmaceutical Research and Manufacturing Association (PhRMA) established a task force in 2004 to investigate, categorize, categorize, and evaluate the toxicity risk of PGI in pharmaceutical products [16]. Showed that most structurally sensitive functional groups are involved in DNA reactions. This group divides DNA into three groups [17]:
- Group 1: N-hydroxyaryl, alkyl aminals, aza-aryl N-oxide, alkylation and aminals, purines or pyrimidines, intercalators, etc. Aromatic group.
- Group 2: Alkyl and aryl groups such as aldehydes, N-methylol, N-nitrosamines, nitrogen compounds, carbamates, epoxides, aziridine, propiolactone, propisolones, haloethyl beta, hydrazine, and azo compounds.
- Group 3: Heteroaromatic groups include reactive Michael acceptors, alkyl phosphonates or sulfonate esters, haloalkanes, and basic halides (alkyl and aryl-CH2). PhRMA divides impurities into five categories [15].
- Class 1: Genotoxic (mutagenic) and carcinogenic pollutants. These pollutants represent the most serious threat and their elimination is the most important challenge for the conversion process. If this is not possible, the TTC concept should be adopted as a last resort.
- Class 2: Genotoxic (mutagenic) but pollutant with unknown carcinogenic potential. This waste is regulated by the TTC principle.
- Class 3: Contains signal compounds of unknown API structure and genotoxic (mutagenic) potential. This group includes impurities and functional parts that may be related to genotoxicity depending on their composition.
- Class 4: Contains errors in the API custom alert structure. This group includes functional and structural generalized anxiety disorders.
- Class 5: Waste without a warning structure is considered normal waste and managed according to ICH guidelines.
- Class 3 or Class 4 compounds are assigned to Class 2 and Class 5 if genotoxicity has not been tested.
B. USFDA Draft Guidance
In December 2008, the USFDA distributed a draft rule named "Suggested Approach for Genotoxic and Cancer-causing Pollutants in Medication Substances and Items".
As of late, this rule has been supplanted by the ICH M7 rule on "Assessment and Control of DNA-Responsive Debasements (PGIs) in Drug Items to Restrict Potential Malignant growth Dangers." The USFDA rules give explicit suggestions to the wellbeing capability of genotoxic pollutants. known or thought. These rules portray different techniques for describing and lessening the potential disease chances related with genotoxic and cancer-causing pollutions. Suggested approaches incorporate (1) counteraction of genotoxic and cancer-causing contaminations; b) decrease of levels of genotoxic and cancer-causing pollutions (most extreme passable day to day target measure of 1.5 g/day), (iii) extra recognizable proof of genotoxic and cancer-causing dangers, and (iv) contemplations for adaptability of way to deal with better help fitting debasement particular [18, 19].
1) ICH guidelines
According to ICH guidelines, impurities in drug substances and drug products are non-toxic and do not cause adverse effects. (a) 0.10% or 1.0 mg per day (whichever is less) 0.15% or 1.0 mg (whichever is less) > 2 g per day 0.03% 0.05% 0.05% a) Dose which is ordained every day. (b) Higher reporting limits must be scientifically justified. c) If the impurities are highly toxic, there may be a lower limit. Benefits for patients. For example, ICH Q3A15 regulates impurities in new drug substances by setting limits for reporting, identifying and detecting impurities. ICH Q3B16 and ICH Q3C17 are similar guidelines for controlling impurities and residual solvents in new drugs. Based on the potential risk to human health, waste solutions are divided into three categories [20].
Class I solvents should be avoided and daily exposure limits should be allowed for class II solvents, but there is no health limit for class III solvents with an exposure of 50 mg per day. ICH Q3D is currently under development and includes elements of heavy metal pollutants and limits [21]. The currently published ICH guidelines for impurity limits are not appropriate for most genotoxic contaminants. The main problem of genotoxic contamination is that drug synthesis requires the use of reactive substances that can interact with human DNA, cause mutations, and even cause cancer at very low levels. Therefore, genotoxic contamination should be prevented or reduced as much as possible below the defined limit [22].
