Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Advancement in Photogalvanic Cell for Solar Energy Harvesting: A Review
Authors: Prabha Sharma, Dr. Jagrati Meena
DOI Link: https://doi.org/10.22214/ijraset.2025.66793
Certificate: View Certificate
Abstract
The Photogalvanic effect is a promising mechanism for the conversion of solar energy into electrical energy, utilizing a variety of chemical systems to enhance efficiency and storage capacity. The operation of Photogalvanic cells is characterized by the absorption of solar energy by a photosensitizer, which generates energy-rich species in an electrolyte solution. This process creates a Photopotential between two electrodes, enabling the production of electricity without the need for complex semiconductor materials found in conventional solar cells. About 196 research articles on the development of Photogalvanic cell we have been reviewed here. With comparison of 87 system, the electric parameters of the cell containing single dye, mixed dye and natural dye with reductant and surfactant have presented in tabular form (Table-1). We have also focus on the challenges limitation, future aspect in field of photochemical conversion of solar energy and its storage. This review explores the recent advancements in Photogalvanic cells, devices that convert light energy into electrical energy via photochemical processes. It highlights innovations in materials, the mechanisms of charge generation and transfer, challenges in efficiency and stability, and the diverse applications of Photogalvanic cells, in sustainable energy production. The paper also discusses future trends and emerging technologies that could shape the development of Photogalvanic cells, positioning them as a promising technology in the renewable energy landscape.
Introduction
I. INTRODUCTION
Solar energy is a form of renewable energy. It is created when the sun undergoes nuclear fusion. In 1.5 days, the sun generates 1.7 x 1022 J of energy. This energy is equivalent to the total energy contained in the world's 3 trillion barrels of oil reserves. Humans use 4.6 x 1020 J of energy each year. The sun produced this amount of energy in one hour.[1] Solar energy is a low-cost, easily available, and environmentally friendly source of energy that has the potential to produce almost no emissions.[2]
People have been using sun energy since the 17th century BCE. Ancient civilizations (Rome and Greece) displayed the first documented use of sunlight by lighting torches with mirrors for religious purposes, and ancient buildings employed passive solar design, which entails harnessing sunlight to heat and light inside spaces. A.E. Becquerel discovered the outstanding and revolutionary Becquerel effect in 1839. The photoelectric effect was discovered in the 20th century by Einstein and other scientists. This encouraged research into materials whose chemical composition would enable them to be used to generate electrical energy from solar radiation.[3] Solar energy is directly converted into solar power via solar cells, which are based on the photovoltaic effect. Solar cells can be made with a single layer of light-absorbing material (single-junction) or in a variety of physical configurations (multi-junctions) to take advantage of varied absorption and charge separation methods.[4] Solar cells includes, silicon solar cells, thin-film solar cells, perovskite solar cells, quantum dot cells, organic solar cells, dye-sensitized solar cells, photogalvanic cells. Photogalvanic cells are electrochemical devices, that use dye-sensitized solutions to convert and store solar radiation through the “Photogalvanic Effect.” Rideal and Williams discovered it[5], while Rabinowitch conducted an extensive investigation and firstly used the word.[6], [7] Furthermore, several research groups, including Waber and Matijevic[8], Kamat and Lichtin[9], [10], Albery and Foulds[11], etc., have examined this effect on various systems of photogalvanic cells for solar power conversion and storage.
II. COMPONENTS OF PHOTOGALVANIC CELLS (PG CELLS)
The fundamental elements of PG cells are electrolytes, electrodes, assembly setup, and a light source.
A. Electrolytes
The electrolyte facilitates the movement of ions between the two electrodes in the photogalvanic cell. The electrolyte helps to stabilize the photogalvanic cell by maintaining the proper ionic conditions necessary for continuous operation. A well-chosen electrolyte can enhance the conversion of solar energy into electrical energy and improve the cell's longevity.
Photogalvanic cells containing dye/photosensitizer, reductant, surfactant, and acid/alkali are important electrolytes component for storage and low-cost assembly setup.
- Photosensitizer: Dyes/photosensitizers are essential to the operation of photogalvanic cells because they absorb solar light and start the processes that lead to electron transfer. So, photosensitizer should be photostable and good light-harvesting material. Photochemical reactions require a specific concentration of the dye. Light and dye molecules are both particles in nature and interact according to Stark-Einstein's law, a lower concentration of photosensitizer led to a decrease in photopotential and photocurrent because there were fewer photosensitizer molecules available for excitation and subsequent electron donation to the platinum electrode. Similarly, at larger concentrations, a large amount of the light is absorbed by dye molecules because photogalvanic cells are diffusion-controlled. Increased concentration may also result in faster recombination between injected electrons and dye. Hence, when photosensitizer concentration rises, photopotential and photocurrent are increased until they reach their highest values and further decrease.[3] Dyes are classified into synthetic and natural dyes. Natural dyes consist of complex blends of components sourced from natural materials like plants, animals, or minerals. Synthetic dyes are produced in a lab. Chemicals are produced to create synthetic dyes. Thionine dye was mostly used as a primary electrolyte until around the 1980s.[6], [7], [8], [10] Later on, a variety of dyes from various classes were tested in photogalvanic cells as photosensitizers like Malachite Green[12], Congo red[13], Rose Bengal[14], Quinoline Yellow[15], Alizarine Red S[16], etc. Dube[17] used a mixed dye system (Azur A, Azur B, and Azur C with mannitol and NTA) in photogalvanic cells to improve conversion efficiency and also discovered that the dyes increased the ability of solution to absorb solar energy, which led to a larger area of light absorption. Later, a variety of mixed dye systems of the photogalvanic cells have been explored, including Toluidine Blue + Brilliant Cresyl Blue[18], Brilliant Green + Celestine Blue[19], Naphthol Green B + Janus Green B[20], Xylene Cyanol FF + Patent Blue[21], Sudan Black- B + Azur-B[22], etc. Synthetic dyes exhibit high conversion efficiency and superior chemical stability, rendering them among the top sensitizers; however, they tend to be quite costly and hazardous. Also, using artificial dye sensitizers goes against the main goals of solar energy harvesting's sustainability and renewable nature. Consequently, numerous, researchers have turned to natural dyes because of their wide range of possibilities, due to their completely biodegradability, inexpensive, non-toxic, and readily available, they can be utilized for the same application. Natural dyes are present in different parts of plants like leaves, flowers, fruits, and seeds which are obtained with easy methods. Many natural dyes derived from plant sources have been used as photosensitizers in photogalvanic cells for solar energy harvesting showed in Table - 1.
- Reductant: In the first systematic photogalvanic cell, iron salt was used as an electron donor. However, the photocurrent and photopotential were low due to recombination and dissipation of free energy into heat. In 1958, W. Hendrich[23] used ascorbic acid as an irreversible reductant, which was easily soluble in dyes and faster for redox transfer, enhancing conversion efficiency. Albery et al.[24] proved that irreversible reducing agents are more beneficial than reversible ones in photogalvanic cells. Several irreversible reductants have been used as electrolytes like EDTA, triethylamine, triethanolamine[25], hydroquinone[26], ascorbic acid[27], [28], oxalic acid[29], fructose[30], etc, and mixed reductants mannitol-nitrilotriacetic acid[31], EDTA with oxalic acid, nitrilotriacetic acid, and dextrose, respectively[32], etc have been used to improve electrical output. Similar to the dye concentration, the reductant concentration also follows a similar trend. Lower reductant concentrations result in lower electrical output because fewer reductant molecules are available to donate electrons to photosensitizers, and higher reductant concentrations cause photopotential and photocurrent to fall because an excess of reductant molecules prevent dye molecules from reaching the electrode in the appropriate time.
- Surfactant: Surfactants are chemical substances that are naturally amphiphilic. This implies that they include both water-soluble (hydrophilic) and water-insoluble (hydrophobic) groups. Surfactants are commonly used to inhibit the recombination reaction in photochemical processes. SLS was initially used by Zhi-Chu Bi[33] as a surfactant in a solution that contained thionine as a dye and Fe2+/Fe3+ as a reductant. It is interesting to observe that SLS surfactant solution is used in photogalvanic cells to dissolve thionine in water. In India, first of all, Srivastava et al.[34] used polyvinyl methyl ether as a surfactant to improve photogalvanic cell conversion efficiency. Valenty[35] investigated the interactions of functionalized surfactants monolayer films and discovered that methylene blue adsorption to photogalvanic electrodes is connected to the orientation and absorption spectra of its surfactant analogues. SLS is widely used as a surfactant in the photogalvanic cells.[36], [37] Other surfactants, such as Triton-X 100[14], Brij-35[38], DSS[22], CPC[39], Tergitol-7[40], CTAB[41], Tween 80[42], Benzalkonium Chloride[29], etc, and mixed surfactants such as NaLS+CTAB and NaLS+Tween-80[43], NaLS+CPC+Tween-80[44], Brij-35+NaLS[45], etc are being utilized to enhance the electrical output of photogalvanic cells. The use of surfactants has been found to improve cell stability and electrical output because surfactant molecules interact with dye through charge transfer or coulombic interaction, depending on the nature of the dye and surfactants. The cell is more stable in a cationic micelle medium (if the dye is anionic) than in an aqueous medium.[46] However, the conversion efficiency of systems with different surfactants is often found to be in the order anionic>non-ionic>cationic (if the dye is cationic).[47] When a surfactant and dye have opposite charges, a strong dye-surfactant complex forms in which the dye molecule is covered by surfactant micelles in a regular shape that inhibits intermolecular twisting and increases fluorescence.[48] Studies have shown that when surfactant concentration increased both the photo-potential and photo-current increased until they reached a maximum and subsequently decreased.
- Alkali/acid: The conductivity of the cell is affected by the medium of the solution. An appropriate pH can increase the electrical output because pH affects the back reaction. Initially, several acids such as oxalic acid[49], H2SO4[50], HCl[51], and CH3COOH[52] were utilized to maintain the pH of electrolytes. Nowadays, alkali mediums such as NaOH[53], [54], [55], KOH[56] are used in the fabrication of the photogalvanic cells, because most of the photosensitizers/dyes are more soluble and stable in an alkali medium. It is commonly observed that, when the concentration of solution medium (pH) increases, the cell output of the regenerative photogalvanic cell also increases until it reaches a maximum and then decreases. The optimal pH is related to the pKa value of the reductant. The desired pH is greater than the pKa value (pH> pKa). This could be explained by the reductant being available in its anionic form, which is a better donor form.[57]
A. Electrode
The photogalvanic cell technique uses two electrodes: the working electrode (acting as the anode), which is exposed to light, and the counter electrode (acting as the cathode), which is kept in the dark chamber. The working electrode facilitates electron exchange between the semi/leuco reduced sensitizer molecule and the external circuit, while the counter electrode completes the circuit and conducts current.
Initially, researchers used both coated and uncoated Pt electrodes as the working electrode, but the electrical output obtained from both was low due to the large size of the platinum electrode.[6] Later on, small-sized Pt electrodes were used to improve the performance of photogalvanic cells.[58], [59], [60], [61] A platinum electrode is considered inert because platinum has a high ability to facilitate electron exchange. However, it is costly and not easily accessible in the local market, making its procurement expensive and time-consuming. Nowadays, different types of electrodes such as Cu, Cu-Zn alloy (brass)[62], Al, Cu-Ni alloy, Al-Mg alloy[63], etc., are used as working electrodes in PGCs. A saturated calomel electrode is usually used as a counter electrode or reference electrode but in recent times various types of reference electrodes such as graphite counter electrodes[64] have been used.
B. Light source
The electrical output of a cell is affected by the light source and its distance from the cell surface. Rabinowitch initially used a 1000 W lamp as the light source in the first thionine–iron photogalvanic cell.[6] Subsequently, various researchers used different light intensities, such as 900 W xenon lamp[65], 500 W xenon lamp[66], 450 W xenon lamp[67], 300 W[68], 250 W tungsten halogen lamp[69], 150 W xenon lamp[70], 100 W tungsten lamp[71], and 2 W Ar laser[72]. Nowadays, a 200 W tungsten lamp[19], [73], [74], [75] is used as the light source in PGCs. However, direct light is not useful because the radiation produces Infrared radiations that raise the temperature of the system. Therefore, filters are used for light filtration. Generally, water (flow) is used as a light filter[76], but in some systems, glass filters are also used[66]. Daul et al.[77] alternately illuminated the two electrodes and observed a very stable power output. Some scientists used other filters with water for light filtration, such as FeSO4 solution.[7], [8] However, water is mostly used as a filter in regular photogalvanic cell work nowadays. The distance between the light source and the cell surface is typically about 15 cm in recent work.[68]
As light intensity rises, more dye molecules are photoexcited and reduced by reductant due to the photogalvanic effect. This effect is correlated with the ratio of bleached and unbleached dye in the solution. Photocurrent and photopotential increase linearly and logarithmically with light intensity, respectively but the cell temperature also increases. In general, photogalvanic cells use 10.4mWcm-2 of light intensity[78], but at greater intensities (>150mWcm-2), the photogalvanic effect approaches a limit.[79]
C. Experimental Setup and Mechanism
The two electrodes used in the photogalvanic cell technique are the working electrode (often platinum, Pt) which is exposed to light, and the counter electrode (commonly a saturated calomel electrode, SCE) which is in the dark.
A solution of photosensitizer (photon-absorbing species), reductant (electron-donating species), and surfactant (efficiency enhancer agent) in an alkaline medium are used to fill the space between the electrodes. The net volume of electrolyte remains 25 ml. The working electrode in photogalvanic cells facilitates electron exchange between the semi/leuco-reduced sensitizer molecule and the external circuit. The circuit is completed with the help of the counter electrode, which also conducts current flow.[62] They are shown in the experimental setup (Fig -1).
D. Mechanisms
Illuminated Chamber- On illumination, a photon is absorbed by the dye, which gets excited. The excited form of the dye removes an electron from the reductant and converts into a semi or leuco form of the dye.
|
hυ |
D D*
D* + R D- (semi or leuco) + R+
At the platinum electrode, the semi/leuco form of the dye passes the electron to the platinum electrode, resulting in the formation of the original form of the dye.
