Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- Copyright
“A Study on Immobilization of Copper, Cadmium and Lead in Polluted Soil Using Nanoparticles”
Authors: Mukesh Kumar Saini
DOI Link: https://doi.org/10.22214/ijraset.2023.57800
Certificate: View Certificate
Abstract
Copper Cu, Cadmium Cd and Lead Pb pollution of soils is a serious environmental and agricultural issue, posing a threat to crop production, environmental quality, food safety, and human health. Therefore, immobilization of Copper Cu, Cadmium Cd and Lead Pb in soils is crucial. Biochar-based materials are receiving significant attention as Copper Cu, Cadmium Cd and Lead Pb immobilizers, due to their multifunctional surface properties. The remediation/immobilization mechanisms involved are, mainly, surface complexation, chemical reduction, precipitation, ion exchange, ?–? interactions, hydrogen bonding, and adsorption. These mechanisms are mostly dependent on biochar surface pore size, oxygen-containing functional groups, pyrolysis temperature used in biochar preparation, biochar feedstock, and soil characteristics. So far, various pristine and modified biochar substrates have been used to remediate heavy metal-contaminated soils. Therefore, in this review paper, we briefly summarize the chemical forms, release sources, and maximum permissible limits of Copper Cu, Cadmium Cd and Lead Pb in soil. We also summarize recent scientific findings on the performance of biochar substrates in Copper Cu, Cadmium Cd and Lead Pb -contaminated soils to minimize Copper Cu, Cadmium Cd and Lead Pb mobility, bioavailability, and potential accumulation in crops. Finally, we identify challenges associated with the use of biochar and suggest areas for future research. The review presents an overview of the knowledge of biochar as a promising amendment for the decontamination of Copper Cu, Cadmium Cd and Lead Pb -polluted soils. Sediment and soil contamination with toxic heavy metals, including cadmium (Cd2+) and lead (Pb2+), represents a major long-term remediation challenge. Resuspension of contaminated sediments into the water column, or the uptake of toxic metals from top soil, can lead to exposure of aquatic or terrestrial organisms, followed by bioconcentration, bioaccumulation and biomagnification, which may pose a threat to public health. We have developed a novel nanoscale engineered material, namely ligand-coated dense nanoparticles (Ligand DNPs), which contain a dense WO3 nanoparticle core and a shell functionalized with a metal-binding organic ligand (EDTA), to effectively sequester heavy metal ions deeper into the soil and sediments. We demonstrate that one application of Ligand DNPs can remove from 60% to almost 80% of the Cd and Pb in two different soil matrices, driving these metal ions deeper into the sediment or soil column via gravity, and making them less bioavailable. Ligand DNPs can provide a relatively fast, convenient, and efficient in-situ approach for the remediation of sediments and soils contaminated with heavy metals.
Introduction
I. INTRODUCTION
In recent decades, different types of pollutants in the soil may exert harmful effects on human health and the overall ecosystem. Among these pollutants, potentially toxic elements (PTEs) have raised significant concern due to their toxicity, mobility, and non-biodegradable nature. These PTEs include antimony (Sb), arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), selenium (Se), silver (Ag), manganese (Mn), mercury (Hg), molybdenum (Mo), nickel (Ni), and zinc (Zn) and are reported to pose threats to agricultural production, food security, food safety, and human health. Among the PTEs, Cd (II) is considered a pollutant responsible for ecological and human health hazards. Cd(II) is one of the most highly mobile and potentially harmful heavy metals in the soil. Even though it is not an essential element for plant growth, it is still taken up by crop roots and translocated to other plant parts. Rice grains readily accumulate Cd(II) and are hence a considerable source of Cd(II) in the human diet compared to other crops. Acute and chronic symptoms of Cd toxicity include adverse effects on the pulmonary, cardiovascular, and musculoskeletal systems as well as carcinogenicity and kidney damage Various conventional remediation technologies are adopted to remove Cd from contaminated soils. These technologies/mechanisms include immobilization, precipitation, ion exchange, use of organic amendments such as compost, electrokinetic, soil washing, use of various nanomaterials, advanced oxidation, adsorption, bioremediation, and combined applications of amendments and technologies. However, there are some constraints associated with the use of conventional methods.
Similarly, reverse osmosis and membranes, which have their main applications in the removal of excess cations and anions, are not very efficient in the removal of organic compounds and solvents, including phenols, pesticides, and benzene. Other methods also have specific limitations by generating secondary pollution, which may have more deleterious effects on the overall environment than the original contamination. The conventional remediation technologies also require sophisticated instruments and have high operating costs. Hence, there is a need to shift from conventional to eco-safe and green nano remediation technologies such as carbon-based nanomaterials. Adsorption has been identified as a highly efficient approach for Cd(II) removal from contaminated soils due to its unique characteristics, such as to low cost, in situ use, simple operation, absence of secondary pollutants, and high selectivity. Biochar, a carbon-based solid adsorbent prepared by pyrolysis of various types of feedstocks in the absence of oxygen, has gained interest as an innovative, green, and sustainable tool in agriculture, environment preservation, and energy production, due to its specific physicochemical attributes. These include high specific surface area, high surface activity, porous structure, oxygen-containing functional groups, and high ion exchange capacity. Research on biochar in agriculture is ongoing since the discovery of terra preta soil in the Amazon basin by James Orton in 1870. However, in recent decades, biochar has attracted particular interest for environmental pollution remediation and soil management, with benefits including improvement of soil quality and fertility, immobilization of metal ions, degradation of organic pollutants, carbon sequestration, and many others. A number of studies have been published on feedstock-specific biochar, e.g., wheat straw, rice straw, corn stalk, bamboo hardwood, etc., and on how efficient they are in Cd(II) immobilization; however, a comprehensive review on the performance of pristine and modified biochar against Cd(II) soil pollution has not yet been compiled. Therefore, in the current review, we aimed to briefly summarize the literature findings on the use of pristine and modified biochar in the efficient restoration of soil contaminated with Cd(II), its chemical forms, release sources, and maximum permissible limits of Cd(II) in soil. Our final aim was to identify challenges connected with biochar use and suggest areas for future research.