2) EMEA guideline
The European Medicines Agency (EMEA) guidelines provide a framework and practical approach on how to address genotoxic impurities in new active ingredients. According to the guidelines, "Toxicological assessment of genotoxic impurities and the determination of acceptable limits for such impurities in active ingredients is a difficult problem and is not discussed in detail in the current ICH Q3X guidelines. The TTC limit for genotoxic impurities is considered to be 1.5 μg/mg/drug this drug is accepted by the majority. " Product Day, Medicinal Products for Human Use (CHMP), 2006 Classification of hazardous compounds (lifetime risk less than 1) as genotoxic (pollutant) in vitro or in vivo genotoxicity test using DNA reagent. Considered mutagenic and carcinogenic in EMEA / CHMP, 2006) [23,24].
3) Toxicological Background
Current observations show that genotoxic compounds (in vivo) can cause DNA damage at any exposure level and that such damage can lead to tumorigenesis. Therefore, there is no established threshold for genotoxic carcinogens and it is reasonable to assume that any level of exposure is a risk. However, when it comes to genotoxic events, there is evidence that the mechanism causes biologically significant threshold effects. This is especially true for non-DNA targets and compounds that can be neutralized before hitting the critical target. The regulatory approach for such chemicals can be based on establishing no-effect levels (NOELs) and using uncertainty factors. Even for compounds that can interact with DNA molecules, some protective mechanisms are effective at low doses, so linear extrapolation to very low exposure levels (to humans) in high-dose studies may not be appropriate. However, experimentally proving the existence of a genotoxic threshold for a particular mutagen is currently very difficult. Therefore, if there is sufficient evidence to support a range of genotoxic compounds and it is difficult to determine a safe dose, the concept of acceptable risk should be adopted [25,26].
III. CLASSFICATION OF GENOTOXIC IMPURITIES
1) Alkyl halides
Methyl halide, ethyl halide, and propyl halide are widely used as industrial alkylating agents. Although the mechanism of toxicity is not fully understood, it directly alkylates biologically active macromolecules such as proteins and DNA.
Sources of alkyl halides in the API stream include not only the direct use of alkyl halides, but also the leaching of hydrogen halides and ammonia salts from alcohol solutions [27].
2) Dialkyl Sulfates
The most common dialkyl sulfates used in the pharmaceutical industry are methyl and ethyl sulfate, the latter of which is used as a chemical weapon. Dimethyl sulfate (DMS), a strong methylating agent, is used to introduce methyl groups to atoms characterized by lone pairs, such as oxygen, nitrogen, carbon, sulfur, phosphorus, and some metals. DMS is preferred over alkyl halide-based methylating agents because the reaction is faster and the production of side products is less.
A para-sympathomimetic drug that acts as a reverse acetylcholinesterase inhibitor in the final synthesis step of quaternary aromatic amines. Dimethyl sulfate is used in the final step of API synthesis to form neostigmine, a quaternary ammonium salt [28].
3) Epoxides
Epoxides are the simplest cyclic ethers with three ring atoms. Due to the large ring strain associated with the three-membered ring, epoxides are highly reactive molecules and are therefore often used as reagents for API production. It readily participates in epoxide ring-opening reactions with alcohols, amines, halides, organometallics, cyanides, sulfides, aromatics, and reactive methylene groups. On the other hand, the high reactivity of this compound leads to genotoxicity because the two electrophilic carbon atoms of this compound can react with the nucleophilic center of DNA to form alkylation products. Substituted epoxides such as 2,3-epoxypropanol (glycidol), 1-chloro-2,3-epoxypropane (epichlorohydrin), or 1,2-epoxy-3-butene during API synthesis. Epoxide ring opening reactions are localized and functional, so they can act as coupling agents or form heterocycles [29].
4) Hydrazine
The toxicity of hydrazine and its derivatives is due to the formation of carbocations, carbon-based radicals, and oxygen-based radicals, which are highly reactive species. DNA alkylation and other DNA damage have been reported for these reactive intermediates [30].
The formulation of Sildenafil, also known as Viagra, used to treat erectile dysfunction, involves the formation of a substituted pyrazole ring with the help of hydrazine [31].
5) Aromatic Amine
Aromatic amines are generally not genotoxic, but electrophilic species are produced after metabolic activation. The main metabolic pathway of aromatic amines is oxidation to produce N-hydroxy compounds related to acetate, sulfate, or glucuronide. Binding then produces nitrene ions (ArN + H), which are considered active DNA-bound genotoxins [32].