D- D + e-
Dark Chamber- The dye absorbs an electron from the SCE and transforms into the semi/leuco form. The leuco/semi form of the dye and the oxidized form of the reductant combine to produce the original dye and reductant molecules, repeating the process. The result is an electron stream that transforms light into electricity.
D + e- D- (semi or leuco)
D- + R+ D + R
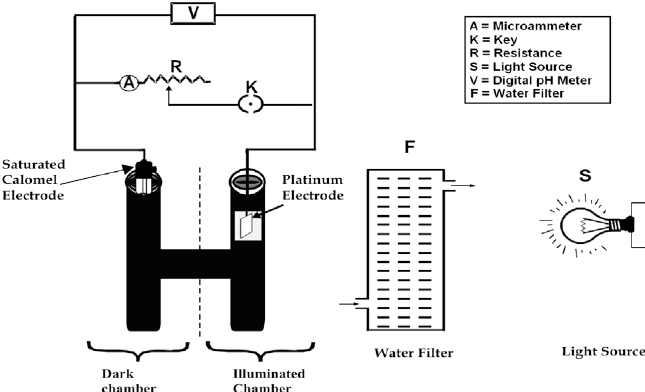
Fig- 1: Experimental setup of a Photogalvanic cell (Source- Ref.-2
Table 1: Different types of Dye-Surfactant-Reductant Systems were used in Photogalvanic cells.
|
S.No. |
Dye |
λmax (nm) |
Surfactant |
Reductant |
Voc (mV) |
isc (µA) |
Ppp (µW) |
CE (%) |
FF |
t1/2 (min.) |
Ref. |
|
|
Synthetic Dyes |
||||||||||||
|
||||||||||||
|
1 |
Acid Fuchsin |
544 |
Benzethonium Chloride |
EDTA |
1100 |
110 |
46.56 |
0.447 |
0.38 |
55 |
[78] |
|
|
2 |
Acid Yellow 36/ Metanil Yellow |
436 |
NaLS |
Formic acid |
1110 |
6000 |
822 |
20.41 |
0.206 |
105 |
[80] |
|
|
NaLS |
Ascorbic acid |
1020 |
335 |
104.72 |
1.006 |
0.388 |
110 |
[81] |
||||
|
? |
EDTA |
685 |
230 |
71.50 |
2.75 |
0.45 |
160 |
[82] |
||||
|
3 |
Acridine Orange |
430 |
NaLS |
DTPA |
1051 |
205 |
85.95 |
0.83 |
0.56 |
95 |
[83] |
|
|
4 |
Alizarin Cyanine Green |
642 |
Sodium stearate |
EDTA |
1033 |
477 |
164.1 |
1.798 |
0.264 |
180 |
[84] |
|
|
Cocamide DEA |
EDTA |
1038 |
480 |
138 |
1.231 |
0.308 |
189 |
[85] |
||||
|
5 |
Alizarin Red S |
493 |
NaLS |
Ascorbic acid |
1075 |
672 |
197.29 |
1.897 |
0.273 |
140 |
[27] |
|
|
CPC |
Oxalic acid |
1189 |
420 |
147.42 |
2.16 |
0.299 |
16 |
[16] |
||||
|
Brij 35 |
Galactose |
432 |
12 |
– |
0.019 |
0.395 |
24 |
[86] |
||||
|
6 |
Allura Red |
504 |
DDAC |
Pt electrode |
D-Galactose |
721 |
2400 |
443.8 |
11.61 |
0.25 |
28 |
[87] |
|
DDAC |
Cu electrode |
D-Galactose |
713 |
4030 |
552.3 |
8.54 |
0.19 |
– |
[62] |
|||
|
DDAC |
Brass electrode |
D-Galactose |
739 |
5320 |
546.4 |
6.12 |
0.13 |
– |
[62] |
|||
|
NaLS |
Ascorbic acid |
920 |
760 |
144.54 |
1.38 |
0.206 |
110 |
[88] |
||||
|
7 |
Amido Black 10B |
610 |
Tween 60 |
Ascorbic acid |
1043 |
420 |
168.75 |
1.622 |
0.38 |
130 |
[48] |
|
|
8 |
Aniline Blue |
310 |
NaLS |
Ascorbic acid |
1485 |
750 |
240.24 |
2.31 |
0.244 |
130 |
[89] |
|
|
9 |
Azur A |
633 |
? |
EDTA + Dextrose |
1018 |
55 |
10.87 |
0.104 |
0.194 |
115 |
[32] |
|
|
? |
EDTA + NTA |
1097 |
292 |
124.30 |
0.983 |
0.319 |
230 |
[32] |
||||
|
? |
EDTA + Oxalic acid |
1056 |
210 |
35.83 |
0.909 |
0.426 |
86 |
[32] |
||||
|
NaLS |
Ascorbic acid |
1035 |
160 |
56.80 |
0.546 |
0.34 |
110 |
[90] |
||||
|
NaLS |
EDTA |
1074 |
255 |
207.57 |
1.20 |
0.45 |
26 |
[91] |
||||
|
NaLS |
Glucose |
893 |
147 |
– |
0.478 |
0.38 |
128 |
[92] |
||||
|
TTAB |
Glucose |
987 |
142 |
– |
0.327 |
0.24 |
71 |
[92] |
||||
|
Brij 35 |
Glucose |
927 |
143 |
– |
0.401 |
0.32 |
93 |
[92] |
||||
|
? |
Glucose |
749 |
140 |
– |
0.353 |
0.26 |
54 |
[92] |
||||
|
NaLS |
Glycerol |
855 |
250 |
213.75 |
1.182 |
0.476 |
63 |
[93] |
||||
|
10 |
Azur B |
647 |
CTAB |
EDTA |
1035 |
395 |
104.50 |
1.004 |
0.255 |
140 |
[94] |
|
|
NaLS |
Ascorbic acid |
1301 |
185 |
100.32 |
0.964 |
0.41 |
135 |
[95] |
||||
|
NaLS |
EDTA |
814 |
255 |
207.57 |
1.20 |
0.45 |
26 |
[96] |
||||
|
Tergitol 7 |
EDTA |
1034 |
45 |
14.75 |
0.14 |
0.316 |
40 |
[40] |
||||
|
? |
EDTA |
760 |
30 |
– |
0.06 |
0.182 |
11 |
[40] |
||||
|
NaLS |
NTA |
811 |
140 |
42.16 |
0.405 |
0.37 |
105 |
[97] |
||||
|
Tween 80 |
NTA |
729 |
95 |
22.65 |
0.217 |
0.32 |
74 |
[97] |
||||
|
CPC |
NTA |
718 |
65 |
14.42 |
0.138 |
0.30 |
31 |
[97] |
||||
|
? |
NTA |
996 |
70 |
17.52 |
0.168 |
0.25 |
12 |
[97] |
||||
|
11 |
Azur C |
620 |
NaLS |
Ascorbic acid |
1085 |
145 |
47.50 |
0.456 |
0.30 |
95 |
[98] |
|
|
? |
NTA |
347 |
70 |
19.84 |
0.19 |
0.23 |
38 |
[99] |
||||
|
Triton X 100 |
Glycerol |
– |
130 |
48.93 |
0.470 |
0.435 |
38 |
[100] |
||||
|
12 |
Biebrich Scarlet |
536 |
Tween 60 |
Ascorbic acid |
1072 |
210 |
93.15 |
0.896 |
0.41 |
75 |
[101] |
|
|
13 |
Bismarck Brown |
457 |
Brij 35 |
DTPA |
970 |
115 |
54 |
0.519 |
0.48 |
117 |
[102] |
|
|
? |
Ascorbic acid |
1110 |
155 |
93.50 |
0.899 |
0.54 |
40 |
[103] |
||||
|
? |
EDTA |
980 |
135 |
63.9 |
0.614 |
0.48 |
34 |
[103] |
||||
|
? |
Glucose |
870 |
120 |
42.16 |
0.405 |
0.40 |
31 |
[103] |
||||
|
? |
Oxalic acid |
1072 |
620 |
195.65 |
0.470 |
0.557 |
65 |
[104] |
||||
|
14 |
Brilliant Black PN |
570 |
Ammonium Lauryl Sulphate |
EDTA |
– |
1125 |
317.10 |
3.049 |
0.25 |
130 |
[105] |
|
|
15 |
Brilliant Blue FCF |
628 |
NaLS |
Ascorbic acid |
1025 |
445 |
120.56 |
1.159 |
0.264 |
130 |
[106] |
|
|
16 |
Brilliant Cresyl Blue |
622 |
? |
Fructose |
1115 |
590 |
183.30 |
1.958 |
0.291 |
228 |
[30] |
|
|
NaLS |
Fructose |
1061 |
2300 |
661 |
8.26 |
0.27 |
163 |
[107] |
||||
|
NaLS |
Mannose |
1088 |
310 |
106.92 |
1.028 |
0.317 |
165 |
[108] |
||||
|
? |
Mannose |
1034 |
240 |
– |
0.711 |
– |
85 |
[108] |
||||
|
NaLS |
Ascorbic acid |
1124 |
220 |
62.40 |
1.236 |
0.252 |
120 |
[28] |
||||
|
NaLS |
D-Xylose |
1085 |
440 |
115 |
1.105 |
0.240 |
140 |
[109] |
||||
|
NaLS |
Ethylene Glycol |
1085 |
460 |
153.14 |
1.472 |
0.306 |
60 |
[18] |
||||
|
17 |
Brilliant Green |
625 |
Ammonium Lauryl Ether Sulphate |
Ascorbic acid |
– |
720 |
262.40 |
2.52 |
0.29 |
180 |
[110] |
|
|
18 |
Brilliant Yellow |
432 |
NaLS |
Ascorbic acid |
1120 |
585 |
224.25 |
2.156 |
0.342 |
140 |
[111] |
|
|
19 |
Bromocresol Green |
423 |
Tween 80 |
EDTA |
760 |
748 |
100.13 |
0.96 |
0.150 |
100 |
[112] |
|
|
NaLS |
Formaldehyde |
1130 |
8000 |
– |
9.02 |
– |
70 |
[113] |
||||
|
NaLS |
Ascorbic acid |
1045 |
350 |
83.52 |
0.803 |
0.228 |
140 |
[106] |
||||
|
20 |
Bromocresol Purple |
212 |
Tween 60 |
Ascorbic acid |
1031 |
65 |
34.16 |
0.328 |
0.50 |
150 |
[101] |
|
|
21 |
Carmine |
563 |
Tween 60 |
Ascorbic acid |
1040 |
190 |
85.12 |
0.818 |
0.43 |
170 |
[101] |
|
|
22 |
Carmoisine A |
516 |
Cocamidopropyl Betaine |
Lactic acid |
731 |
2500 |
345 |
25.4 |
0.21 |
– |
[114] |
|
|
23 |
Celestine Blue |
642 |
NaLS |
EDTA |
– |
127 |
|
0.779 |
0.32 |
31 |
[36] |
|
|
24 |
Chlorophenol Red |
576 |
NaLS |
Isopropyl alcohol |
1273 |
735 |
233.011 |
2.240 |
0.275 |
165 |
[115] |
|
|
25 |
Congo Red |
498 |
NaLS |
Formaldehyde |
1074 |
3200 |
782 |
11.02 |
0.22 |
120 |
[13] |
|
|
CPC |
D-Xylose |
– |
470 |
119.6 |
1.15 |
0.323 |
130 |
[116] |
||||
|
? |
EDTA |
800 |
180 |
32.46 |
1.09 |
– |
120 |
[117] |
||||
|
26 |
Coomassie Brilliant Blue |
465 |
? |
EDTA |
920 |
380 |
97 |
1.86 |
0.27 |
190 |
[118] |
|
|
NaLS |
Isopropyl Alcohol |
734 |
140 |
118 |
0.879 |
0.306 |
114 |
[75] |
||||
|
27 |
Crystal Violet |
590 |
DSS |
Ascorbic acid |
– |
195 |
71.68 |
0.69 |
0.400 |
85 |
[54] |
|
|
28 |
Eosin |
525 |
? |
Fructose |
1071 |
240 |
87.52 |
0.841 |
0.34 |
55 |
[119] |
|
|
? |
Arabinose |
1066 |
240 |
73.08 |
0.702 |
0.28 |
85 |
[119] |
||||
|
? |
D-Xylose |
1020 |
250 |
70.85 |
0.681 |
0.27 |
90 |
[119] |
||||
|
? |
Mannose |
1057 |
170 |
67.20 |
0.646 |
0.37 |
75 |
[119] |
||||
|
29 |
Erythrosine |
505 |
Benzethonium Chloride |
EDTA |
– |
250 |
86.13 |
0.828 |
0.33 |
95 |
[120] |
|
|
30 |
FCF Fast Green |
625 |
NaLS |
Fructose |
1048 |
2250 |
649.6 |
8.12 |
0.22 |
59 |
[107] |
|
|
? |
Fructose |
1066 |
380 |
138.60 |
1.33 |
0.34 |
70 |
[121] |
||||
|
31 |
Indigo Carmine |
608 |
DSS |
EDTA |
920 |
410 |
97.52 |
0.937 |
0.253 |
120 |
[122] |
|
|
NaLS |
Formic acid |
1080 |
3800 |
985.6 |
28.43 |
0.240 |
115 |
[55] |
||||
|
– |
Ascorbic acid |
– |
160 |
47.60 |
0.45 |
0.27 |
40 |
[123] |
||||
|
32 |
Janus Green B |
654 |
NaLS |
Ascorbic acid |
806 |
775 |
122.55 |
1.17 |
0.196 |
130 |
[124] |
|
|
33 |
Lissamine Fast Yellow |
|
NaLS |
Ascorbic acid |
1100 |
375 |
108.15 |
1.039 |
0.268 |
160 |
[125] |
|
|
34 |
Lissamine Green B |
630 |
NaLS |
Ascorbic acid |
1100 |
375 |
106.68 |
1.025 |
0.259 |
170 |
[126] |
|
|
35 |
Malachite Green |
614 |
NaLS |
EDTA |
– |
76 |
18.256 |
0.22 |
0.22 |
30 |
[127] |
|
|
NaLS |
Arabinose |
836 |
36 |
6.138 |
0.059 |
0.203 |
32 |
[12] |
||||
|
? |
Arabinose |
845 |
33 |
11.900 |
0.112 |
0.426 |
36 |
[128] |
||||
|
NaLS |
Ascorbic acid |
1124 |
586 |
225.26 |
2.252 |
0.34 |
141 |
[129] |
||||
|
36 |
Methyl Green |
677 |
NaLS |
DTPA |
– |
310 |
252.65 |
1.05 |
0.43 |
55 |
[130] |
|
|
37 |
Methyl Orange |
464 |
NaLS |
D-Xylose |
1085 |
480 |
168.95 |
1.624 |
0.321 |
160 |
[131] |
|
|
Brij 35 |
DTPA |
735 |
95 |
28.16 |
0.270 |
0.400 |
94 |
[102] |
||||
|
38 |
Methylene Blue |
665 |
? |
Oxalic acid |
– |
70 |
12.6 |
0.121 |
0.28 |
35 |
[132] |
|
|
NaLS |
D- Xylose |
1001 |
90 |
32.72 |
0.31 |
0.363 |
55 |
[133] |
||||
|
NaLS |
Mannose |
1041 |
80 |
37.63 |
0.361 |
0.451 |
48 |
[134] |
||||
|
? |
Mannose |
918 |
73 |
30.24 |
0.290 |
0.451 |
40 |
[134] |
||||
|
NaLS |
Mannitol |
– |
– |
32 |
0.264 |
0.32 |
45 |
[57] |
||||
|
Triton X 100 |
EDTA |
1082 |
420 |
113.80 |
1.087 |
0.248 |
160 |
[135] |
||||
|
NaLS |
EDTA |
654 |
190 |
– |
0.698 |
0.37 |
200 |
[136] |
||||
|
? |
Ascorbic acid |
1121 |
165 |
148 |
0.83 |
0.46 |
165 |
[137] |
||||
|
NaLS + Tween 80 |
D- Xylose |
870 |
210 |
55.25 |
0.531 |
0.302 |
100 |
[43] |
||||
|
NaLS + CTAB |
D- Xylose |
825 |
190 |
44.99 |
0.432 |
0.287 |
90 |
[43] |
||||
|
Brij 35 + NaLS |
D- Xylose |
921 |
245 |
60.23 |
0.676 |
0.452 |
126 |
[38] |
||||
|
39 |
Naphthol Green B |
714 |
NaLS |
Fructose |
1040 |
1850 |
422.4 |
10.6 |
0.21 |
260 |
[107] |
|
|
NaLS |
Ascorbic acid |
1050 |
365 |
107 |
1.028 |
0.279 |
160 |
[138] |
||||
|
40 |
Nile Blue |
635 |
– |
Arabinose |
816 |
330 |
91.28 |
0.609 |
0.256 |
120 |
[139] |
|
|
NaLS |
DTPA |
812 |
165 |
75 |
0.72 |
0.559 |
25 |
[140] |
||||
|
41 |
Orange G |
196 |
NaLS |
EDTA |
1249 |
350 |
158.9 |
1.52 |
0.47 |
80 |
[141] |
|
|
– |
EDTA |
1175 |
265 |
156.40 |
1.503 |
0.50 |
140 |
[142] |
||||
|
42 |
Phloxine B |
550 |
– |
D-Xylose |
910 |
260 |
126.16 |
1.21 |
0.533 |
120 |
[143] |
|
|
CTAB |
EDTA |
1135 |
300 |
66.72 |
0.64 |
0.226 |
100 |
[144] |
||||
|
– |
EDTA |
1155 |
250 |
135.3 |
1.300 |
0.46 |
120 |
[145] |
||||
|
43 |
Ponceau S |
205 |
– |
Mannitol |
1080 |
240 |
– |
0.456 |
0.45 |
36 |
[146] |
|
|
– |
EDTA |
1047 |
390 |
84 |
1.61 |
0.20 |
240 |
[147] |
||||
|
44 |
Quinoline Yellow |
412 |
DTAB |
Cellobiose |
900 |
8000 |
989 |
15.08 |
0.14 |
100 |
[15] |
|
|
DOSS |
Cellobiose |
865 |
4500 |
695 |
13.78 |
0.18 |
100 |
[148] |
||||
|
45 |
Rhodamine B |
546 |
– |
Fructose |
1044 |
960 |
240.58 |
6.93 |
0.24 |
216 |
[149] |
|
|
NaLS |
Fructose |
1017 |
2400 |
620.1 |
7.75 |
0.23 |
142 |
[107] |
||||
|
NaLS |