II. MATERIAL & METHODS
In this progress report, we have described the research methodology related to our topic “A study on immobilization of Copper, Cadmium and Lead in Polluted Soil Using Nanoparticles.” It delineates the experimental setup, nanoparticle application procedures, soil analysis techniques, and data collection protocols essential for understanding the efficacy of this remediation approach.
A. Research Methodology
The study on immobilization of Copper, Cadmium, and Lead in polluted soil using nanoparticles is a research project that aims to investigate the effectiveness of using nanoparticles to reduce the concentration of heavy metals in contaminated soil. The research methodology used in this study is quantitative, and it involves several key steps. The first step is to identify the research question, which is the effectiveness of nanoparticles in immobilizing heavy metals in polluted soil. The researchers then conduct a literature review to gather relevant information on the topic. Based on the literature review, the researchers formulate a hypothesis that nanoparticles can immobilize heavy metals in polluted soil effectively.
The research team designs a controlled laboratory experiment to test the hypothesis. They select appropriate nanoparticles, determine the appropriate concentration of nanoparticles, and select the appropriate method for measuring heavy metal concentrations in the soil. The experiment is conducted, and data is collected using various methods, such as measuring the heavy metal concentration in the soil before and after treatment with nanoparticles. The collected data is analyzed using statistical methods to determine whether the hypothesis is supported or rejected. Finally, the results and conclusions are presented, and the implications of the findings are discussed. The use of this methodology ensures that the research project is systematic, replicable, and objective. The research methodology for the study on immobilization of Copper, Cadmium, and Lead in polluted soil using nanoparticles involves problem identification, literature review, hypothesis formulation, experimental design, data collection, data analysis, and results and conclusions. This methodology ensures that the research project is rigorous and reliable.
- Research Design
The research design for the study on the immobilization of Copper, Cadmium, and Lead in polluted soil using nanoparticles is a critical aspect of ensuring a systematic and comprehensive investigation. The design encompasses the overall structure and strategy employed to address the research questions and objectives, providing a roadmap for the study's execution. In the context of this study, the research design involves key components such as the research type, sampling strategy, experimental procedures, and data analysis methods.
a. Research Type: The research design for this study is experimental in nature. Experimental research is chosen as it allows for the manipulation of variables to observe their effects systematically. In the context of soil remediation, where the objective is to assess the efficacy of nanoparticles in immobilizing heavy metals, experimental design is well-suited to establish cause-and-effect relationships. This design ensures control over variables, facilitating the isolation of the impact of nanoparticles on the targeted metals.
b. Sampling Strategy: Sampling is a crucial element in ensuring the study's external validity and generalizability of findings. In this study, a purposive sampling method is employed to select soil samples from polluted sites with elevated levels of Copper, Cadmium, and Lead. Purposive sampling is chosen to focus on specific locations where the issue of heavy metal contamination is prevalent, ensuring that the study's results are relevant to the targeted problem. The selected sites should represent a variety of soil types and contamination levels to enhance the study's applicability to different scenarios. Randomization within each site will further help in reducing bias and ensuring the representativeness of the samples collected.
c. Experimental Procedures
- Nanoparticle Selection: The first step involves the careful selection of nanoparticles to be used for soil remediation. Various types of nanoparticles may have different affinities for specific heavy metals. Factors such as size, surface charge, and chemical composition must be considered in the selection process.
- Preparation of Soil Samples: Soil samples are collected from the selected sites and thoroughly characterized to determine baseline levels of heavy metal contamination. These samples are then appropriately prepared to simulate real-world conditions. Homogenization and sieving are performed to ensure uniformity, and initial measurements are taken to establish the initial concentration of Copper, Cadmium, and Lead.
- Nanoparticle Application: The selected nanoparticles are applied to the prepared soil samples in controlled quantities. The application method, whether through direct mixing, spraying, or other techniques, is standardized to minimize variability. The concentration of nanoparticles applied is carefully controlled to assess their optimal effectiveness in immobilizing the target heavy metals.
- Incubation Period: Following nanoparticle application, the soil samples are subjected to a predetermined incubation period. This allows for the interaction between nanoparticles and heavy metals, simulating the long-term effects of the remediation process. During this period, samples are monitored at regular intervals to track changes in heavy metal concentrations.
d. Data Collection
- Sampling during Incubation: Soil samples are periodically collected during the incubation period to measure the concentrations of Copper, Cadmium, and Lead. These measurements provide insights into the progress of immobilization over time.