6) Boronic acid
Boronic acid was recently tested and identified as a new family of bacterial mutagens. However, there is no direct evidence of direct covalent bonding between them and DNA. Recently, 12 of thirteen boric acids tested by O'Donovan et al. Losartan, an angiotensin II receptor antagonist, is used to treat high blood pressure. The diagram below shows the synthetic steps involved in the synthesis of losartan developed by Merck research chemists such as Suzuki. First, tertly protected phenyltetrazole was ortholyzed with butyl ether, then quenched with triisopropyl borate, and after treatment with aqueous ammonia chloride solution, boronic acid compound was obtained. Another major Suzuki interface with these derivative boronic acids is involved in coupling reactions with imidazole alcohols [33].
7) Sulfonates Esters and Their Precursors
Sulphonic acid esters are alkylating agents and potentially genotoxic compounds. They are called alkylating agents because of their ability to add alkyl residues to the active nucleophilic sites of DNA bases or after metabolic activation [34].
8) Nitrosamines
Nitrosamines or N-nitrosamines are molecules that contain a nitrogen functional group (N-NO). N-nitrosamines are polar, hydrophilic molecules with high vapor pressure and very high solubility in water. N-nitroso compounds are a large group of strong carcinogens, including N-nitrosamines.
These N-nitrosamines are derived from alkyl, alkaryl, aryl or cyclic amines. Common groups derived from N-alkyl amides, N-alkylureas, N-alkyl carbamates, and N-nitrosamides. These compounds are associated with nitrosamine pollutants that can be mutagenic, teratogenic, and carcinogenic to humans [35].
ICH M7(R1) classifies nitrosamine pollutants as Class 1 genotoxic pollutants based on carcinogenicity and mutagenicity data in mice. These are known to be mutagenic and carcinogenic. These nitrosamine impurities affect genetic material by causing mutations through chromosome breakage, rearrangements, covalent bonds, or duplicated DNA. Changes in genetic material caused by exposure to small amounts of nitrosamine pollutants can lead to cancer. Therefore, the detection of nitrosamine impurities in pharmaceutical products is important to ensure public safety [36].
IV. TECHNIQUES TO DETECT GENOTOXIC IMPURITIES
Contaminant analysis and analytical methods that are sensitive, selective and reliable are essential to develop a rational and adequate management strategy for GTI. The choice of method for GTI analysis depends on the expected concentration of these pollutants, target definition, and regulatory compliance. Therefore, the chosen method must provide reliable analytical data and the chosen method can be used for typical test loads. Analytical methods are based on genotoxic and chemical characteristics of GTI, including different compositions, reactivity and sensitivity to the chosen detection method, and possible matrix effects (shown in Table 5).
Quantification of ppm levels of GTI in pharmaceuticals poses several challenges for analytical chemists, including:
- Selection of appropriate analytical methods to develop methods according to GTI characteristics (volatility, thermal stability, presence of chromophores, hydrophobicity, etc.).
- GTI characteristics, such as GI reactivity and stability, must be determined during the development of the method to provide the necessary evidence for reproducibility and precision.
- Choose a sensitive analytical method, clinical dose and study duration in the development of the method.
- The developed analytical method includes parameters for choosing the detection method (ultraviolet [UV], light scattering, electrochemical detection, mass spectrometry, etc.). For example, the matrix impedance is the inverse of the matrix quotient:
- Isolation of target analytes by sample preparation.
- Chromatographic solution or
- Using more selective detectors.
- Various analytical methods can be used to analyze GTI. Due to the different structure of the matrix and the complexity of the matrix, it is difficult to choose the ideal method. However, no single approach can be used to solve all problems. Over time, isolation methods, linear methods, and software based on silicon drug design have been widely used [38].
- Many analytical laboratories in the pharmaceutical industry follow a consistent strategy to develop the two-step GTI method:
- Evaluation of analytical volatility to choose a chromatographic method.
- Choose a detection method based on the properties of the analyte, such as the presence of chromophores or halogen atoms in the molecule.