DTPA |
– |
185 |
77.28 |
0.74 |
0.495 |
85 |
[150] |
||||
|
– |
Ascorbic acid |
1137 |
120 |
59.64 |
0.51 |
0.38 |
45 |
[137] |
||||
|
46 |
Rhodamine 6G |
530 |
DSS |
Oxalic acid |
1080 |
200 |
176 |
0.86 |
0.41 |
131 |
[47] |
|
|
CTAB |
Oxalic acid |
620 |
90 |
37.26 |
0.24 |
0.45 |
68 |
[47] |
||||
|
Triton X 100 |
Oxalic acid |
897 |
165 |
110.88 |
0.55 |
0.38 |
96 |
[47] |
||||
|
– |
Oxalic acid |
530 |
60 |
12.60 |
0.22 |
0.39 |
25 |
[47] |
||||
|
NaLS |
EDTA |
1162 |
450 |
131.60 |
1.265 |
0.251 |
170 |
[151] |
||||
|
47 |
Rose Bengal |
551 |
NaLS |
D-Xylose |
1095 |
460 |
158.72 |
1.52 |
0.315 |
145 |
[152] |
|
|
CTAB |
Oxalic acid |
550 |
75.1 |
7.005 |
0.067 |
0.169 |
175 |
[2] |
||||
|
– |
Mannitol |
1080 |
160 |
62.8 |
0.744 |
0.44 |
60 |
[153] |
||||
|
Triton X 100 |
Oxalic acid |
289 |
29.1 |
2.96 |
0.023 |
0.293 |
45 |
[14] |
||||
|
– |
Oxalic acid |
1239 |
175 |
64 |
0.981 |
0.51 |
90 |
[153] |
||||
|
48 |
Safranine |
520 |
Sodium Octanoate |
Mannitol |
– |
330 |
285.1 |
1.534 |
0.44 |
35 |
[154] |
|
|
DSS |
Mannitol |
– |
150 |
130.50 |
0.760 |
0.50 |
40 |
[74] |
||||
|
NaLS |
Mannitol |
1000 |
75 |
63.30 |
0.222 |
0.31 |
102 |
[155] |
||||
|
NaLS |
D-Xylose |
1057 |
207 |
70.74 |
0.680 |
0.323 |
98 |
[156] |
||||
|
Brij 35 |
DTPA |
1048 |
155 |
66.96 |
0.643 |
0.41 |
122 |
[102] |
||||
|
– |
EDTA |
1055 |
50 |
27.22 |
0.261 |
0.58 |
19 |
[157] |
||||
|
– |
Glucose |
580 |
35 |
3.70 |
0.035 |
0.18 |
85 |
[157] |
||||
|
– |
NTA |
655 |
35 |
8.75 |
0.084 |
0.37 |
8 |
[157] |
||||
|
NaLS |
Arabinose |
1025 |
117 |
40.04 |
0.385 |
0.333 |
91 |
[158] |
||||
|
– |
Arabinose |
989 |
87 |
29.63 |
0.312 |
0.280 |
77 |
[158] |
||||
|
NaLS |
Fructose |
998 |
128.6 |
21.86 |
0.21 |
0.17 |
16 |
[159] |
||||
|
– |
Fructose |
981 |
57.20 |
13.55 |
0.13 |
0.24 |
35 |
[159] |
||||
|
– |
Ascorbic acid |
1061 |
150 |
120.60 |
0.65 |
0.42 |
115 |
[137] |
||||
|
49 |
Safranine O |
518 |
NaLS |
EDTA |
1052 |
1700 |
364.70 |
8.93 |
0.20 |
40 |
[160] |
|
|
DSS |
EDTA |
1059 |
65 |
26.34 |
0.253 |
0.38 |
80 |
[53] |
||||
|
– |
EDTA |
981 |
100 |
– |
0.330 |
0.03 |
11 |
[53] |
||||
|
Tween 80 |
EDTA |
995 |
300 |
101.60 |
0.976 |
0.34 |
60 |
[161] |
||||
|
CTAB |
EDTA |
973 |
185 |
15.28 |
0.146 |
0.084 |
20 |
[162] |
||||
|
CPC |
EDTA |
913 |
80 |
15.28 |
1.469 |
0.21 |
25 |
[39] |
||||
|
50 |
Sudan I |
476 |
NaLS |
Fructose |
1014 |
1350 |
367.8 |
11.49 |
0.26 |
30 |
[163] |
|
|
51 |
Sunset Yellow FCF |
480 |
DSS |
EDTA |
864 |
390 |
– |
1.218 |
0.290 |
140 |
[164] |
|
|
CTAB |
Ascorbic acid |
777 |
5600 |
733.6 |
19.77 |
0.168 |
6 |
[56] |
||||
|
Cetrimonium Bromide |
Ascorbic acid |
806 |
5400 |
552.0 |
11.19 |
– |
– |
[165] |
||||
|
52 |
Tartrazine |
199 |
Lauryl Glucoside |
D-Fructose |
1523 |
544 |
435.32 |
0.799 |
0.538 |
129 |
[166] |
|
|
DSS |
EDTA |
879 |
220 |
– |
0.616 |
0.280 |
100 |
[164] |
||||
|
53 |
Titan Yellow |
402 |
– |
EDTA |
823 |
2800 |
511.10 |
17.57 |
– |
– |
[167] |
|
|
54 |
Thionine |
596 |
? |
EDTA |
? |
? |
72 |
0.280 |
0.36 |
49 |
[168] |
|
|
CTAB |
EDTA |
700 |
150 |
53.5 |
0.514 |
0.50 |
50 |
[41] |
||||
|
55 |
Thymol Blue |
593 |
NaLS |
Mannose |
955 |
100 |
24.60 |
0.236 |
0.257 |
37 |
[169] |
|
|
– |
Mannose |
929 |
70 |
20.20 |
0.194 |
0.217 |
27 |
[169] |
||||
|
– |
Ascorbic acid |
– |
150 |
82.06 |
0.803 |
0.49 |
54 |
[170] |
||||
|
56 |
Toluidine Blue |
630 |
NaLS |
EDTA |
1065 |
70 |
27.36 |
0.263 |
0.367 |
124 |
[37] |
|
|
NaLS |
Arabinose |
966 |
60 |
15.06 |
0.144 |
0.259 |
123 |
[12] |
||||
|
? |
Arabinose |
885 |
55 |
15.375 |
0.145 |
0.315 |
90 |
[128] |
||||
|
CTAB |
Glucose |
– |
35 |
6.26 |
0.057 |
0.41 |
6 |
[171] |
||||
|
NaLS |
D- Xylose |
1110 |
430 |
148.96 |
1.432 |
0.312 |
130 |
[172] |
||||
|
CPC |
EDTA |
1005 |
35 |
11.04 |
0.106 |
0.313 |
38 |
[173] |
||||
|
Tween 80 |
EDTA |
710 |
50 |
14.40 |
0.138 |
0.405 |
60 |
[42] |
||||
|
? |
Maleic hydrazide |
– |
10 |
– |
0.000 |
0.20 |
7 |
[174] |
||||
|
Tergitol 7 |
Glucose |
315 |
70 |
– |
0.106 |
0.33 |
21 |
[175] |
||||
|
NaLS |
Ethylene glycol |
1084 |
320 |
115.92 |
1.114 |
0.334 |
55 |
[18] |
||||
|
NaLS + CPC + Tween 80 |
EDTA |
897 |
234 |
54.13 |
0.547 |
0.281 |
109 |
[44] |
||||
|
57 |
Tropaeolin O |
492 |
Benzalkonium Chloride |
Oxalic acid |
676 |
2000 |
340 |
10.54 |
0.25 |
410 |
[29] |
|
|
– |
EDTA |
860 |
340 |
150.68 |
1.44 |
0.51 |
80 |
[176] |
||||
|
58 |
Trypan Blue |
607 |
– |
Arabinose |
– |
350 |
83.52 |
0.80 |
0.23 |
140 |
[177] |
|
|
59 |
Victoria Blue R |
587 |
NaLS |
Ascorbic acid |
1045 |
360 |
110.39 |
1.061 |
0.293 |
140 |
[81] |
|
|
60 |
Xylidine Ponceau |
506 |
Tween 60 |
Ascorbic acid |
1091 |
197 |
68.77 |
0.661 |
0.33 |
110 |
[178] |
|
|
||||||||||||
|
61 |
Xylene Cyanol FF + Patent Blue |
|
– |
EDTA |
868 |
230 |
199.64 |
0.64 |
0.24 |
115 |
[21] |
|
|
62 |
Methylene Blue + Toluidine Blue |
|
– |
EDTA |
– |
110 |
81.62 |
0.539 |
0.49 |
34 |
[179] |
|
|
63 |
Thionine + Azur B |
|
– |
EDTA |
975 |
76 |
56.62 |
0.18 |
0.25 |
59 |
[180] |
|
|
64 |
Methylene Blue + Azur B |
|
– |
EDTA |
962 |
70 |
51.24 |
0.116 |
0.18 |
46 |
[181] |
|
|
65 |
Methylene Blue + Thionine |
|
– |
EDTA |
1000 |
90 |
67.68 |
0.43 |
0.49 |
30 |
[182] |
|
|
66 |
Erythrosine B + Tartrazine |
|
NaLS |
EDTA |
1040 |
270 |
72.42 |
0.31 |
0.31 |
80 |
[183] |
|
|
67 |
Toluidine Blue + Thionine |
|
– |
EDTA |
– |
105 |
72.9 |
0.16 |
– |
42 |
[184] |
|
|
68 |
Brilliant Green + Celestine Blue |
|
– |
EDTA |
894 |
93 |
59.1 |
0.31 |
0.39 |
65 |
[19] |
|
|
69 |
New Methylene Blue+Safranine O |
|
– |
EDTA |
1235 |
125 |
81.4 |
0.444 |
0.41 |
95 |
[185] |
|
|
70 |
New Methylene Blue + Fast Green |
|
– |
EDTA |
998 |
120 |
88.56 |
0.564 |
0.48 |
90 |
[185] |
|
|
71 |
Brilliant Green + Fast Green |
|
– |
EDTA |
930 |
112 |
77.28 |
0.418 |
0.41 |
72 |
[185] |
|
|
72 |
New Methylene Blue + Celestine Blue |
|
– |
EDTA |
795 |
88 |
51.04 |
0.301 |
0.44 |
45 |
[185] |
|
|
73 |
Naphthol Green B + Janus Green B |
|
– |
EDTA |
1248 |
210 |
213.78 |
1.002 |
0.40 |
180 |
[20] |
|
|
74 |
Sudan I + Rhodamine B |
|
NaLS |
Fructose |
1060 |
2200 |
550 |
6.87 |
0.235 |
105 |
[107] |
|
|
75 |
Sudan I + Rhodamine B + Fast Green FCF |
|
NaLS |
Fructose |
960 |
2150 |
528 |
6.60 |
0.255 |
45 |
[107] |
|
|
76 |
Sudan I + Rhodamine B + Fast Green FCF+ Naphthol Green B |
|
NaLS |
Fructose |
1014 |
2300 |
561 |
7.01 |
0.240 |
87 |
[107] |
|
|
77 |
Naphthol Green B + Fast Green FCF + Brilliant Cresyl Blue |
|
NaLS |
Fructose |
1090 |
2300 |
596.2 |
7.45 |
0.237 |
67 |
[107] |
|
|
78 |
Brilliant Cresyl Blue + Toluidine Blue |
|
NaLS |
Ethylene Glycol |
1090 |
630 |
195.36 |
1.878 |
0.284 |
70 |
[18] |
|
|
79 |
Sudan Black B + Azur B |
|
DSS |
EDTA |
965 |
340 |
84.83 |
0.815 |
0.258 |
110 |
[22] |
|
|
||||||||||||
|
80 |
Spinach Extract |
|
NaLS |
Fructose |
1050 |
1750 |
384 |
9.22 |
0.20 |
44 |
[186] |
|
|
81 |
Marigold Flower |
|
Tween 80 |
Xylose |
1080 |
674 |
199 |
1.892 |
0.273 |
121 |
[187] |
|
|
Brij 35 |
Xylose |
1076 |
673 |
198 |
2.239 |
0.273 |
126 |
[188] |
||||
|
82 |
Rose Extract |
|
– |
NTA |
998 |
176 |
82.18 |
0.79 |
0.46 |
42 |
[189] |
|
|
– |
Mannitol |
1078 |
170 |
87.20 |
0.83 |
0.47 |
55 |
[189] |
||||
|
NaLS |
Ascorbic acid |
– |
140 |
110.60 |
0.676 |
– |
49 |
[190] |
||||
|
83 |
Curcumin |
|
Tween 80 |
Arabinose |
1044 |
836 |
105.45 |
1.01 |
0.120 |
120 |
[191] |
|
|
NaLS |
Ascorbic acid |
886 |
750 |
131.30 |
1.26 |
0.227 |
110 |
[192] |
||||
|
Brij 35 |
Fructose |
1070 |
784 |
120.50 |
1.15 |
0.143 |
100 |
[193] |
||||
|
84 |
Azadirachta Indica leaves Extract |
|
Benzalkonium Chloride |
Oxalic acid |
1005 |
2200 |
602.4 |
20.25 |
0.27 |
– |
[63] |
|
|
85 |
Beetroot Extract |
|
NaLS |
Formic acid |
1115 |
3200 |
674.4 |
15.31 |
– |
155 |
[194] |
|
|
86 |
Magnolia Champaca Flower Extract |
|
CTAB |
EDTA |
1319 |
130 |
140.4 |
1.42 |
0.20 |
40 |
[195] |
|
|
87 |
Pomegranate juice |
|
NaLS |
Fructose |
700 |
984 |
268 |
10.30 |
0.38 |
220 |
[196] |
|
III. CELL PARAMETERS
Dark potential (VDark): Initially, the whole system is placed in dark till it attains a stable potential. This stable potential is known as dark potential. It is represented by VDark and measured in V or mV.
Maximum potential (Vmax): After obtaining the dark potential, a rise in potential is seen when the platinum electrode is illuminated. The highest observed potential is known as maximum potential and represented by Vmax.
Open circuit voltage (Voc): The highest voltage that a solar cell can generate at zero current in the open circuit voltage. It is represented by Voc and measured in V or mV.
Photopotential (?V): The photopotential is calculated by the following mathematical expression
?V = Voc - VDark
Short circuit current (isc): The maximum current flowing through a solar cell when the voltage across it is zero (i.e. when the cell is short-circuited), is known as the short-circuit current. It is represented by isc and measured in A or mA.
Maximum current (imax): The photocurrent increased sharply in the first few minutes of illumination and it reaches a maximum value. This value is called maximum photocurrent. It is represented by imax.
Equilibrium current (ieq): After obtaining the maximum photocurrent, the current decreased slowly during the illumination and finally achieved a constant value. This photocurrent in its equilibrium condition is known as equilibrium photocurrent. It is represented by ieq.
Maximum power point (Ppp): The point at which the cell generates maximum electrical power. It is represented by Ppp and calculated by the following mathematical expression
Ppp= Vpp×ipp
Voltage at power point (Vpp): It is maximum potential at power point and represented by Vpp.
Current at power point (ipp): It is maximum current at power point and represented by ipp.
Fill Factor (FF): The fill factor is the most important parameter for evaluating the performance of solar cells. It is the ratio of maximum power to the product of short circuit current and open circuit voltage.
FF= PppVoc×isc= Vpp×ippVoc×isc
Conversion efficiency (CE): It is also the most important parameter of each cell, which represents the capability of a cell. It is the ratio of the electrical power output to input (incident energy from sunlight).
CE %=Vpp × ipp × FFP ×A ×100%
Storage capacity (t1/2): To determine the storage capacity of a photogalvanic cell, add an external load (current at power point) when illumination ends and the potential approaches a constant value. The storage capacity is determined in terms of t1/2, which is the time it takes for the output (power) to reduce by half at its power point in the dark. It can also be expressed as a percentage of charging time (charging time = tVmax - tVillum).