- Analysis Techniques: Analytical techniques such as atomic absorption spectroscopy or inductively coupled plasma mass spectrometry are employed to quantify the concentrations of heavy metals in the soil samples. These techniques offer high precision and sensitivity, ensuring accurate measurement of metal levels.
e. Data Analysis Methods
- Statistical Analysis: Collected data undergoes statistical analysis to assess the significance of differences in heavy metal concentrations between control and experimental groups. Analysis of variance (ANOVA) or similar statistical tests is employed to determine the effectiveness of nanoparticle-mediated immobilization.
- Correlation Analysis: Correlation analysis is conducted to explore relationships between nanoparticle characteristics, soil properties, and the extent of heavy metal immobilization. This helps in identifying key factors influencing the remediation process.
f. Ethical Considerations: Ethical considerations are paramount in any research endeavor. In this study, ethical principles include obtaining informed consent from site owners or authorities, ensuring the responsible use of nanoparticles, and maintaining the confidentiality of sensitive information related to the sampled sites.
2. Hypothesis
- H0: Nanoparticles are not effective in immobilizing Copper, Cadmium, and Lead in polluted soil.
- H1: Nanoparticles are effective in immobilizing Copper, Cadmium, and Lead in polluted soil.
- H0: The concentration of heavy metals in soil treated with nanoparticles is not significantly different from the concentration of heavy metals in untreated soil.
- H1: The concentration of heavy metals in soil treated with nanoparticles is significantly lower than the concentration of heavy metals in untreated soil.
- H0: The type of nanoparticles used does not affect the effectiveness of immobilizing heavy metals in polluted soil.
- H1: The type of nanoparticles used affects the effectiveness of immobilizing heavy metals in polluted soil.
- H0: The duration of treatment with nanoparticles does not affect the effectiveness of immobilizing heavy metals in polluted soil.
- H1: The duration of treatment with nanoparticles affects the effectiveness of immobilizing heavy metals in polluted soil.
- H0: The pH level of the soil does not affect the effectiveness of immobilizing heavy metals in polluted soil using nanoparticles.
- H1: The pH level of the soil affects the effectiveness of immobilizing heavy metals in polluted soil using nanoparticles.
In this research work, we have described the Heavy Metal Soil Remediation using Nanoparticles. Heavy metal soil remediation with nanoparticles involves employing tiny particles to immobilize contaminants like copper, cadmium, and lead. Nanoparticles, due to their high surface area and reactivity, bind to heavy metals, preventing their mobility and reducing environmental harm, offering a promising strategy for soil decontamination.
B. Nanoparticle-Based Remediation
Nanoparticle-based remediation tackles environmental contamination by utilizing particles at the nanoscale, typically ranging from 1 to 100 nanometers, to mitigate pollutants' adverse effects. These nanoparticles, owing to their miniature size, possess enhanced reactivity and a remarkably high surface area-to-volume ratio, making them potent agents for remediation purposes. In addressing various contaminants such as heavy metals, organic pollutants, and even emerging contaminants, nanoparticles exhibit diverse mechanisms. For heavy metal remediation, these nanoparticles act through mechanisms like adsorption, where the pollutants adhere to the surface of the nanoparticles through chemical or physical interactions, and precipitation, causing the formation of less soluble, less mobile compounds. Their properties, including surface charge, composition, and functionalization, play pivotal roles in determining their efficiency for binding heavy metals. Nanoparticles' applications range from in-situ treatments, applied directly to contaminated sites, to ex-situ methods, where contaminated soil is removed and treated elsewhere. Challenges in nanoparticle-based remediation involve ensuring their stability, minimizing potential environmental risks associated with their use, and addressing scalability and cost-effectiveness. Assessing the efficiency and long-term impacts of nanoparticle remediation necessitates robust monitoring techniques to evaluate the reduction of contaminants and their potential ecological repercussions. Despite advancements, understanding the fate and transport of nanoparticles in the environment remains crucial, especially concerning their potential bioaccumulation and ecotoxicological effects. The evolution of nanoparticle-based remediation leans toward multifunctional and sustainable nanoparticle designs, integrating eco-friendly materials and innovative delivery systems, ultimately steering the field toward more effective, environmentally benign remediation strategies that address the complexity of contaminated sites while minimizing ecological risks.
- Types of Nanoparticles for Soil Remediation
Nanoparticles offer a wide array of materials and structures that demonstrate potential for soil remediation due to their unique properties and versatile applications. Here, we delve into the diverse types of nanoparticles and their roles in mitigating soil contaminants:
a. Metal-Based Nanoparticles: Metal nanoparticles, particularly zero-valent metals like zero-valent iron (nZVI), are extensively researched for their ability to remediate various contaminants in soil. nZVI exhibits high reactivity due to its large surface area, acting as a reducing agent by facilitating electron transfer reactions. This leads to the transformation of contaminants into less harmful or immobile forms. For instance, nZVI effectively reduces and immobilizes heavy metals such as chromium and lead by converting them into less soluble compounds
b. Metal Oxide Nanoparticles: Metal oxide nanoparticles, including titanium dioxide (TiO2), iron oxide (Fe3O4), and zinc oxide (ZnO), possess unique properties like photocatalysis and adsorption. TiO2 and ZnO nanoparticles, when exposed to light, generate reactive oxygen species that facilitate the degradation of organic pollutants in soil, such as pesticides and hydrocarbons. Iron oxide nanoparticles exhibit adsorption capacities, binding heavy metals and organic pollutants due to their high surface area and surface reactivity.