A. Genotoxic Impurities and Principle of Genotoxicity
Genotoxic pollutants and chemicals can have mutagenic and DNA-damaging effects. Genotoxicity tests are performed such as in vitro and in vivo tests. In general, this test has been shown to identify compounds that cause genetic damage through different mechanisms [39]. The purpose of this test is to determine the risk associated with DNA damage and stabilization. DNA damage can occur in a number of ways, including genetic mutation, large-scale chromosomal damage, or recombination. In addition, genetic influences and various disease processes can be studied. The entire implementation process is complex and genetic variation is partly responsible [40].
Changes in the number of chromosomes may be related to tumor progression. Carcinogenicity can be predicted by genotoxicity tests. Potential mutagenic impurities in pharmaceuticals can be identified by conventional mutagenetic studies. More detailed information on this is provided in the ICH S2 Guidelines on genotoxicity testing and data interpretation. The safe dose of chemicals / solutions can be calculated using various QSAR-based software such as MDL, DS TOPKAT, Tox box and Leadcope. Estimated safe doses were calculated for acrylonitrile (7.6 μg/day), 2-amino-4-nitrophenol (1007 μg/day), and nitrobenzene (31 μg/day) and met the safe carcinogenic dose limit in animals. From this study, it can be concluded that in silico detection of mutagenic structural defects provides a sensitive and conservative method for the detection of genotoxic contaminants [41].
In the absence of additional genotoxicity literature, the detection of contamination using the reverse mutation method of bacteria (Ames test) should be considered [42]. In recent years, much attention has been paid to new strategies developed to combat genotoxic pollutants or genotoxic pollutants arising from drug synthesis. These genotoxic pollutants can be introduced as starting materials, reagents, intermediate catalysts, side products, degradation products, and isomers [43,44].
B. Analysis of Genotoxic Impurities
Analyze contaminants using sensitive, selective, and reliable analytical methods to develop GTI management strategies. The GTI analysis method is determined by the expected levels of these pollutants that meet target standards and regulatory requirements. Analytical procedures should provide a detection limit of 1 to 5 ppm (0.0001 to 0.0005% w/w). Additional organic impurities such as by-products may be more abundant in the lower concentration range, so such low levels require not only sensitive analytical equipment but also greater selectivity. Low-level impurities can be treated with large amounts of API [45]. Gas chromatography (GC) and liquid chromatography (LC), often used with mass spectrometry (MS) detection, are two analytical methods for GI determination. Below are some common examples of Gistrace analysis using GC and LC. The possibility of online reaction monitoring has also been discussed [46].
The European Agency for the Evaluation of Medicinal Products (EMEA) has developed guidelines to limit genotoxic impurities in pharmaceutical formulations based on the concept of toxicity concerns (TTC) (1). With the "general warning function" (2), the pollutant is measured at a level below the TTC, equivalent to a daily intake of 1.5 g (for lifetime exposure). In practice, this means that genotoxic impurities in medicinal substances or pharmaceutical products must be detected at lower levels than conventional impurity assessment methods. In general, GI (dissolved mass or specific solubility) can be determined at a drug concentration of 10 ng/g to 1000 mg/g (10 to 1000 ppb). As a result, it is difficult to develop methods to detect genotoxic contamination in drug testing. HPLC (using a UV/Vis detector) and GC (using a FID detector) are the most commonly used methods to detect genotoxic pollutants. Mass spectrometers have been used as detectors in recent years to achieve better sensitivity and selectivity [47].
C. Herbal Medicine and Genotoxicity
The use of herbal medicine to prevent and treat disease has a long history. The history of its use goes back to mankind and the earliest written records of antiquity, the Middle Ages, and modern times. [48] ??It has been a major part of human civilization since ancient times. The World Health Organization reports that about 80% of the world's population relies on medicinal plants for primary health care. Since then, herbs have been widely used throughout the world, especially in Western countries [49]. For example, 71% of the Canadian population (IPSOS-Reid, 2005) IPSOSReid, 2005 Medicine. Approximately 19% of US adults use herbal medicinal products [51,52], and the market size in the US has increased from $23 billion in 2000 to $5 billion in 2011 (NBJ). Plants from Africa and Asia cost an average of $1 billion in 2004 [53].