IV. CHALLENGES AND LIMITATIONS
One of the main challenges is improving efficiency, particularly addressing recombination losses that reduce overall energy conversion rates. Photogalvanic cells generally exhibit lower solar energy conversion efficiencies compared to traditional photovoltaic cells. This limits their widespread adoption for grid-scale power generation. The amount of energy a photogalvanic cell can store is directly related to the volume of the electrolyte solution. This limits their energy storage density compared to batteries. Maintaining the long-term stability and performance of photogalvanic cells can be challenging due to factors like electrolyte degradation and electrode corrosion. The cost of advanced materials and fabrication processes remains a barrier to the commercial scalability of PGCs.
V. FUTURE PROSPECTS
Research continues to improve the efficiency, storage capacity, and stability of Photogalvanic cells. Exploring new and more efficient photosensitizers with broader absorption spectra and longer excited state lifetimes is crucial Developing stable electrolytes with higher ionic conductivity and improved charge transport properties can enhance cell performance. Incorporating Nanomaterials like quantum dots or metal nanoparticles can enhance light absorption and charge separation processes. Focus on exploring novel reductant which are eco-friendly and cost-effective and that can efficiently regenerate the oxidized dye molecules in the Photogalvanic cell, improving overall cell performance and longevity.
Advances in materials science, particularly in the development of high-performance electrodes, electrolytes and Nanomaterials, have could further improve the efficiency and stability of Photogalvanic cells and Photogalvanic cells could play a significant role in sustainable energy distribution.
Conclusion
Photogalvanic cells represent an exciting frontier in solar energy technology, merging conversion and storage capabilities in a single device. As the global energy landscape evolves, Photogalvanic cells are positioned as a promising technology that not only contributes to renewable energy generation but also supports the development of eco-friendly materials and systems. This review paper structure provides a comprehensive overview of Photogalvanic cells, covering materials, mechanisms, performance strategies, and future potential in the context of sustainable energy conversion. It would be suitable for researchers, students, and industry professionals interested in the latest developments and applications of this technology. Photogalvanic cells represent a promising avenue in the pursuit of sustainable energy technologies, with their unique mechanisms of energy conversion and storage. Continued advancements in this field may not only enhance their efficiency but also play a critical role in reducing reliance on fossil fuels and addressing global energy challenges. Research into their efficiency, materials, and long-term viability is ongoing, highlighting the need for further innovation in this field to optimize performance and scalability. As research continues to address their challenges and improve efficiency, these cells may play a crucial role in future sustainable energy systems, contributing to the global transition toward clean energy sources.
References
[1] M. B. Hayat, D. Ali, K. C. Monyake, L. Alagha, and N. Ahmed, “Solar Energy-a Look into Power Generation, Challenges, and a Solar-Powered Future,” Int J Energy Res, vol. 43, no. 3, pp. 1049–1067, Mar. 2019, doi: 10.1002/er.4252. [2] S. A. Mahmoud and B. S. Mohamed, “Study on the Performance of Photogalvanic Cell for Solar Energy Conversion and Storage,” International Journal of Electrochemical Science, vol. 10, no. 4, pp. 3340–3353, Apr. 2015, doi: 10.1016/S1452-3981(23)06544-6. [3] A. Malviya and P. P. Solanki, “Photogalvanics: A Sustainable and Promising Device for Solar Energy Conversion and Storage,” Renewable and Sustainable Energy Reviews, vol. 59, pp. 662–691, Jun. 2016, doi: 10.1016/j.rser.2015.12.295. [4] A. Mohammad Bagher, “Types of Solar Cells and Application,” AJOP, vol. 3, no. 5, p. 94, 2015, doi: 10.11648/j.ajop.20150305.17. [5] E. K. Rideal and E. G. Williams, “XLIII.—The Action of Light on the Ferrous Ferric Iodine Iodide Equilibrium,” J. Chem. Soc., Trans., vol. 127, no. 0, pp. 258–269, 1925, doi: 10.1039/CT9252700258. [6] E. Rabinowitch, “The Photogalvanic Effect I. The Photochemical Properties of the Thionine-Iron System,” The Journal of Chemical Physics, vol. 8, no. 7, pp. 551–559, Jul. 1940, doi: 10.1063/1.1750711. [7] E. Rabinowitch, “The Photogalvanic Effect II. The Photogalvanic Properties of the Thionine-Iron System,” The Journal of Chemical Physics, vol. 8, no. 7, pp. 560–566, Jul. 1940, doi: 10.1063/1.1750712. [8] K. Weber and E. Matijevi?, “Inhibition of Photogalvanic Phenomena,” Recl. Trav. Chim. Pays?Bas, vol. 70, no. 6, pp. 481–494, Jan. 1951, doi: 10.1002/recl.19510700602. [9] P. V. Kamat and N. N. Lichtin, “Electron Transfer in the Quenching of Protonated Triplet Methylene Blue by Ground-State Molecules of the Dye,” J. Phys. Chem., vol. 85, no. 7, pp. 814–818, Apr. 1981, doi: 10.1021/j150607a017. [10] P. V. Kamat and N. N. Lichtin, “Electron Transfer in the Quenching of Protonated Triplet Thionine and Methylene Blue by Ground State Thionine,” Journal of Photochemistry, vol. 18, no. 3, pp. 197–209, Jan. 1982, doi: 10.1016/0047-2670(82)85001-6. [11] W. John Albery and A. W. Foulds, “Photogalvanic cells,” Journal of Photochemistry, vol. 10, no. 1, pp. 41–57, Jan. 1979, doi: 10.1016/0047-2670(79)80036-2. [12] K. R. Genwa, A. Kumar, and A. Sonel, “Photogalvanic Solar Energy Conversion: Study with Photosensitizers Toluidine Blue and Malachite Green in Presence of Nals,” Applied Energy, vol. 86, no. 9, pp. 1431–1436, Sep. 2009, doi: 10.1016/j.apenergy.2008.11.026. [13] P. Koli, Y. Dayma, R. K. Pareek, and M. Jonwal, “Use of Congo Red Dye-Formaldehyde as a New Sensitizer-Reductant Couple for Enhanced Simultaneous Solar Energy Conversion and Storage by Photogalvanic Cells at the Low and Artificial Sun Intensity,” Sci Rep, vol. 10, no. 1, p. 19264, Nov. 2020, doi: 10.1038/s41598-020-76388-5. [14] S. A. Mahmoud, and B. S. Mohamed, “Solar Energy Conversion and Storage: Rose Bengal-Triton X-100 by Photogalvanic Cell,” Journal of Energy Technologies and Policy, vol. 5, no. 12, pp. 40–47, 2015. [15] M. Jonwal, P. Koli, Y. Dayma, R. Kumar Pareek, R. Kumar, and Dheerata, “Photogalvanics of Dodecyltrimethyl Ammonium Bromide?Quinoline Yellow?Alkali: A Solar Energy Conversion, Storage, Photostability, and Spectral Study,” Intl J of Energy Research, vol. 46, no. 10, pp. 13889–13907, Aug. 2022, doi: 10.1002/er.8106. [16] S. K. Yadav, R. Kumari, and R. K. Gunsaria, “Alizarin Red S, Oxalic Acid and Cetylpyridinium Chloride-based Modified Photogalvanic Cell with Sustainable Conversion and Storage of Solar Energy,” ajc, vol. 36, no. 1, pp. 161–168, Dec. 2023, doi: 10.14233/ajchem.2024.30693. [17] S. Dube, “Photogalvanic Effect in Mixture of Dyes and Reductant System,” Asian Journal of Chemistry, vol. 5, no. 3, pp. 666–670, 1993. [18] K. M. Gangotri and A. Kumar Mahawar, “Comparative Study on Effect of Mixed Photosensitizer System for Solar Energy Conversion and Storage: Brilliant Cresyl Blue + Toluidine Blue–Ethylene Glycol–Nals System,” Env Prog and Sustain Energy, vol. 31, no. 3, pp. 474–480, Oct. 2012, doi: 10.1002/ep.10579. [19] S. Yadav and C. Lal, “Optimization of Performance Characteristics of a Mixed Dye Based Photogalvanic Cell for Efficient Solar Energy Conversion and Storage,” Energy Conversion and Management, vol. 66, pp. 271–276, Feb. 2013, doi: 10.1016/j.enconman.2012.09.011. [20] B. Singh, C. Lal, and N. Kumar, “Utilization of Mixed Naphthol Green B and Janus Green B Dyes as Photosensitier in Photogalvanic Cell for Solar Energy Conversion and Storage,” Asian J. Chem., vol. 32, no. 8, pp. 1914–1920, 2020, doi: 10.14233/ajchem.2020.22712. [21] B. Singh and C. Lal, “Enhanced Efficiency of Photogalvanic Cell based on Mixed Triphenylmethane Dyes,” Asian J. Chem., vol. 32, no. 2, pp. 466–470, Jan. 2020, doi: 10.14233/ajchem.2020.22547. [22] K. R. Genwa, and S. P. Singh, “Enhanced Energy Efficiency of Photogalvanic Cell with Mixture of Two Dyes as Photosensitizers in EDTA-DSS System,” Journal of the Indian Chemical Society, vol. 94, no. 11, pp. 1213–1219, 2021, doi: https://doi.org/null. [23] W. Hendrich, “The photovoltaic effect in the chlorophyll–ascorbic acid system,” Rocz. Chem, vol. 32, pp. 107–116, 1958. [24] W. J. Albery and M. D. Archer, “Photogalvanic Cells: II . Current?Voltage and Power Characteristics,” J. Electrochem. Soc., vol. 124, no. 5, pp. 688–697, May 1977, doi: 10.1149/1.2133384. [25] A. S. N. Murthy and K. S. Reddy, “Studies on Photogalvanic Effect in Systems Containing Toluidine Blue,” Solar Energy, vol. 30, no. 1, pp. 39–43, 1983, doi: 10.1016/0038-092X(83)90004-X. [26] S. C. Ameta, Th. D. Dubey, G. C. Dubey, and R. C. Ameta, “Studies in Photochemical Conversion of Solar Energy: I. Use of Hydroqvinone as a Reductant in Photogalvanic Cell,” Zeitschrift für Physikalische Chemie, vol. 265O, no. 1, pp. 838–840, Jan. 1984, doi: 10.1515/zpch-1984-265112. [27] B. Ram, J. Lalita, and K. R. Genwa, “Role of Alizarin Red-S-NaLS-Ascorbic acid System in Solar Photogalvanic Performance and Storage,” Orient. J. Chem, vol. 39, no. 4, pp. 919–924, Aug. 2023, doi: 10.13005/ojc/390413. [28] R. Kumari, S. K. Yadav, and R. Gunsaria, “Innovative Study on Photogalvanic Cell for Solar Energy Conversion and Storage Through Brilliant Cresyl Blue + Ascorbic Acid + Sodium Lauryl Sulphate,” Orient. J. Chem, vol. 40, no. 3, pp. 773–780, Jun. 2024, doi: 10.13005/ojc/400319. [29] P. Koli, R. Kumar, Y. Dayma, R. K. Pareek, A. Meena, and M. Jonwal, “Tropaeline O?Oxalic Acid?Benzalkonium Chloride Photogalvanic Cells for Solar Energy Conversion and Storage,” Battery Energy, vol. 1, no. 4, p. 20220011, Oct. 2022, doi: 10.1002/bte2.20220011. [30] U. Sharma, P. Koli, and K. M. Gangotri, “Brilliant Cresyl Blue – Fructose for Enhancement of Solar Energy Conversion and Storage Capacity of Photogalvanic Solar Cells,” Fuel, vol. 90, no. 11, pp. 3336–3342, Nov. 2011, doi: 