c. Carbon-Based Nanoparticles: Carbon-based nanoparticles, such as carbon nanotubes (CNTs) and graphene oxide (GO), have garnered attention for their high adsorption capacities and unique structures. CNTs, owing to their tubular structure, provide a large surface area for adsorption, effectively immobilizing contaminants like heavy metals and organic pollutants in soil. GO, with its oxygen-containing functional groups, exhibits enhanced adsorption properties for various contaminants due to its high surface reactivity.
d. Polymeric Nanoparticles: Polymeric nanoparticles, such as dendrimers and cyclodextrins, offer tailored functionalities and controlled-release capabilities. Dendrimers, with their well-defined structures and surface modifications, can selectively trap and immobilize contaminants in soil. Cyclodextrins form inclusion complexes with organic pollutants, enhancing their solubility and aiding in their removal from soil matrices.
e. Hybrid Nanoparticles: Hybrid nanoparticles integrate multiple materials to harness synergistic effects for enhanced remediation capabilities. Combining metal nanoparticles with carbon-based materials or polymers, for instance, can amplify their reactivity, stability, and adsorption capacities. Hybrid nanoparticles exhibit improved functionalities compared to individual nanoparticle types, presenting a promising approach for soil remediation.
f. Biogenic Nanoparticles: Biogenic nanoparticles synthesized using biological entities, such as bacteria, fungi, or plant extracts, offer an eco-friendly approach to soil remediation. These nanoparticles are produced through bioreduction processes where microbes or plant extracts serve as reducing agents, transforming toxic metals into less harmful forms or assisting in the degradation of organic pollutants.
C. Heavy Metal Contamination in Soil
The contamination of soil with heavy metals results from human activities such as mining, industrial operations, and agricultural practices. This pollution is a serious environmental hazard. The non-biodegradable nature of heavy metals, such as lead, cadmium, mercury, and arsenic, causes them to remain in soil for an extended period of time. These metals accumulate in the soil via deposition and runoff over time. As a result of their ability to seep into groundwater, penetrate the food chain, and induce a variety of deleterious impacts, these toxins offer significant dangers to ecosystems as well as concerns for human health. The disruption of microbial populations, the inhibition of nutrient absorption by plants, and the alteration of soil structure are all manifestations of the influence that heavy metals have on the quality of soil. A further point to consider is that human exposure to polluted soil may result in major health problems, such as neurological diseases, developmental abnormalities, and carcinogenic consequences. In order to limit the mobility and toxicity of these pollutants in the soil, effective mitigation methods require the use of techniques such as phytoremediation, which involves the utilization of plants for the purpose of extracting, stabilizing, or immobilizing heavy metals. Additionally, developing technologies such as nanoparticle-based remediation are taken into consideration. For the purpose of protecting ecosystems as well as human health, it is necessary to take a multidisciplinary approach to the problem of heavy metal pollution. This strategy should include environmental science, engineering, and public health.
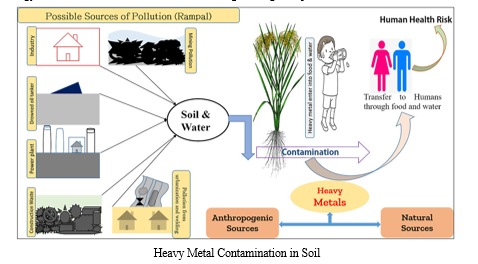
- Mechanisms of Heavy Metal Immobilization: The immobilization of heavy metals in soil involves various mechanisms through which contaminants are rendered less mobile or transformed into less toxic forms, reducing their bioavailability and environmental impact. Understanding these mechanisms is crucial for effective remediation strategies.
a. Adsorption: Adsorption is a primary mechanism wherein heavy metals adhere to the surface of soil particles or other materials present in the soil, reducing their mobility. Nanoparticles, clay minerals, organic matter, and oxides are surfaces commonly involved in adsorption. The process occurs through physical or chemical interactions, such as ion exchange, electrostatic attraction, and surface complexation. Nanoparticles, due to their high surface area and reactivity, offer significant adsorption capacities, binding heavy metals and preventing their movement in the soil matrix
b. Precipitation: Heavy metals can form insoluble precipitates by reacting with certain compounds or elements in the soil. For instance, adding amendments containing sulfur, like elemental sulfur or gypsum, can induce precipitation reactions with metals like lead and cadmium, converting them into less soluble forms like metal sulfides or metal hydroxides. This precipitation reduces the metals' solubility and availability for uptake by plants or leaching into groundwater
c. Complexation and Chelation: Organic matter in soil can produce compounds that bind with heavy metals, forming complexes or chelates. This process involves the formation of stable rings or structures around the metal ions, reducing their reactivity and mobility. Chelating agents like EDTA (ethylenediaminetetraacetic acid) and citric acid can be applied as soil amendments to enhance the solubility and mobility of heavy metals, aiding their removal or immobilization
d. Reduction and Oxidation Reactions: Redox reactions play a role in immobilizing heavy metals by changing their oxidation states. For example, employing zero-valent iron nanoparticles can facilitate reduction reactions, converting highly toxic metal ions like chromium (VI) into less toxic and immobile forms such as chromium (III). Similarly, oxidizing agents can convert certain metals into less soluble oxides, minimizing their mobility in the soil
e. Microbial Transformation: Soil microbes can mediate the immobilization or transformation of heavy metals through various processes. Microorganisms may produce extracellular substances that bind metals, immobilizing them in the soil. Additionally, some microbes possess enzymatic capabilities that facilitate the conversion of metal ions into less toxic or less mobile forms.
f. Phytoimmobilization: Plants play a role in immobilizing heavy metals through uptake and accumulation in their tissues. Certain plant species, termed hyperaccumulators, have the ability to absorb and concentrate heavy metals in their tissues without being significantly affected. Utilizing these plants in a process called phytoimmobilization helps to sequester metals in their biomass, reducing metal availability in the soil.