In addition, the WHO recommends the use of traditional Chinese medicine in the primary health care system in poor countries [54]. Although herbal remedies are effective and documented, their toxicity is often unknown. Furthermore, unlike modern pharmaceutical research and development, the toxicity of traditional herbal medicines is often unknown. On the other hand, many people are apathetic and believe that because these things have been used before, they must be safe. Advances in genomics, proteomics, and metabonomics in the post-genomics and bioinformatics era may be important in determining genotoxicity, teratogenicity, and nephrotoxicity of plant-based therapies [55].
Conclusion
Identification and regulation of genotoxins by synthetic processes is difficult due to their variable properties and different routes of entry. Therefore, synthetic pathways should be tested for structural defects associated with genotoxicity. This review article describes genotoxic contaminants (GTI), their sources, classification, and current regulatory approaches to control genotoxic contaminants in pharmaceuticals. This review article provides a brief overview of the various GTI models and includes examples of each class. Since it is very difficult for researchers to determine the trace level of GTI, the detection method is also shown in the diagram. Almost all GTIs are mutagenic, but in the case of nitrosamines there is no strong evidence that they are mutagenic or carcinogenic, literature showing genotoxicity. Therefore, it is considered mutagenic and carcinogenic. Various management strategies to reduce addictive GTI are also discussed. Balancing risk and cost in drug compound development requires a multidisciplinary approach involving experts in toxicology, synthetic chemistry, and analytical chemistry. Finally, it is fair to conclude that a safe product has expected benefits and risks commensurate with the available alternatives.
References
[1] Sharma A. Kumar, SK. Review on identification and quantification of genotoxic impurities. International Journal of Health Sciences, 2022;6(S7):4043–4065. [2] “pharmaceutical cGMPs for the 21 st century-a risk-based approach final report,” 2004. [3] J. V. B.-H. A. I. J. for the P. of and undefined 2014, “Philosophy of science and philosophy of chemistry,” lirias.kuleuven.be, 2014;20:11–57. [4] Ahuja S. and Alsante K., Handbook of isolation and characterization of impurities in pharmaceuticals. 2003. [5] Abdin AY., Yeboah P., Jacob C., “Chemical Impurities: An Epistemological Riddle with Serious Side Effects,” Int. J. Environ. Res. Public Heal. 2020;17:1030. [6] Bercu JP., Dobo KL., Gocke E., Mcgovern TJ., “Overview of genotoxic impurities in pharmaceutical development,” Int. J. Toxicol., 2009;28(6):468–478. [7] Sultan S, Tengli A, Aksila G. Analytical Assessment of Genotoxic Impurities in Pharmaceuticals by Gas Chromatographic Techniques. JPA. 2019;8(1):22-32. [8] Matveeva OA and Kovaleva EL. Modern approaches to estimating the content of genotoxic impurities in drugs. Pharm Chem J. 2016;49:765-770. [9] “Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7(R1) Current Step 4 version,” 2017. [10] Holm R., Elder DP., “Analytical advances in pharmaceutical impurity profiling,” Eur. J. Pharm. Sci., 2016;87:118–135. [11] Pounikar AR., Umekar MJ., Gupta KR., “Genotoxic impurities: an important regulatory aspect,” Asian J. Pharm. Clin. Res., 2020;5:10–25. [12] Leistner A., Haerling S., Kreher JD., Becker I., Jung D., Holzgrabe U., “Risk assessment report of potential impurities in cetirizine dihydrochloride,” J. Pharm. Biomed. Anal., 2020;189.85. [13] Liu KT, Chen CH. Determination of impurities in pharmaceuticals: Why and how? In: Quality Management and Quality Control: New Trends and Developments. London, United Kingdom: Intech Open; 2019. [14] Gosar A, Sayyed H, Shaikh T. Genotoxic impurities and its risk assessment in drug compounds. Drug Design Intellect Properties Int J 2018;2:227-32. [15] Gupta et al. Genotoxic impurities: an important