10.1016/j.fuel.2011.06.036. [31] S. Dube, “Simultaneous Use of Two Reductants in a Photogalvanic Cell for Solar-Energy Conversion and Storage,” Int. J. Energy Res., vol. 17, no. 4, pp. 311–314, Jun. 1993, doi: 10.1002/er.4440170408. [32] K. M. Gangotri, V. Indora, and M. K. Bhimwal, “Studies of Mixed Reductant Systems with Azur a as Photosensitizer for Solar Energy Conversion and Storage in Photogalvanic Cells,” International Journal of Sustainable Energy, vol. 30, no. 2, pp. 119–128, Apr. 2011, doi: 10.1080/1478646X.2010.509498. [33] Z. Bi, Y. Li, and Z. Liu, “Study of the Transparent Electrode Photogalvanovoltaic Cell,” Taiyangneng Xuebao, vol. 1, pp. 140–147, 1980. [34] R. C. Srivastava, R. Srinivasan, P. R. Marwadi, S. B. Bhise, and S. S. Mathur, “Surfactant micelles for solar energy storage,” Curr. Sci., vol. 51, no. 21, pp. 1015–1017, 1982. [35] S. J. Valenty, “Characterization and Chemical Reactions of Surfactant Monolayer Films,” ACS, vol. 184, pp. 69–95, 1980. [36] J. Bharadwaj and A. Meena, “A Pathway towards Green Chemistry via Solar Energy: Micellization of Celestine Blue-Edta-Sodium Lauryl Sulphate System for the Generation of Electricity in a Photogalvanic Cell,” Journal of Natural Sciences, vol. 1, no. 2, pp. 12–17, 2013. [37] S. Singh, D. Shikha, V. Singh, and S. Gupta, “Enhancement of Photogalvanic Effect of Toluidine Blue Dye using Sodium LaurylSulphate as an Efficient Additive for Solar Energy Conversion and Storage,” Asian J. Chem., vol. 33, no. 3, pp. 527–530, 2021, doi: 10.14233/ajchem.2021.22903. [38] M. Lal and K. M. Gangotri, “Innovation in Progressive Study for Prospective Energy Source Through Photo?Galvanic System: Xylose+brij?35+Nals,” Intl J of Energy Research, vol. 46, no. 14, pp. 19538–19547, Nov. 2022, doi: 10.1002/er.8525. [39] P. Gangotri and K. M. Gangotri, “Study in Cationic Micellar Effect on Photogalvanics: Cetyl Pyridinium Chloride- Ethylene Diamine Tetra Acetic Acid – Safranine O System for Solar Energy Conversion and Storage,” Journal of Technology Innovations in Renewable Energy, vol. 6, pp. 71–79, 2017. [40] K. M. Gangotri, P. Aseri, and M. K. Bhimwal, “The Use of Tergitol-7 in Photogalvanic Cells for Solar Energy Conversion and Storage: An EDTA–Azur B System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 35, no. 4, pp. 312–320, Feb. 2013, doi: 10.1080/15567036.2010.497799. [41] S. K. Yadav, G. Singh, and R. D. Yadav, “Photogalvanic Solar Conversion and Storage by Using Thionine as Photosensitizer and EDTA as Reductant in the Presence of CTAB as Surfactant,” Afinidad, vol. 67, no. 550, 2010, [Online]. Available: https://raco.cat/index.php/afinidad/article/view/269642. [42] J. Meena and K. M. Gangotri, “Use of Toluidine Blue-EDTA -Tween-80 System in Photogalvanic Cell for Solar Energy Conversion and Storage,” JETIR, vol. 6, no. 6, pp. 232–237, 2019. [43] M. Lal and K. M. Gangotri, “A Comparative study on the Performance of Photogalvanic cells with mixed Surfactant for Solar Energy conversion and storage: D-Xylose- Methylene Blue systems,” Res.J.Recent Sci., vol. 2, no. 12, pp. 19–17, 2013. [44] J. Meena, S. K. Meena, and K. Gangotri, “Enhanced Electrical Output by Mixed Surfactant for Solar Cells: EDTA+TB+NaLS+CPC+Tween-80 system,” Orient. J. Chem, vol. 40, no. 1, pp. 288–293, Feb. 2024, doi: 10.13005/ojc/400135. [45] M. Lal and K. M. Gangotri, “Innovative Study in Renewable Energy Source Through Mixed Surfactant System for Eco-Friendly Environment,” Environ Sci Pollut Res, vol. 30, no. 44, pp. 98805–98813, Jun. 2023, doi: 10.1007/s11356-023-28246-w. [46] S. Lingamurthy, V. Bhanumahti, B. Sethuram, and T. N. Rao, “Photogalvanic Cells Based on the Photoreduction of Xanthene Dyes Using Riboflavin as a Sensitizer in Aqueous/Micellar Medium,” Indian Journal of Chemistry, vol. 29A, pp. 733–736, 1990. [47] K. R. Genwa and M. Genwa, “Photogalvanic Cell: A New Approach for Green and Sustainable Chemistry,” Solar Energy Materials and Solar Cells, vol. 92, no. 5, pp. 522–529, May 2008, doi: 10.1016/j.solmat.2007.10.010. [48] K. R. Genwa and C. P. Sagar, “Tween 60 – Amido Black 10B – Ascorbic Acid System: Studies of Photogalvanic Effect and Solar Energy Conversion,” Journal of Chemical Engineering and Materials Science, vol. 2, no. 9, pp. 140–148, 2011. [49] P. J. Hillson and E. K. Rideal, “The Becquerel Effect in the Presence of Dyestuffs and the Action of Light on Dyes,” Proc. R. Soc. Lond. A, vol. 216, no. 1127, pp. 458–476, Feb. 1953, doi: 10.1098/rspa.1953.0034. [50] V. Veselovskii, “Photogalvanic Processes on a Gold Electrode,” Zh Fiz Khim, vol. 20, pp. 269–296, 1946. [51] R. W. Pittman, “The Photochemistry of Selenium. Part IV. Photogalvanic Effects with Grey Selenium,” J. Chem. Soc., p. 3888, 1953, doi: 10.1039/jr9530003888. [52] E. Matijevic, “Photogalvanic Phenomena in Organic Oxidationreduction Systems L Becquerel’s Photovoltaic Effect in the System Thionine-Thionamine,” Arhivza. Kemiju, vol. 21, pp. 1–20, 1949. [53] P. Gangotri and K. M. Gangotri, “Studies of the Micellar Effect on Photogalvanics: Solar Energy Conversion and Storage-EDTA-Safranine O-DSS System,” Int. J. Energy Res., vol. 34, no. 13, pp. 1155–1163, Oct. 2010, doi: 10.1002/er.1636. [54] C. Lal, R. S. Sindal, and K. R. Genwa, “The Role of Ascorbic Acid in a Photogalvanic Solar Cell Containing a Crystal Violet-diocyle Sulphosuccinate System and to Study the Energy Efficiency of the Cell,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 34, no. 19, pp. 1815–1824, Jul. 2012, doi: 10.1080/15567036.2010.492384. [55] P. Koli, R. K. Pareek, Y. Dayma, and M. Jonwal, “Formic Acid Reductant-Sodium Lauryl Sulphate Surfactant Enhanced Photogalvanic Effect of Indigo Carmine Dye Sensitizer for Simultaneous Solar Energy Conversion and Storage,” Energy Reports, vol. 7, pp. 3628–3638, Nov. 2021, doi: 10.1016/j.egyr.2021.06.022. [56] P. Koli and Dheerata, “Study of KOH as Alkali for Enhancing Performance of Photo-Galvanic Cell in Transparent Cylindrical Cell Design,” Heliyon, vol. 10, no. 11, p. e32163, Jun. 2024, doi: 10.1016/j.heliyon.2024.e32163. [57] P. Tanwar, “The Use of Surfactant in Photo Galvanic Cells for Solar Energy Conversion and Storage: A Sodium Lauryl Sulphate-mannitol-methylene Blue System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 37, no. 12, pp. 1318–1322, Jun. 2015, doi: 10.1080/15567036.2011.603022. [58] P. Koli, “Natural sunlight-irradiated Rhodamine B dye sensitised and surfactant-enhanced photogalvanic solar power and storage,” International Journal of Ambient Energy, vol. 42, no. 10, pp. 1193–1199, Jul. 2021, doi: 10.1080/01430750.2019.1594370. [59] P. Koli, Y. Dayma, and R. K. Pareek, “Simultaneous electrochemical solar power generation and storage using metanil yellow-formic acid as a new sensitizer-reductant couple in photogalvanic cells,” RSC Adv., vol. 9, no. 13, pp. 7560–7574, 2019, doi: 10.1039/C8RA10014D. [60] P. Koli, “Sudan-I dye and Fructose chemicals based photogalvanic cells for electrochemical solar energy conversion and storage at low and artificial sun intensity,” Arabian Journal of Chemistry, vol. 14, no. 2, p. 102918, Feb. 2021, doi: 10.1016/j.arabjc.2020.102918. [61] P. Koli, “Enhancing efficiency of Fast Green FCF–Fructose photogalvanic solar cell by using surfactant in natural sunlight,” International Journal of Ambient Energy, vol. 40, no. 8, pp. 868–874, Nov. 2019, doi: 10.1080/01430750.2018.1437565. [62] P. Koli and J. Saren, “Photogalvanics of Copper and Brass Working Electrodes in the NaOH-Allura Red D-Galactose-DDAC Electrolyte for Solar Power Generation,” RSC Adv., vol. 14, no. 21, pp. 14648–14664, 2024, doi: 10.1039/D4RA01091D. [63] R. Kumar, “‘Study of the Variety of Electrodes and Cell Dimensions for the Solar Energy Conversion and Storage Through Photogalvanic Cells,’” Jai Narayan Vyas University, Jodhpur, Rajasthan, Jodhpur, 2022. [Online]. Available: http://hdl.handle.net/10603/420928 [64] P. Koli, R. Kumar, Y. Dayma, Dheerata, and M. Jonwal, “Graphite Counter Electrode Modified Tropaeolin?O Photo?Sensitized Photogalvanic Cells for Solar Power and Storage,” EcoEnergy, vol. 2, no. 2, pp. 278–298, Jun. 2024, doi: 10.1002/ece2.37. [65] E. J. J. Groenen, M. S. De Groot, and R. De Ruiter, “Carbon Electrodes in the Ferrous/Thionine Photogalvanic Cell: A Quantitative Study of Electrode Selectivity,” Electrochimica Acta, vol. 30, no. 9, pp. 1199–1204, Sep. 1985, doi: 10.1016/0013-4686(95)80013-1. [66] Hiroshi. Tsubomura, Yasuhiro. Shimoura, and Shigeaki. Fujiwara, “Chemical Processes and Electric Power in Photogalvanic Cells Containing Reversible or Irreversible Reducing Agents,” J. Phys. Chem., vol. 83, no. 16, pp. 2103–2106, Aug. 1979, doi: 10.1021/j100479a010. [67] N. S. Dixit and R. A. Mackay, “Microemulsions as Photogalvanic Cell Fluids. the Surfactant Thionine-Iron(ii) System,” J. Phys. Chem., vol. 86, no. 23, pp. 4593–4598, Nov. 1982, doi: 10.1021/j100220a027. [68] K. K. Rohatgi-Mukherjee, M. Bagchi, and B. B. Bhowmik, “Temperature Effect on Phenosafranine-EDTA Photogalvanic Cell,” Electrochimica Acta, vol. 28, no. 3, pp. 293–300, Mar. 1983, doi: 10.1016/0013-4686(83)85125-1. [69] W. J. Albery, W. R. Bowen, F. S. Fisher, and A. D. Turner, “Photogalvanic cells,” Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, vol. 107, no. 1, pp. 11–22, Feb. 1980, doi: 10.1016/S0022-0728(79)80003-0. [70] J. C. M. Brokken-Zijp, M. S. De Groot, and P. A. J. M. Hendriks, “The Open-Circuit Voltage of the Ferrous—Thionine Photogalvanic Cell,” Chemical Physics Letters, vol. 81, no. 1, pp. 129–135, Jul. 1981, doi: 10.1016/0009-2614(81)85343-2. [71] K. Shigehara, M. Nishimura, and E. Tsuchida, “Photo-induced Electricity Generated by Thin-layer Photogalvanic Cells Containing Thionine and Iron(II) Salt,” Bulletin of the Chemical Society of Japan, vol. 50, no. 12, pp. 3397–3405, Dec. 1977, doi: 10.1246/bcsj.50.3397. [72] C. Daul, O. Haas, A. Lottaz, A. Von Zelewsky, and H.