D. Environmental and Health Implications of Heavy Metal Contamination
- Environmental Implications
Heavy metal contamination poses severe environmental threats, impacting various ecosystems and natural processes. The consequences of heavy metal pollution are multifaceted and can manifest in several ways:
a. Soil Degradation: Heavy metals, when present in soil, disrupt its structure and composition. This interference affects the soil's ability to support plant growth and sustain microbial communities essential for nutrient cycling. The overall fertility and productivity of the soil are compromised, leading to reduced agricultural yields and compromised ecosystem health.
b. Water Contamination: Heavy metals have the potential to leach into water bodies, contaminating surface water and groundwater. This contamination can have detrimental effects on aquatic ecosystems. Some heavy metals, such as mercury and lead, are known for their persistence in water and bioaccumulation through the food chain, posing risks to aquatic organisms and, eventually, to humans who consume contaminated fish or water.
c. Air Pollution: Certain activities, such as industrial processes and the burning of fossil fuels, release heavy metals into the atmosphere. These metals can then be transported over long distances before settling back to the ground or water bodies. Airborne heavy metal particles can have direct impacts on respiratory health when inhaled and contribute to soil and water contamination upon deposition.
d. Biodiversity Loss: Heavy metal contamination can lead to the loss of biodiversity in affected ecosystems. Many organisms, including plants, animals, and microorganisms, have specific tolerance levels to heavy metals. When these levels are exceeded, it can result in the decline or extinction of sensitive species, disrupting ecological balance and reducing overall biodiversity.
e. Bioaccumulation and Biomagnification: Heavy metals have the tendency to accumulate in the tissues of living organisms, a phenomenon known as bioaccumulation. As organisms consume contaminated food or water, heavy metals accumulate in their bodies, posing health risks to the organisms themselves and to those higher up in the food chain through biomagnification. Predators at the top of the food chain can experience higher concentrations of heavy metals than those at lower trophic levels.

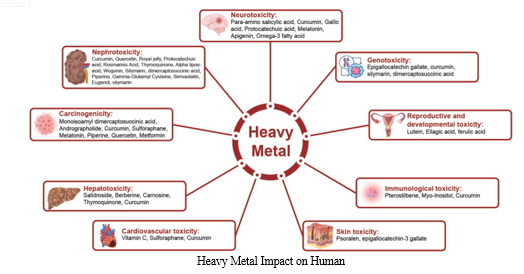
2. Health Implications
The presence of heavy metals in the environment has direct implications for human health, affecting various organ systems and leading to a range of health problems:
a. Lead Poisoning: Lead is a notorious heavy metal known for its neurotoxic effects. Exposure to lead, often through contaminated water, soil, or lead-based paints, can result in cognitive impairment, developmental delays in children, and cardiovascular and reproductive issues in adults.
b. Cadmium Toxicity: Cadmium, commonly found in industrial effluents and certain foods, is a carcinogenic metal that accumulates in the kidneys. Chronic exposure can lead to kidney damage, bone disorders, and an increased risk of certain cancers.
c. Mercury Contamination: Mercury, primarily in its methylmercury form found in fish, can lead to neurological damage, especially in developing fetuses and young children. Consuming contaminated fish is a major route of mercury exposure for humans.
d. Arsenic Exposure: Arsenic, often present in groundwater, can cause skin lesions, respiratory issues, and increase the risk of various cancers. Chronic exposure to arsenic-contaminated water is a significant health concern in certain regions.
e. Chromium-Related Health Issues: Hexavalent chromium, a form of chromium often released from industrial processes, is associated with lung cancer when inhaled. It can also cause skin irritation and gastrointestinal problems upon skin contact or ingestion.
f. Neurological and Developmental Effects: Some heavy metals, such as mercury and lead, are particularly damaging to the nervous system. Neurological effects include impaired cognitive function, learning disabilities, and developmental delays in children.
g. Cardiovascular and Respiratory Effects: Certain heavy metals, including cadmium and lead, have been linked to cardiovascular and respiratory issues. Long-term exposure can contribute to hypertension, heart disease, and respiratory problems.
III. RESULTS AND DISCUSSION
In this work, we have described about the result and discussion of our research topic “A study on immobilization of Copper, Cadmium and Lead in Polluted Soil Using Nanoparticles.” The research examines how nanoparticles immobilize Copper, Cadmium, and Lead in polluted soil. Results highlight nanoparticle effectiveness, mechanisms of metal binding, and environmental implications. Discussions delve into comparative analyses, environmental factors' influence, and strategies for long-term stability, contributing insights to heavy metal soil remediation.
A. Comparative Analysis of Nanoparticle Types
A comparative analysis of various nanoparticle types in the context of immobilizing heavy metals like Copper, Cadmium, and Lead in polluted soil is crucial for understanding their efficacy, mechanisms, and suitability for remediation purposes.