regulatory aspect. Asian J Pharm Clin Res, 2020;13(6):10-25. [16] Muller L, Mauthe RJ, Riley CM, Andino MM, Antonis DD, Beels C, et al. A rationale for determining, testing and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity, regul. Toxicol Pharmacol 2006;44:198-211. [17] Center for Drug Evaluation and Research, Food and Drug Administration. Guidance (Draft) for Industry Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches. United States: Center for Drug Evaluation and Research, Food and Drug Administration; 2008. [18] Patel AB, Asnani AH, Vyas AJ, et al. A Brief Review on Genotoxic impurities in Pharmaceuticals. Asian Journal of Pharmaceutical Research. 2021;11(3):187-3. [19] Reddy AVB et al. Identification, control strategies, and analytical approaches for the determination of potential genotoxic impurities in pharmaceuticals: A comprehensive review. The Journal of Separation Science. 2015;38(5):764–79. [20] International Conference on Harmonisation Guideline on Impurities in New Drug Products, Q3B(R2); 2006. Available from: https://www.ema. europa.eu/en/documents/scientific-guideline/ich-q-3-b-r2-impuritiesnew-drug-products-step-5en.pdf. [Last accessed on 2019 Oct 31]. [21] Dhara Vashi, Suresh Kumar. Impurity Identification and Characterization of some Anti-Diabetic Drugs using various Analytical Methods. Asian J. Pharm. Res. 2019;9(4):243-248. [22] Chandana OS, Babu R. Stability Indicating HPLC Method Development and Validation for Thalidomide and its Impurity Determination. Asian J. Pharm. Ana. 2016;6(2):115-118. [23] Johnson DB., Sai KP., Verma MA., Tamil Selvan. A. Antimutagenic Activity of Terminalia chebula Fruit Extract. Research J. Pharmacognosy and Phytochemistry 2010;2(6):455-459. [24] Prasad P, Agrawal RC., Roy A, Gupta N. Antimutagenic effects of Tribulus terristris fruit extract on Micronucleus. Research J. Science and Tech. 2012;4(3):140-142. [25] Dobo K.L., Greene N., Cyr M.O., Caron S., Ku W.W., The application of structure-based assessment to support safety and chemistry diligence to manage genotoxic impurities in active pharmaceutical ingredients during drug development, Reg. Tox Pharm. 2006;44:282-293. [26] Kroes R., Kozianowski G., Threshold of toxicological concern (TTC) in food safety assessment, Toxicol Letters. 2002;127:43-46. [27] Haney BP et al. Controlling the Genotoxins Ethyl Chloride and Methyl Chloride Formed during the Preparation of Amine Hydrochloride Salts from Solutions of Ethanol and Methanol. Organic Process Research and Development. 2009;13(4):786-791. [28] Fleming NW, Henderson TR, Dretchen KL. Mechanisms of respiratory failure produced by neostigmine and diisopropyl fluorophosphate. Europian Journal of Pharmacology. 1991;195:85–91. [29] Benigni R and Bossa C. Mechanisms of chemical carcinogenicity and mutagenicity: A review with implications for predictive toxicology. Chem Reviews. 2011;111(4):2507–36. [30] Kovacic P and Jacintho JD. Mechanisms of Carcinogenesis: Focus on Oxidative Stress and Electron Transfer. Current medicinal chemistry. 2001;8(7):773-796. [31] Terrett NK et al. Sildenafil (Viagra(TM)), a potent and selective inhibitor of type 5 CGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorganic and Medical Chemistry Letter. 1996;6(15):1819–1824. [32] Snodin DJ. Genotoxic impurities: From structural alerts to qualification. Organic Process Research and Development. 2010;14(4):960–976. [33] O’Donovan MR et al. Boronic acids-A novel class of bacterial mutagen. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2011;724(1–2):1–6. [34] Gocke E at al. Literature review on the genotoxicity, reproductive toxicity, and carcinogenicity of ethyl methanesulfonate. Toxicological Letters. 2009;190(3):254–265. [35] European Medicines Agency. Lessons learnt from presence of N-nitrosamine impurities in sartan medicines. Available on: https://www.ema.europa.eu/en/documents/report/lessons -learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf [36] Shaikh T, Gosar A, Sayyed H. Nitrosamine Impurities in Drug Substances and Drug Products. Journal of Advances in Pharmacy Practices. 