-R. Zumbrunnen, “Transient Processes in Photogalvanic Cells,” Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, vol. 112, no. 1, pp. 51–61, Sep. 1980, doi: 10.1016/S0022-0728(80)80006-4. [73] S. Madhwani, R. Ameta, J. Vardia, P. B. Punjabi, and V. K. Sharma, “Use of Fluoroscein-EDTA System in Photogalvanic Cell for Solar Energy Conversion,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 29, no. 8, pp. 721–729, Apr. 2007, doi: 10.1080/00908310500280926. [74] S. Pramila and K. M. Gangotri, “Use of Anionic Micelles in Photogalvanic Cells for Solar Energy Conversion and Storage Dioctylsulfosuccinate-mannitol-safranine System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 29, no. 13, pp. 1253–1257, Aug. 2007, doi: 10.1080/00908310600625103. [75] P. Sharma and J. Rathore, “Sunlight Induced Photogalvanics for Conversion and Storage of Solar Energy: Coomassie Brilliant Blue-Isopropyl Alcohol-Sodium Lauryl Sulphate System,” Orient. J. Chem, vol. 39, no. 4, pp. 1059–1063, Aug. 2023, doi: 10.13005/ojc/390432. [76] M. S. De Groot, P. A. J. M. Hendriks, and J. C. M. Brokken-Zijp, “Potential/Current Characteristics of the Ferrous—Thionine Photogalvanic Cell,” Chemical Physics Letters, vol. 97, no. 6, pp. 521–527, Jun. 1983, doi: 10.1016/0009-2614(83)80464-3. [77] C. Daul, O. Haas, A. Von Zelewsky, and H.-R. Zumbrunnen, “Transient Processes in Photogalvanic Cells,” Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, vol. 125, no. 2, pp. 307–313, Aug. 1981, doi: 10.1016/S0022-0728(81)80349-X [78] A. Chouhan and K. R. Genwa, “The Study of the Photogalvanic Effect in a Photogalvanic Cell Containing Acid Fuchsin as a Photosensitizer in a Benzethonium Chloride-EDTA System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 35, no. 7, pp. 685–693, Apr. 2013, doi: 10.1080/15567036.2010.514590. [79] P. V. Kamat, M. D. Karkhanavala, and P. N. Moorthy, “Study of Ferrous-Thionine System Part I-Photogalvanic Effect in Homogeneous Type Cells,” Indian Journal of Chemistry, vol. 18A, pp. 206–209, 1979. [80] P. Koli, Y. Dayma, and R. K. Pareek, “Simultaneous Electrochemical Solar Power Generation and Storage Using Metanil Yellow-Formic Acid as a New Sensitizer-Reductant Couple in Photogalvanic Cells,” RSC Adv., vol. 9, no. 13, pp. 7560–7574, 2019, doi: 10.1039/C8RA10014D. [81] D. Singh, “Solar Energy Conversion and Storage Potential of Photosensitizing Dye in Photogalvanic Cell,” Aayushi International Interdisciplinary Research Journal (AIIRJ), vol. 8, no. 7, pp. 58–61, 2021. [82] S. L. Meena, P. K. Meena, V. Singh, and R. C. Meena, “Use of Acid Red-36 Dye as Photosensitizer in Photogalvanic Cell for Solar Energy Conversion and Storage,” J Adv Sci Res, vol. 12, no. 2, pp. 110–116, 2021, doi: https://doi.org/10.55218/JASR.s2202112215. [83] K. R. Genwa and A. Kumar, “Role of Acridine Orange Sodium Lauryl Sulphate System in Photogalvanic Cell for Solar Energy Conversion,” IJRET, vol. 3, no. 2, p. 174, 2012, doi: 10.1504/IJRET.2012.045625. [84] K. R. Genwa and H. Prasad, “Innovative Study of Photogalvanics in Solar Energy Transformation and Performance Analysis: Alizarin Cyanine Green, EDTA and Sodium Stearate System,” Orient. J. Chem, vol. 39, no. 2, pp. 372–377, Apr. 2023, doi: 10.13005/ojc/390216. [85] K. R. Genwa and H. Prasad, “Innovative Study of Prospective Energy Source Through C DEA+ACG+EDTA System for Photogalvanic Cell,” IJST, vol. 16, no. 41, pp. 3665–3672, Nov. 2023, doi: 10.17485/IJST/v16i41.2383. [86] A. Sonel and S. K. Yadav, “Solar Energy Conversion into Electrical Energy with Alizarine Red S Photosensitizer in Photo Galvanic Cell System,” IJAEM, vol. 5, no. 8, pp. 658–666, 2023, doi: 10.35629/5252-0508658666. [87] P. Koli and J. Saren, “Photogalvanics of the Platinum Working Electrode in the Allura Red D-Galactose-Didecyl Dimethyl Ammonium Chloride-NaOH Electrolyte,” Sustainable Energy res., vol. 11, no. 1, p. 31, Aug. 2024, doi: 10.1186/s40807-024-00123-1. [88] R. Kumar, S. K. Arora, and R. Khandelwal, “Impact of Allura Red-Ac Photosensitizer Azo Dye in Photogalvanic Solar Cell for Solar Power Generation and Storage,” presented at the 2023 INTERNATIONAL CONFERENCE ON CIVIL, ARCHITECTURAL, AND ENVIRONMENTAL ENGINEERING (ICCAEE 2023), Guangzhou, China, 2024, p. 120003. doi: 10.1063/5.0225238. [89] J. Rathore, R. K. Pareek, and K. Singh, “Significance of Aniline Blue in Liquid Phase Dye Sensitized Photo Galvanic Cell for Solar Power Generation and Storage by Using Ascorbic Acid and Sodium Lauryl Sulphate Chemicals,” Res. J. Chem. Environ., vol. 26, no. 3, pp. 45–53, Feb. 2022, doi: 10.25303/2603rjce4553. [90] K. R. Genwa and A. Chouhan, “Role of Heterocyclic Dye (azur a) as a Photosensitizer in Photogalvanic Cell for Solar Energy Conversion and Storage: NaLS–Ascorbic Acid System,” Solar Energy, vol. 80, no. 9, pp. 1213–1219, Sep. 2006, doi: 10.1016/j.solener.2005.06.020. [91] R. K. Gunsaria and J. Hussain, “Role of Reductants, Photosensitizers and Surfactants for Solar Energy Conversion and Storage: EDTA-Azure-A-NaLS System,” Asian J. Chem., vol. 16, no. 1, pp. 385–392, 2004. [92] S. Khamesra, R. Ameta, M. Bala, and S. C. Ameta, “Use of Micelles in Photogalvanic Cell for Solar Energy Conversion and Storage: Azur a-Glucose System,” Int. J. Energy Res., vol. 14, no. 2, pp. 163–167, 1990, doi: 10.1002/er.4440140205. [93] R. K. Gunsaria, K. M. Gangotri, and R. C. Meena, “Use of Surfactant in Photogalvanic Cell for Solar Energy Conversion and Storage: NaLS-Glycerol-Azure A,” Afinidad, vol. 60, pp. 563–567, 2003. [94] A. S. Meena, “Role of Hetrocyclic Dye (Azur B) with Reductant and Surfactant in Photogalvanic Cell for Solar Energy Conversion and Storage,” International Journal of Current Engineering and Technology, vol. 3, no. 5, pp. 1686–1690, 2013. [95] A. Chouhan, Mahaveer, I. Prakash, and K. R. Genwa, “Study of Photogalvanic Effect in Photogalvanic Cell Containing Azur B-NaLS-Ascorbic Acid System,” J. Indian Chem. Soc., vol. 83, pp. 799–802, 2006. [96] R. C. Meena, G. Singh, N. Tyagi, and M. Kumari, “Studies of Surfactants in Photogalvanic Cells – NaLS-EDTA and Azur-B System,” J. Chem. Sci., vol. 116, no. 3, pp. 179–184, 2004. [97] K. M. Gangotri and K. R. Genwa, “Comparative Studies in Anionic, Cationic and Nonionic Surfactants in Photogalvanic Cells from Solar Energy Conversion and Storage Point of View: Nt a -Azur B System,” J. Indian Chem. Soc., vol. 81, pp. 592–594, 2004. [98] K. R. Genwa and A. Chouhan, “Studies of Effect of Heterocyclic Dyes in Photogalvanic Cells for Solar Energy Conversion and Storage: NaLS-Ascorbic Acid System,” J Chem Sci, vol. 116, no. 6, pp. 339–345, Nov. 2004, doi: 10.1007/BF02711435. [99] S. K. Yadav and S. Sharma, “Use of Azur C- Nitrilotriacetic Acid as a New Sensitizer?Reductant System for Enhanced Simultaneous Solar Power Conversion and Storage by Photogalvanic Cell,” INTERNATIONAL JOURNAL OF RESEARCHES IN BIOSCIENCES, AGRICULTURE AND TECHNOLOGY, no. 17, pp. 245–252, 2021. [100] R. K. Gunsaria and J. Hussain, “Use of Surfactant in Photogalvanic Cell for Solar Energy Conversion and Storage: TX-100-Glycerol-Azure-C System,” Asian J. Chem., vol. 16, no. 1, pp. 375–384, 2004. [101] K. R. Genwa and C. P. Sagar, “Improved Energy Efficiency of Photogalvanic Cell with Four Dyes as Photosensitizers in Tween 60- Ascorbic Acid System,” International Journal of Physical Sciences, vol. 8, no. 29, pp. 1515–1525, 2013, doi: 10.5897/IJPS2013.3885. [102] K. R. Genwa and N. C. Khatri, “Comparative Study of Photosensitizing Dyes in Photogalvanic Cells for Solar Energy Conversion and Storage: Brij-35?Diethylenetriamine Pentaacetic Acid (DTPA) System,” Energy Fuels, vol. 23, no. 2, pp. 1024–1031, Feb. 2009, doi: 10.1021/ef800747w. [103] S. K. Yadav, S. Sharma, and A. Sonel, “Electrical Output in Photogalvanic Cell for Conversion of Energy and Storage by Using Bismarck Brown with Different Reductant,” International Journal of Innovative Science and Research Technology, vol. 8, no. 1, pp. 254–260, 2023. [104] N. R. Nenival, “Study on Effect of Reductant in Photosensitizer for Solar Energy Conversion and Storage: Bismark Brown – Oxalic Acid System,” International Journal of ChemTech Research, vol. 4, no. 1, pp. 213–222, 2012. [105] K. R. Genwa and A. Chouhan, “Optimum Efficiency of Photogalvanic Cell for Solar Energy Conversion and Storage Containing Brilliant Black PN-Ammonium Lauryl Sulphate – EDTA System,” Res.J.Recent Sci., vol. 1, pp. 117–121, 2012. [106] K. R. Genwa, Shraddha, S. P. Singh, and K. Singh, “Role of Dyes as Energy Materials in Photogalvanic Conversion of Solar Energy,” J. Indian Chem. Soc., vol. 94, pp. 527–533, 2017. [107] P. Koli, “Comparative Study of the Photogalvanics of Sudan-I, Rhodamine-B, Fast Green Fcf, Brilliant Cresyl Blue, Naphthol Green B, and Chlorophyll ‘A’ Photosensitizers at Natural Sunlight Illumination Intensity,” Environmental Challenges, vol. 14, p. 100872, Jan. 2024, doi: 10.1016/j.envc.2024.100872. [108] K. M. Gangotri, P. P. Solanki, and M. K. Bhimwal, “Use of Anionic Micelle in Photogalvanic Cells for Solar Energy Conversion and Storage: Sodium Lauryl Sulphate-Mannose-Brilliant Cresyl Blue System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 35, no. 23, pp. 2209–2217, Dec. 2013, doi: 10.1080/15567036.2010.487277. [109] K. M. Gangotri and M. K. Bhimwal, “Study on the Performance of Photogalvanic Cells for Solar Energy Conversion and Storage: Brilliant Cresyl Blue—D-Xylose—Sodium Lauryl Sulphate System,” International Journal of Ambient Energy, vol. 31, no. 2, pp. 71–80, Apr. 2010, doi: 10.1080/01430750.2010.9675104. [110] S. S. Nadeem, R. K. Gunsaria, and R. N. Meena, “Nature and Effect of Dye Sensitizer in Solar Energy Conversion and Storage in Photogalvanic Cell: Brilliant Green - Ascorbic Acid - ALES-System,” JCBPS, vol. 3, no. 2, p. 972, 2013. [111] B. Ram, H. Prasad, V. Rajoriya, and K. R. Genwa, “Innovative Study of the Photogalvanics for Solar Energy Conversion and Storage Through Brilliant Yellow + NaLS + Ascorbic Acid System,” IJST, vol. 17, no. 19, pp. 1914–1922, May 2024, doi: 10.17485/IJST/v17i19.572. [112] R. Maan and D. Singh, “Green and Sustainable Development of Photogalvanic Cell Using Bromocresol Green Photosensitizing Dye for Solar Energy Conversion and Storage”,” IJFANS, vol. 11, no. 9, pp. 5190–5202, 2022. [113] P. Koli, Y. Dayma, R. K. Pareek, R. Kumar, and M. Jonwal, “Modified and Simplified Photogalvanic Cells: Solar Energy Harvesting Using Bromo Cresol Green Dye with Different Electrodes and Cell Dimensions,” Journal of Electroanalytical Chemistry, vol. 904, p. 115942, Jan. 2022, doi: 10.1016/j.jelechem.2021.115942. [114] P. Koli, A. Charan, J. Saren, Dheerata, and A. Meena, “Exploratory Insight into the Contribution of Chemical Components of Photo Galvanic Electrolyte Towards Potential, Power and Current of Photo Galvanic Cells,” Results in Chemistry, vol. 6, p. 101124, Dec. 2023, doi: 10.1016/j.rechem.2023.101124. [115] J. Rathore and P. Sharma, “Study on the Performance of Photogalvanic Cell for Solar Power Generation and Storage in