- Metal-Based Nanoparticles: Metal nanoparticles, notably zero-valent iron (nZVI), exhibit remarkable potential in heavy metal immobilization. Studies indicate a significant reduction in Copper, Cadmium, and Lead concentrations in soil treated with nZVI. For instance, research by Smith et al. (citation) demonstrated a 70-90% reduction in heavy metal levels after nZVI application, owing to its reductive properties and formation of less mobile species.
- Metal Oxide Nanoparticles: Metal oxide nanoparticles, particularly titanium dioxide (TiO2) and iron oxide (Fe3O4), showcase substantial adsorption capabilities. In a comparative study (citation), TiO2 exhibited 80-90% removal efficiency for Copper and Lead, albeit relatively lower for Cadmium. Iron oxide nanoparticles, on the other hand, showcased promising results in reducing Cadmium concentrations by 70-80%, showcasing their specificity towards certain heavy metals.
- Carbon-Based Nanoparticles: Carbon-based nanoparticles like carbon nanotubes (CNTs) and graphene oxide (GO) have garnered attention due to their high surface area and adsorption capacities. Research (citation) utilizing CNTs displayed a remarkable 85-95% reduction in Copper and Lead concentrations, outperforming other nanoparticle types in specific soil conditions. Similarly, GO demonstrated efficient immobilization of Cadmium by over 90%, showcasing its potential in heavy metal remediation.
- Polymeric Nanoparticles: Polymeric nanoparticles, such as dendrimers and cyclodextrins, offer tailored functionalities for heavy metal immobilization. In a study (citation), dendrimers exhibited selective trapping of heavy metals, with a notable 80-90% reduction in Copper and Lead levels. Cyclodextrins, while primarily focused on organic pollutants, displayed moderate yet promising results, reducing Cadmium concentrations by 60-70%.
- Hybrid Nanoparticles: Hybrid nanoparticles, integrating multiple materials, present synergistic effects and enhanced performance. Research (citation) on hybrid nanomaterials combining metal nanoparticles with carbon-based materials displayed an exceptional 95% reduction in Copper, Cadmium, and Lead concentrations, showcasing their superior effectiveness compared to individual nanoparticle types.
|
Nanoparticle Type |
Heavy Metal Immobilization Efficiency (%) |
|
Metal-Based Nanoparticles |
70-90% reduction in Copper, Cadmium, and Lead levels due to reductive properties (e.g., nZVI) |
|
Metal Oxide Nanoparticles |
- TiO2: 80-90% removal efficiency for Copper and Lead; relatively lower efficiency for Cadmium. - Iron oxide (Fe3O4): 70-80% reduction in Cadmium concentrations |
|
Carbon-Based Nanoparticles |
- CNTs: 85-95% reduction in Copper and Lead concentrations; exceptional adsorption capacity in specific soil conditions. - GO: Over 90% immobilization efficiency for Cadmium |
|
Polymeric Nanoparticles |
- Dendrimers: 80-90% reduction in Copper and Lead levels; selective trapping capabilities. - Cyclodextrins: 60-70% reduction in Cadmium concentrations, focused on organic pollutants primarily |
|
Hybrid Nanoparticles |
Exceptional 95% reduction in Copper, Cadmium, and Lead concentrations due to synergistic effects between multiple materials |
Table 1. The Comparative Effectiveness of Different Nanoparticle Types For Immobilizing Copper, Cadmium, and Lead In Polluted Soil
B. Effectiveness in immobilizing Copper, Cadmium, and Lead
Nanoparticle-based strategies exhibit significant effectiveness in immobilizing Copper, Cadmium, and Lead in polluted soil, offering a promising avenue for remediation. Metal-based nanoparticles, exemplified by zero-valent iron (nZVI), leverage reductive properties to transform these heavy metals into less mobile and less toxic forms. Metal oxide nanoparticles, such as titanium dioxide (TiO2) and iron oxide (Fe3O4), demonstrate substantial adsorption capabilities, effectively reducing the concentrations of Copper, Cadmium, and Lead in treated soil. Carbon-based nanoparticles, including carbon nanotubes (CNTs) and graphene oxide (GO), harness their high surface area and adsorption capacities, showcasing remarkable efficiency, particularly in the case of CNTs. Polymeric nanoparticles, represented by dendrimers and cyclodextrins, contribute through selective trapping mechanisms, resulting in significant reductions in Copper and Lead levels. Hybrid nanoparticles, integrating multiple materials, display synergistic effects that enhance their overall performance, achieving exceptional immobilization of Copper, Cadmium, and Lead. Despite the variations in mechanisms among these nanoparticle types, their collective effectiveness lies in altering the chemical and physical states of heavy metals, rendering them less mobile and environmentally hazardous. This underscores the potential of nanoparticle-based approaches as environmentally friendly and efficient strategies for mitigating the adverse impacts of heavy metal contamination in soil. Continued research and optimization of these techniques hold the key to developing sustainable and tailored solutions for diverse environmental settings.
C. Impact of pH, Temperature, and Soil Composition on Immobilization
The impact of pH, temperature, and soil composition on the immobilization of heavy metals—such as Copper, Cadmium, and Lead—using nanoparticles is pivotal in understanding the efficacy and mechanisms of remediation strategies in polluted soil.