2019;2(1):48–57. [37] Pierson DA, Olsen BA, Robbins DK, DeVries KM, Varie DL. Approaches to assessment, testing decisions, and analytical determination of genotoxic impurities in drug substances. Org Process Res Dev 2009;13:285-91. [38] Helmy R, Strickfuss S, Al-Sayah M, Hamilton S, Bu X, Lee C, et al. Quantification of Genotoxic Impurities in Active Pharmaceutical Ingredients. United Kingdom; Taylor & Francis Group; 2015;293. [39] Baker A. Development of a strategy for analysis of genotoxic impurities. Genotoxic impurities, strategies for identification and control. Wiley Publication. 2011:281-304. [40] Joshi MD, et al. Selective extraction of genotoxic impurities and structurally alerting compounds using polymeric ionic liquid sorbent coatings in solid-phase microextraction: Alkyl halides and aromatic. J Chromatogr A. 2012;1240:29-44. [41] Liu DQ, et al. Recent advances in trace analysis of pharmaceutical genotoxic impurities. J Pharm Biom Anal. 2010:999– 1014. [42] Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, et al. Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet. Food Chem Toxicol 2004;42:65-83. [43] Raman NVVSS, et al. Strategies for the identification, control and determination of genotoxic impurities in drug substances: A pharmaceutical industry perspective. J Pharm Biomed Anal 2011;55:662-667. [44] Sun M, et al. Matrix deactivation-A general approach to improve stability of unstable and reactive pharmaceutical genotoxic impurities for trace analysis. J Pharma Biom Anal. 2010;52:30-36. [45] David F., Rambla-Alegre M., Vanhoenacker G., Sandra P., “Determination of Genotoxic Impurities in Pharmaceuticals,” LC GC Eur. 2013:31–34. [46] Pierson DA., Olsen BA., Robbins DK., DeVries KM., Varie DL., “Approaches to Assessment, Testing Decisions, and Analytical Determination of Genotoxic Impurities in Drug Substances,” Org. Process Res. Dev. 2008;13(2):285–291, [47] M. Ramble Alegre, F. David, P. Sandra, and G. Vanhoenacker, “Determination of Genotoxic Impurities in Pharmaceuticals,” LCGC Suppl. 2013;31(5):55. [48] Williamson, Elizabeth M. \"Drug interactions between herbal and prescription medicines.\" Drug safety. 2003:15:1075-1092. [49] Jordan et al., Jordan, Scott A., David G. Cunningham, and Robin J. Marles. \"Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment.\" Toxicology and applied pharmacology. 2010:2:198-216. [50] Thomas et al., Thomas, Kate J., J. P. Nicholl, and Patricia Coleman. \"Use and expenditure on complementary medicine in England: a population-based survey.\" Complementary therapies in medicine. 2001;1:2-11. [51] Suryasa, I. W., Rodríguez-Gámez, M., & Koldoris, T. (2021). Get vaccinated when it is your turn and follow the local guidelines. International Journal of Health Sciences, 2021;5(3):10-26 [52] Kenned Kennedy, Jae. \"Herb and supplement use in the US adult population.\" Clinical therapeutics. 2005;11:1847-1858. [53] Patwardhan et al., Patwardhan, Bhushan, Dnyaneshwar Warude, PalpuPushpangadan, and Narendra Bhatt. \"Ayurveda and traditional Chinese medicine: a comparative overview.\" Evidence-based complementary and alternative medicine. 2005;4:465-473. [54] Wakdikar. Wakdikar, Sandhya. \"Global health care challenge: Indian experiences and new prescriptions.\" Electronic Journal of Biotechnology. 2004;3:02-03. [55] Atsamo et al., Atsamo, Albert D., Télesphore B. Nguelefack, Jacques Y. Datté, and Albert Kamanyi. \"Acute and subchronic oral toxicity assessment of the aqueous extract from the stem bark of Erythrina senegalensis DC (Fabaceae) in rodents.\" Journal of Ethnopharmacology. 2011;3:697-702. [56] A REVIEW ON GENOTOXIC IMPURITIES IN PHARMACEUTICAL DOSAGE FORMS AND STRATEGIES FOR IDENTIFICATION
Copyright
Copyright © 2024 S. H. Kalyankar, B. P. Patil, P. K. Chakor, R. C. Jagdale, K. S. Deshmukh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64926
Publish Date : 2024-10-31
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