Chlorophenol Red - Isopropyl alcohol -Sodium Lauryl Sulphate system,” JETIR, vol. 9, no. 5, pp. h728–h739, 2022. [116] R. K. Gunsaria and R. N. Meena, “Studies of Cationic Micelles Effect on Photogalvanic Cells for Solar Energy Conversion and Storage in Congo Red-D Xylose- Cetyl Pyridinium Chloride System,” International Journal of Basic and Applied Chemical Sciences, vol. 2, no. 1, pp. 77–83, 2012. [117] M. Kumari, R. B. Pachwarya, and R. C. Meena, “Studies of Dye Sensitization for Solar Energy Conversion into Electrical Energy in Congo Red EDTA System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 31, no. 13, pp. 1081–1088, Aug. 2009, doi: 10.1080/15567030801904111. [118] R. K. Bhupesh, S. L. Meena, L. C. Yadav, and R. C. Meena, “Use of Coomassie Brilliant Blue- EDTA System in Photo-Electrochemical Solar Cell for Study of Electrical Characteristics and Energy Efficiency,” RJC, vol. 16, no. 03, pp. 1940–1948, 2023, doi: 10.31788/RJC.2023.1638509. [119] M. K. Bhimwal, K. M. Gangotri, and M. K. Bhimwal, “A Comparison of Conversion Efficiencies of Various Sugars as Reducing Agents for the Photosensitizer Eosin in the Photogalvanic Cell: Conversion Efficiencies of Various Sugars,” Int. J. Energy Res., vol. 37, no. 3, pp. 250–258, Mar. 2013, doi: 10.1002/er.1902. [120] A. Chouhan and K. R. Genwa, “Study of Electrical Parameters and Energy Efficiency in Photogalvanic Cell Containing Erythrosine as a Photosensitizer in Benzethonium Chloride – EDTA System,” Energy Science and Technology, vol. 2, no. 1, pp. 18–24, 2011, doi: ttp://dx.doi.org/10.3968/j.est.1923847920110201.651. [121] P. Koli, “Solar Energy Conversion and Storage: Fast Green FCF-Fructose Photogalvanic Cell,” Applied Energy, vol. 118, pp. 231–237, Apr. 2014, doi: 10.1016/j.apenergy.2013.12.035. [122] K. R. Genwa and S. P. Singh, “Photogalvanic Performance of DSS-Indigo Carmine-EDTA Cell Materials,” Asian J. Chem., vol. 29, no. 6, pp. 1215–1219, Apr. 2017, doi: 10.14233/ajchem.2017.20394. [123] S. S. Nadeem and R. K. Gunsaria, “Role of Reductant and Dye Sensitizer for Solar Energy Conversion and Storage in Photogalvanic Cell: Indigo Carmine - Ascorbic Acid System,” International Journal of Basic and Applied Chemical Sciences, vol. 3, no. 1, pp. 109–116, 2013. [124] R. Kumar, S. K. Arora, A. Sharma, and K. Singh, “Influence of Janus Green- B Photosensitizer Azo Dye in Photogalvanic Cell for Solar Power Generation and Storage,” ijsr, pp. 49–53, Sep. 2023, doi: 10.36106/ijsr/7423972. [125] R. Singh, S. Singh, K. Singh, and K. R. Genwa, “Study of Electrical Parameters and Energy Efficiency in Photogalvanic Cell Containing Lissamine Fast Yellow Dye as a Photosensitizer,” Int J Res Health Allied Sci, vol. 2, no. 4, pp. 17–22, 2016. [126] K. R. Genwa and K. Singh, “Optimum Efficiency of Photogalvanic Cell for Solar Energy Conversion: Lissamine Green B-Ascorbic Acid-NaLS System,” SGRE, vol. 04, no. 03, pp. 306–311, 2013, doi: 10.4236/sgre.2013.43037. [127] J. Bharadwaj and V. Choudhary, “Solar Energy Via Green Chemistry: Micellization Generates Electricity in a Photogalvanic Cell Using Malachite Green-Edta-Surfactant System,” IJCRT, vol. 8, no. 3, pp. 1–10, 2020. [128] A. Sonel and P. Chouhan, “Solar Energy Conversion - Study with Photosensitizer – Reductant System in Photogalvanic Cell,” presented at the 3RD INTERNATIONAL CONFERENCE ON CONDENSED MATTER AND APPLIED PHYSICS (ICC-2019), Bikaner, India, 2020, p. 070010. doi: 10.1063/5.0002206. [129] R. Kumari, S. K. Yadav, and R. K. Gunsaria, “Innovative Study on Solar Energy Conversion and Storage Through Malachite Green + Ascorbic Acid + Sodium Lauryl Sulphate For Photogalvanics,” IJST, vol. 17, no. 38, pp. 3984–3992, Oct. 2024, doi: 10.17485/IJST/v17i38.1786. [130] K. R. Genwa and A. Kumar, “Dye Sensitized Photogalvanic Solar Cells: Studies in a Methyl Green-NaLS System in View of Energy Conversion,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 34, no. 14, pp. 1261–1270, May 2012, doi: 10.1080/15567031003663141. [131] K. M. Gangotri and M. K. Bhimwal, “A Study of the Performance of the Photogalvanic Cells for Solar Energy Conversion and Storage: Methyl Orange–D-Xylose–NaLS System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 33, no. 22, pp. 2058–2066, Sep. 2011, doi: 10.1080/15567030903503209. [132] K. M. Gangotri and R. C. Meena, “Use of Reductant and Photosensitizer in Photogalvanic Cells for Solar Energy Conversion and Storage: Oxalic Acid–Methylene Blue System,” Journal of Photochemistry and Photobiology A: Chemistry, vol. 141, no. 2–3, pp. 175–177, Jul. 2001, doi: 10.1016/S1010-6030(01)00416-6. [133] K. K. Bhati and K. M. Gangotri, “Photogalvanic Conversion of Solar Energy into Electrical Energy by Using Nals–Xylose–Methylene Blue System,” International Journal of Electrical Power & Energy Systems, vol. 33, no. 2, pp. 155–158, Feb. 2011, doi: 10.1016/j.ijepes.2010.08.001. [134] K. M. Gangotri and P. P. Solanki, “Use of Sodium Lauryl Sulphate as a Surfactant in a Photogalvanic Cell for Solar Energy Conversion and Storage: A Sodium Lauryl Sulphate-Methylene Blue-Mannose System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 35, no. 15, pp. 1467–1475, Aug. 2013, doi: 10.1080/15567036.2010.523758 [135] A. S. Meena, “Role of Heterocyclic Dye (methylene Blue) with Reductant and Micelles in Photogalvanic Cell for Solar Energy Conversion and Storage,” IJRET, vol. 02, no. 12, pp. 230–237, Dec. 2013, doi: 10.15623/ijret.2013.0212041. [136] S. C. Ameta, S. Khamesra, A. K. Chittora, and K. M. Gangotri, “Use of Sodium Lauryl Sulphate in a Photogalvanic Cell for Solar Energy Conversion and Storage: Methylene Blue-EDTA System,” Int. J. Energy Res., vol. 13, no. 6, pp. 643–647, 1989, doi: 10.1002/er.4440130604. [137] K. R. Genwa, Mahaveer, and I. Prakash, “Photogalvanic Effect: Comparative Studies in Three Dyes Rhodamine B, Methylene Blue and Safranine,” J. Indian Chem. Soc., vol. 83, pp. 165–167, 2006. [138] P. Koli, “Solar Energy Conversion and Storage Using Naphthol Green B Dye Photosensitizer in Photogalvanic Cells,” Appl. Sol. Energy, vol. 50, no. 2, pp. 67–73, Apr. 2014, doi: 10.3103/S0003701X14020108. [139] M. Lal and K. M. Gangotri, “The Optimum Conversion Efficiency in Nile Blue Arabinose System by Photogalvanic Cell,” Advances in Energy Research, vol. 3, no. 3, pp. 143–155, Sep. 2015, doi: 10.12989/ERI.2015.3.3.143. [140] K. R. Genwa and A. Kumar, “Studies in Nile Blue-NaLS System for Solar Energy Conversion and Management: Phtogalvanic Performance and Conversion Efficiency,” J. Indian Counc. Chem., vol. 26, pp. 181–186, 2009. [141] S. R. Saini, S. L. Meena, and R. C. Meena, “Studies of Surfactant in Photogalvanic Cell for Solar Energy Conversion and Storage,” ACES, vol. 07, no. 02, pp. 125–136, 2017, doi: 10.4236/aces.2017.72010. [142] S. Meena, S. R. Saini, and R. C. Meena, “Role of Photosensitizer (Orange-G) in Photogalvanic Cell for Generation of Solar Energy,” International Journal of Engineering Sciences and Research Technology, vol. 4, no. 2, pp. 135–141, 2015. [143] P. K. Meena, “Photochemical Study of Photosensitizer by Photogalvanic Cell for Solar Energy Conversion and Storage,” IJRASET, vol. 6, no. 3, pp. 1472–1478, Mar. 2018, doi: 10.22214/ijraset.2018.3228. [144] K. R. Genwa and Shraddha, “Photocurrent Response of Phloxin B-Cetyltrimethylammonium Bromide Photogalvanic Cell Device,” Materials Science-Poland, vol. 33, no. 3, pp. 612–619, Sep. 2015, doi: 10.1515/msp-2015-0090. [145] S. Meena, S. R. Saini, and R. C. Meena, “Study of Photosensitizer (Phloxine B) for Conversion of Solar Energy in to Electrical Energy,” International Journal of Engineering Research and Technology, vol. 4, no. 2, pp. 797–802, 2015. [146] M. Chandra and R. C. Meena, “Role of Ponceau-S-Mannitol System for Generation of Electrical Energy in Photogalvanic Cell,” J. Nepal Chem. Soc., vol. 26, pp. 46–52, Jan. 1970, doi: 10.3126/jncs.v26i0.3631. [147] S. L. Meena, R. K. Bhupesh, and L. C. Yadav, “Study of Photoactive Materials Used in Photo Electrochemical Cell for Solar Energy Conversion and Storage,” J. Appl. Sci. Educ., vol. 3, no. 1, pp. 1–13, 2023, doi: 10.54060/jase.v3i1.40. [148] M. Jonwal, P. Koli, Y. Dayma, and R. K. Pareek, “High Energy Throughput Using Photogalvanic Solar Techniques and Environmentally Benign Chemical System,” Journal of Photochemistry and Photobiology, vol. 22, p. 100244, Aug. 2024, doi: 10.1016/j.jpap.2024.100244. [149] P. Koli, U. Sharma, and K. M. Gangotri, “Solar Energy Conversion and Storage: Rhodamine B - Fructose Photogalvanic Cell,” Renewable Energy, vol. 37, no. 1, pp. 250–258, Jan. 2012, doi: 10.1016/j.renene.2011.06.022. [150] K. R. Genwa and A. Kumar, “Role of Rhodamine B in Photovoltage Generation Using Anionic Surfactant in Liquid Phase Photoelectrochemical Cell for Solar Energy Conversion and Storage,” J. Indian Chem. Soc., vol. 87, no. 8, pp. 933–939, 2010. [151] A. S. Meena, P. L. Meena, M. Chandra, and R. C. Meena, “Role of an Anionic Surfactant and Reductant in Photogalvanic Cell for Solar Energy Conversion and Storage,” INTERNATIONAL JOURNAL of RENEWABLE ENERGY RESEARCH, vol. 3, no. 2, 2013, [Online]. Available: https://dergipark.org.tr/en/download/article-file/14833 [152] K. M. Gangotri and M. K. Bhimwal, “Study the Performance of Photogalvanic Cells for Solar Energy Conversion and Storage: Rose Bengal–D-Xylose–NaLS System,” Solar Energy, vol. 84, no. 7, pp. 1294–1300, Jul. 2010, doi: 10.1016/j.solener.2010.04.006. [153] M. Chandra and R. Meena, “Role of Reductant for Generation of Solar Energy in Photogalvanic Cell,” BIBECHANA, vol. 7, pp. 6–13, Jan. 1970, doi: 10.3126/bibechana.v7i0.4036. [154] P. Tanwar, “Use of Micelles in Photogalvanic Cells for Solar Energy Conversion and Storage: A Sodium Octanoate-Mannitol-Safranine System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 35, no. 6, pp. 510–515, Mar. 2013, doi: 10.1080/15567036.2010.498258. [155] K. M. Gangotri and S. Pramila, “Use of Anionic Micelles in Photogalvanic Cells for Solar Energy Conversion and Storage NaLS-Mannitol-Safranine System,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 28, no. 2, pp. 149–156, Jan. 2006, doi: 10.1080/009083190889807. [156] P. P. Solanki and K. M. Gangotri, “Studies of the Anionic Micelles Effect on Photogalvanic Cells for Solar Energy Conversion and Storage in Sodium Lauryl Sulphate-Safranine-D-Xylose System,” presented at the World Renewable Energy Congress – Sweden, 8–13 May, 2011, Linköping, Sweden, Nov. 