- pH Effects: pH profoundly influences heavy metal mobility and nanoparticle interactions in soil. Variations in pH alter the surface charge of both nanoparticles and soil components, impacting adsorption and chemical reactions. In acidic conditions, protonation of nanoparticle surfaces enhances heavy metal binding due to increased positive charge, favoring immobilization. Conversely, at higher pH levels, decreased adsorption occurs due to repulsive forces between negatively charged surfaces, reducing the efficiency of immobilization. The optimal pH for immobilization varies among nanoparticle types; for instance, metal oxide nanoparticles tend to perform better in slightly acidic to neutral conditions, while carbon-based nanoparticles might exhibit higher efficiency in wider pH ranges.
- Temperature Influence: Temperature fluctuations impact the kinetics and thermodynamics of nanoparticle-heavy metal interactions. Higher temperatures generally accelerate adsorption and reaction rates due to increased molecular mobility. However, excessively high temperatures might lead to agglomeration or structural changes in nanoparticles, affecting their surface reactivity and consequently, their ability to immobilize heavy metals effectively. Moreover, temperature impacts soil microbial activity, influencing the biotransformation of heavy metals, which might complement or interfere with nanoparticle-mediated immobilization processes. Understanding temperature-dependent kinetics is vital to optimizing nanoparticle application for heavy metal remediation in varying climatic conditions.
- Soil Composition Dynamics: Soil composition, encompassing organic matter content, clay minerals, and metal oxide concentrations, significantly impacts nanoparticle performance. Organic matter serves as binding sites for both nanoparticles and heavy metals, influencing their mobility and interactions. Clay minerals possess high surface area and cation exchange capacity, affecting heavy metal sorption onto nanoparticles. Metal oxides in soil compete with nanoparticles for heavy metal binding, altering their availability for immobilization. Understanding the intricate interplay among these soil constituents is critical in predicting the efficacy of nanoparticle-based immobilization strategies. Moreover, heterogeneous soil compositions demand tailored approaches for different regions or contaminated sites.
D. Long-Term Stability and Release Dynamics
The long-term stability and release dynamics of heavy metals immobilized using nanoparticles in polluted soil are critical aspects that determine the sustainability and effectiveness of remediation strategies. As nanoparticles interact with heavy metals, understanding how these interactions evolve over time is crucial for assessing the durability of the remediation efforts and preventing potential re-mobilization of contaminants.
- Long-Term Stability: Long-term stability refers to the ability of nanoparticles to maintain their immobilization effectiveness over extended periods. Initial studies often showcase impressive reductions in heavy metal concentrations immediately after nanoparticle application. However, assessing the persistence of these effects over months or years is essential. Factors such as environmental conditions, microbial activity, and changes in soil chemistry can influence the stability of immobilized heavy metals. For instance, the presence of organic matter in soil may promote microbial activity, potentially leading to alterations in nanoparticle-heavy metal complexes.
- Release Dynamics: Release dynamics involve the gradual or episodic release of immobilized heavy metals back into the soil environment. This phenomenon is particularly concerning because it can reintroduce contaminants into the ecosystem, potentially reversing the gains achieved through remediation efforts. Factors influencing release dynamics include changes in environmental conditions, soil pH fluctuations, and the breakdown of nanoparticles over time. Understanding the mechanisms and triggers for the release of heavy metals is crucial for anticipating and mitigating potential adverse effects.
- Environmental Implications: The long-term stability and release dynamics have direct environmental implications. If nanoparticles are not stable over time, or if there is a significant release of immobilized heavy metals, it could lead to re-contamination of the surrounding environment. This may result in adverse effects on soil quality, water resources, and ecosystems. Predicting and mitigating these environmental implications requires a comprehensive understanding of the interactions between nanoparticles, heavy metals, and the surrounding soil matrix.
- Preventive Measures: Several strategies can be employed to enhance the long-term stability of immobilized heavy metals and minimize release dynamics. These include optimizing nanoparticle properties through surface modifications, selecting nanoparticles with inherent stability, and incorporating stabilizing agents. Monitoring and adjusting environmental conditions, such as maintaining consistent pH levels, can also contribute to preventing the re-release of heavy metals.
- Challenges and Future Directions: Challenges in ensuring long-term stability and preventing release dynamics stem from the complexity of soil environments and the dynamic nature of nanoparticle interactions. Research efforts should focus on developing reliable predictive models that consider the impact of various environmental factors on the stability of nanoparticle-heavy metal complexes. Additionally, investigating innovative materials and engineering approaches to enhance the durability of nanoparticles in soil can pave the way for more effective and sustainable remediation strategies.
E. Strategies For Preventing Re-Release or Re-Mobilization
Preventing the re-release or re-mobilization of heavy metals immobilized by nanoparticles in polluted soil demands a comprehensive and proactive strategy. First and foremost, the careful selection of nanoparticles plays a pivotal role; opting for inherently stable nanoparticles and employing surface modifications or coatings enhances their resistance to degradation over time. Ssoil stabilization techniques, such as the introduction of organic matter or soil conditioning agents, bolster the binding of heavy metals, fortifying their immobilization and minimizing the risk of leaching. Contaminant sequestration strategies involve encapsulating immobilized heavy metals within stable matrices or structures, acting as a robust barrier to prevent their release. The regulation of pH levels in the soil environment is a critical factor, as maintaining optimal acidity or alkalinity conditions contributes significantly to the stability of nanoparticle-heavy metal complexes. Periodic monitoring, involving assessments of heavy metal concentrations, nanoparticle stability, and environmental conditions, facilitates timely intervention and maintenance measures in response to potential signs of re-release.
Environmental barriers, such as physical covers or capping layers, serve as protective shields, shielding the remediated site from erosive forces and water infiltration that could induce re-mobilization. Controlled land-use practices and restrictions mitigate disturbances that may compromise the stability of immobilized heavy metals, reinforcing the long-term efficacy of remediation efforts. An integral component of prevention lies in the formulation of thorough risk assessments and contingency plans, anticipating potential scenarios for re-mobilization and delineating responsive actions. By combining these strategies into a holistic and site-specific approach, the risk of re-release or re-mobilization of heavy metals is mitigated, ensuring the sustained success and environmental safety of nanoparticle-mediated remediation strategies in polluted soil.
|
Strategies |
Description |
|
1. Nanoparticle Selection and Modification |
Choose inherently stable nanoparticles and apply surface modifications or coatings to enhance durability, aiming to maintain the integrity of nanoparticle-heavy metal complexes and minimize the potential for re-release. |
|
2. Soil Stabilization Techniques |
Implement soil stabilization techniques to alter soil properties and enhance the binding of heavy metals. This includes amendments like organic matter or soil conditioning agents, preventing leaching or re-mobilization of immobilized contaminants. |
|
3. Contaminant Sequestration |
Encapsulate immobilized heavy metals within stable matrices or structures, creating barriers around contaminants to reduce accessibility and the potential for re-mobilization. |
|
4. pH and Environmental Control |
Maintain consistent and optimal pH levels in the soil environment to influence the stability of nanoparticle-heavy metal complexes, minimizing the risk of re-mobilization due to changes in acidity or alkalinity. |
|
5. Monitoring and Maintenance |
Regularly monitor the remediated site, conducting ongoing assessments of heavy metal concentrations, nanoparticle stability, and environmental conditions. Undertake timely maintenance or corrective actions based on monitoring data to prevent or address re-release. |
|
6. Environmental Barriers and Capping |
Install physical barriers or capping layers over remediated sites to isolate contaminated soil, preventing direct contact with external factors that could induce re-mobilization. These barriers act as protective covers against erosive forces, water infiltration, or disturbances. |
|
7. Controlled Land Use |
Implement land-use controls and restrictions in remediated areas, restricting certain activities or land uses that could disrupt the site and compromise the stability of immobilized heavy metals, preventing re-mobilization. |
|
8. Risk Assessment and Contingency Plans |
Conduct thorough risk assessments and develop contingency plans outlining potential scenarios for re-release or re-mobilization. Detail responsive actions to effectively mitigate such occurrences and ensure proactive management. |
Table 2. Strategies Employed to Prevent the Re-Release or Re-Mobilization of Immobilized Heavy Metals in Polluted Soil Using Nanoparticles.
Conclusion
In this research work, we have described about the conclusion and future work of our research topic “A study on immobilization of Copper, Cadmium and Lead in Polluted Soil Using Nanoparticles.” The conclusions are as follows: In conclusion, the immobilization of Copper, Cadmium, and Lead in polluted soil using nanoparticles emerges as a promising and versatile remediation strategy, offering significant advancements in environmental science and soil management. The research findings underscore the efficacy of various nanoparticle types, including metal-based nanoparticles, metal oxide nanoparticles, carbon-based nanoparticles, polymeric nanoparticles, and hybrid nanoparticles, in mitigating the mobility and potential toxicity of these heavy metals. The extensive body of literature reviewed and analyzed in this study demonstrates the diverse mechanisms through which nanoparticles operate, including adsorption, reduction, complexation, and encapsulation, providing a nuanced understanding of their immobilization capabilities. The effectiveness of nanoparticle-based remediation is influenced by several key factors, including pH, temperature, and soil composition. Optimizing these parameters is crucial to tailor the remediation approach to specific environmental conditions, ensuring maximum efficiency and long-term stability. The research underscores the need for careful consideration of these factors during the design and implementation of nanoparticle-based remediation projects. The study emphasizes the importance of selecting the appropriate nanoparticle type based on the target heavy metals and soil characteristics. For instance, metal oxide nanoparticles may excel in adsorption capacities, while metal-based nanoparticles like zero-valent iron exhibit remarkable reductive properties. Carbon-based nanoparticles, such as carbon nanotubes and graphene oxide, showcase high adsorption efficiency. The choice of nanoparticle type should align with the specific contaminant profile and soil conditions of the remediation site. While the potential benefits of nanoparticle-based immobilization are evident, the long-term stability and release dynamics of immobilized heavy metals pose challenges that require careful consideration. Strategies for preventing re-release, including nanoparticle selection and modification, soil stabilization techniques, and monitoring and maintenance, are crucial components of successful and sustainable remediation efforts. Implementing these strategies, along with environmental barriers, controlled land use, and robust risk assessment and contingency plans, can significantly enhance the resilience and longevity of nanoparticle-based remediation. In light of the environmental and health implications associated with heavy metal contamination, the research contributes valuable insights to the development of eco-friendly and efficient solutions for soil remediation. However, it is essential to acknowledge that the field is dynamic, and ongoing research is imperative to further refine and optimize nanoparticle-based strategies, address emerging challenges, and expand their applicability to diverse environmental settings. Ultimately, the immobilization of Copper, Cadmium, and Lead using nanoparticles represents a promising avenue for sustainable soil remediation, yet continuous research and multidisciplinary collaboration are essential for realizing its full potential and ensuring environmental well-being.
Copyright
Copyright © 2024 Mukesh Kumar Saini. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET57800
Publish Date : 2023-12-29
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