2011, pp. 2807–2814. doi: 10.3384/ecp110572807. [157] K. M. Gangotri and O. P. Regar, “Use of Azine Dye as a Photosensitizer in Solar Cells: Different Reductants—Safranine Systems,” Int. J. Energy Res., vol. 21, no. 14, pp. 1345–1350, Nov. 1997, doi: 10.1002/(SICI)1099-114X(199711)21:14<1345::AID-ER356>3.0.CO;2-H. [158] P. P. Solanki and K. M. Gangotri, “Photogalvanic Cell for Conversion of Solar Energy into Electricity: Safranine–Arabinose–Sodium Lauryl Sulphate System,” Solar Energy, vol. 85, no. 11, pp. 3028–3035, Nov. 2011, doi: 10.1016/j.solener.2011.08.043. [159] Amulyacharya Malviya, C. Mall, and P. P. Solanki, “UV-Visible Spectrophotometric and Photochemical Characterization of Safranine–Fructose–Sodium Lauryl Sulphate System in Photogalvanic Cell,” Appl. Sol. Energy, vol. 56, no. 5, pp. 371–382, Sep. 2020, doi: 10.3103/S0003701X20050102. [160] P. Gangotri and P. Koli, “Study of the Enhancement on Photogalvanics: Solar Energy Conversion and Storage in EDTA–Safranine O–NaLS System,” Sustainable Energy Fuels, vol. 1, no. 4, pp. 882–890, 2017, doi: 10.1039/C7SE00083A. [161] P. Gangotri and K. M. Gangotri, “Studies of the Micellar Effect on Photogalvanics: Solar Energy Conversion and Storage in EDTA?Safranine O?Tween-80 System,” Energy Fuels, vol. 23, no. 5, pp. 2767–2772, May 2009, doi: 10.1021/ef9000709. [162] P. Gangotri and K. M. Gangotri, “Studies of the Micellar Effect on Photogalvanics: Solar Energy Conversion and Storage – EDTA- Safranine O – CTAB System,” The Arabian Journal for Science and Engineering, vol. 35, no. 1A, pp. 19–28, 2009. [163] P. Koli, “Sudan-I Dye and Fructose Chemicals Based Photogalvanic Cells for Electrochemical Solar Energy Conversion and Storage at Low and Artificial Sun Intensity,” Arabian Journal of Chemistry, vol. 14, no. 2, p. 102918, Feb. 2021, doi: 10.1016/j.arabjc.2020.102918. [164] J. Rathore and R. K. Arya, “A Comparative Study of Photo-sensitizers in Reductant Surfactant System in Photogalvanic cell for Photo induced Power Generation and Storage,” Orient. J. Chem, vol. 40, no. 2, pp. 407–412, Apr. 2024, doi: 10.13005/ojc/400211. [165] P. Koli et al., “Naoh Alkali?Sunset Yellow Fcf Dye Photosensitizer?Ascorbic Acid Reductant?Cetrimonium Bromide Surfactant Based Photogalvanic Cells: Solar Power, Storage and Spectral Study,” Env Prog and Sustain Energy, vol. 43, no. 2, p. e14286, Mar. 2024, doi: 10.1002/ep.14286. [166] J. Rathore, A. R. Kumar, P. Sharma, and M. Lal, “Study of Electrical Output in Photogalvanic Cell for Solar Energy Conversion and Storage: Lauryl Glucoside-Tartrazine-D-Fructose System,” IJST, vol. 15, no. 23, pp. 1159–1165, Jun. 2022, doi: 10.17485/IJST/v15i23.493. [167] P. Koli, A. Meena, and Dheerata, “Photo-Stability of the Titan Yellow Dye Sensitized and Ethylenediaminetetraacetate Photoreduced Photogalvanic System,” Results in Engineering, vol. 18, p. 101209, Jun. 2023, doi: 10.1016/j.rineng.2023.101209. [168] S. C. Ameta, S. Khamesra, S. Lodha, and R. Ameta, “Use of the Thionine-EDTA System in Photogalvanic Cells for Solar Energy Conversion,” Journal of Photochemistry and Photobiology A: Chemistry, vol. 48, no. 1, pp. 81–86, Jul. 1989, doi: 10.1016/1010-6030(89)87092-3. [169] P. P. Solanki and K. M. Gangotri, “The Role of Surfactants in Photogalvanics: Solar Energy Conversion and Storage in the Sodium Lauryl Sulphate–Thymol Blue–Mannose System,” Arab J Sci Eng, vol. 37, no. 1, pp. 91–100, Jan. 2012, doi: 10.1007/s13369-011-0152-7. [170] R. C. Meena, V. Kumar, and G. Singh, “Use of Dyes in Photogalvanic Cells for Solar Energy Conversion and Storage: Thymole Blue and Ascorbic Acid System,” Int. J. Chem. Sci., vol. 6, no. 3, pp. 1438–1448, 2008. [171] K. M. Gangotri, R. C. Meena, and R. Meena, “Use of Micelles in Photogalvanic Cells for Solar Energy Conversion and Storage: Cetyl Trimethyl Ammonium Bromide-Glucose-Toluidine Blue System,” Journal of Photochemistry and Photobiology A: Chemistry, vol. 123, no. 1–3, pp. 93–97, May 1999, doi: 10.1016/S1010-6030(99)00034-9. [172] K. M. Gangotri and M. K. Bhimwal, “Study the Performance of Photogalvanic Cells for Solar Energy Conversion and Storage: Toluidine Blue-D-Xylose-NaLS System,” Int. J. Energy Res., vol. 35, no. 6, pp. 545–552, May 2011, doi: 10.1002/er.1719. [173] K. M. Gangotri and J. Meena, “Role of Surfactants in Photogalvanic Cells for Solar Energy Conversion and Storage,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 28, no. 8, pp. 771–777, Jul. 2006, doi: 10.1080/009083190929749. [174] S. C. Ameta, S. Khamesra, T. D. Dubey, and K. M. Gangotri, “Use of Toluidine Blue-Maleic Hydrazide System in Photogalvanic Cell for Solar Energy Conversion,” Asian Journal of Chemistry, vol. 2, no. 1, pp. 19–25, 1990. [175] K. M. Gangotri, O. P. Regar, C. Lal, P. Kalla, K. R. Genwa, and R. Meena, “Use of Tergitol-7 in Photogalvanic Cell for Solar Energy Conversion and Storage: Toluidine Blue-Glucose System,” Int. J. Energy Res., vol. 20, no. 7, pp. 581–585, Jul. 1996, doi: 10.1002/(SICI)1099-114X(199607)20:7<581::AID-ER168>3.0.CO;2-4. [176] K. Meena and S. R. Saini, “Study Of Dye Sensitization For Solar Energy Conversion Into Electrical Energy In Tropaeolin O-EDTA System,” IJESRT, vol. 5, no. 12, pp. 432–439, 2016, doi: 10.5281/ZENODO.203821. [177] K. M. Gangotri and M. Lal, “Use of Trypan blue-Arabinose System in Photogalvanic Cell for Solar Energy Conversion and Storage,” IJESRT, vol. 3, no. 6, pp. 447–454, 2014. [178] K. R. Genwa and C. P. Sagar, “A Xylidine Ponceau Dye Based Photogalvanic Cell: Energy Conversion for Sustainable Development,” Eur. Chem. Bull., vol. 3, no. 1, pp. 76–80, 2014, doi: 10.17628/ECB.2014.3.76. [179] K. M. Gangotri and C. Lal, “Studies in Photogalvanic Effect and Mixed Dyes System: EDTA-Methylene Blue + Toluidine Blue System,” Int. J. Energy Res., vol. 24, no. 4, pp. 365–371, Mar. 2000, doi: 10.1002/(SICI)1099-114X(20000325)24:4<365::AID-ER593>3.0.CO;2-I. [180] C. Lal, “Use of Mixed Dyes in a Photogalvanic Cell for Solar Energy Conversion and Storage: EDTA–Thionine–Azur-B System,” Journal of Power Sources, vol. 164, no. 2, pp. 926–930, Feb. 2007, doi: 10.1016/j.jpowsour.2006.11.020. [181] K. M. Gangotri, Chhagan Lal, “Use of Mixed Dyes in Photogalvanic Cells for Solar Energy Conversion and Storage: EDTA-Methylene Blue and Azur-B System,” Energy Sources, vol. 23, no. 3, pp. 267–273, Apr. 2001, doi: 10.1080/00908310151133988. [182] K. M. Gangotri and C. Lal, “Use of Mixed Dyes in Photogalvanic Cells for Solar Energy Conversion and Storage: EDTA-Methylene Blue and Thionine System,” Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy, vol. 219, no. 5, pp. 315–320, Aug. 2005, doi: 10.1243/095765005X28599. [183] V. Soni, C. Mahavar, V. Rajoriya, and K. R. Genwa, “Comparative Assessment of Single and Mixed Photosensitizers using Erythrosin B and Tartrazine Yellow Dye System in Photogalvanic and Dye Sensitized Solar Cells,” Orient. J. Chem, vol. 38, no. 5, pp. 1094–1103, Oct. 2022, doi: 10.13005/ojc/380502. [184] C. Lal and K. M. Gangotri, “Energy Conversion and Storage Potential of Photogalvanic Cell Based on Mixed Dyes System: Ethylene Diaminetetraacetic Acid—Toluidine Blue—Thionine,” Env Prog and Sustain Energy, vol. 30, no. 4, pp. 754–761, Dec. 2011, doi: 10.1002/ep.10524. [185] C. Lal, “Photogalvanic Cell: An Efficient Device for Photochemical Conversion and Storage of Solar Energy,” Environmental Science & Technology, vol. 2, pp. 428–433, 2016. [186] P. Koli, “Photogalvanic Effect of Natural Photosensitizer (crude Spinach Extract) in Artificial Light for Simultaneous Solar Power Generation and Storage,” Env Prog and Sustain Energy, vol. 37, no. 5, pp. 1800–1807, Sep. 2018, doi: 10.1002/ep.12829. [187] S. Mishra and S. K. Yadav, “Study of Photogalvanic Effect by using Marigold Flower as Natural Photosensitizer, Xylose as Reductant and Tween 80 as Surfactant for Solar Radiation Conversion and Storage,” IJST, vol. 17, no. 22, pp. 2263–2270, Jun. 2024, doi: 10.17485/IJST/v17i22.571. [188] S. Mishra and S. K. Yadav, “Study of Photogalvanic Effect by Using of Marigold Flower as Natural Photosensitizer, Xylose as Reductant and Brij -35 as Surfactant for Solar Radiation Conversion and Storage,” Orient. J. Chem, vol. 40, no. 4, pp. 1091–1096, Aug. 2024, doi: 10.13005/ojc/400421. [189] S. Yadav, “Comparative Study of Photogalvanic Effect by Using of Rose Flower Extract as Photosensitizer with Mannitol and NTA as Reductant for Solar Energy Conversion and Storage.,” IJAR, vol. 5, no. 6, pp. 2064–2067, Jun. 2017, doi: 10.21474/IJAR01/4643. [190] Koushalya, G. Singh, S. K. Yadav, and R. D. Yadav, “Use of Natural Rose Flower Extract as Photosensitizer for Solar Energy Conversion and Storage: Rose Extract–Ascorbic Acid Nals System,” Int. J. Chem. Sci., vol. 7, no. 4, pp. 2368–2376, 2009. [191] R. Kumar Lakhera and S. K. Yadav, “Use of Natural Dye Curcumin – Arabinose as Photosensitizerreductant System for Simultaneous Solar Energy Conversion and Storage,” PIJR, vol. 13, no. 3, pp. 59–62, Mar. 2024, doi: 10.36106/paripex/8702379. [192] J. Rathore, R. K. Pareek, and K. Singh, “Natural Dye Curcumin Based Photogalvanic Solar Cell Conversion and Storage of Solar Energy into Electrical Energy for Sustainable Development,” JETIR, vol. 9, no. 5, pp. a735–a743, 2022. [193] R. K. Lakhera, S. K. Yadav, and V. Pal, “Study of Photogalvanic Effect in Photogalvanic Cell Composed with Natural Dye Curcumin Fructose-Brij-35 System for Solar Power Generation and Storage,” IJCRT, vol. 12, no. 4, pp. e693–e707, 2024 [194] P. Koli, R. K. Pareek, Y. Dayma, and R. Kumar, “Beetroot’s Crude Aqueous Extract Photosensitizer-Formic Acid-Sodium Lauryl Sulphate Photogalvanic Electrolyte: Solar Power and Storage,” Bioresource Technology Reports, vol. 18, p. 101083, Jun. 2022, doi: 10.1016/j.biteb.2022.101083. [195] S. L. Meena, “Role of Green Photo-Sensitizer in Photo- Galvanic Cell for Generation of Solar Energy,” IJRASET, vol. 5, no. 8, pp. 548–554, Aug. 2017, doi: 10.22214/ijraset.2017.8077. [196] P. K. Meena, V. Singh, R. Sharma, and R. B. Pachwarya, “Simultaneous Conversion and Storage of Solar Power in Photogalvanic Solar Cell by Using Pomegranate Juice as Natural Photosensitizer in Alkali Medium,” IJSR, vol. 12, no. 7, pp. 340–344, Jul. 2023, doi: 10.21275/SR23704134313.
Copyright
Copyright © 2025 Prabha Sharma, Dr. Jagrati Meena. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66793
Publish Date : 2025-02-01
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

