Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Advances in Optical Sensors for Magnesium Ion Detection
Authors: Irfana Nahid, Ajit Kumar
DOI Link: https://doi.org/10.22214/ijraset.2025.66807
Certificate: View Certificate
Abstract
Magnesium ions (Mg²?) play a crucial role in various biological, environmental, and industrial processes, making their accurate detection essential for medical diagnostics, water quality monitoring, and agricultural applications. Optical sensors have emerged as a powerful tool for Mg²? detection due to their high sensitivity, selectivity, and real-time monitoring capabilities. This review highlights recent advances in optical sensing technologies for Mg²? detection, including fluorescence, colorimetric, and plasmonic sensors. Key developments in sensor design, novel recognition elements, and nanomaterial-based enhancements are discussed. Additionally, challenges such as interference from other cations, detection limits, and practical applicability are addressed. Future directions focus on improving selectivity, miniaturization, and integration with wearable and point-of-care diagnostic platforms. These advancements pave the way for more efficient and reliable Mg²? sensing technologies across diverse fields.
Introduction
I. INTRODUCTION
Magnesium ions (Mg²?) are essential for various physiological functions and play a critical role in both cellular processes and the functioning of the body as a whole [1]. Magnesium ions are vital for processes like cell proliferation, apoptosis, enzymatic reactions, and DNA stabilization [2]. Mg²? mediates ~300 enzymatic reactions and is crucial for cellular metabolism [3]. Intracellular Mg²? levels are tightly regulated (0.5–0.7 mM) via transport, buffering, and organelle compartmentalization [4].
Moreover, Magnesium ions (Mg²?) are generally considered to have a relatively low environmental impact compared to other ions and metals. However, like any substance, the extraction, production, and disposal of magnesium-based compounds can have ecological consequences [5]. The environmental impact of magnesium ions is influenced by several factors, including their use in various industries, the mining of magnesium ores, and their potential for contamination in natural ecosystems.
Magnesium is extensively used in industrial processes such as cathodic galvanic protection and dry batteries [6]. Industrial waste discharge can contribute to magnesium contamination in the environment. Low magnesium levels in plants can impair their growth, reducing agricultural productivity and quality [7]. High environmental concentrations of Mg²? can become toxic to both plants and animals [8]. Therefore, the development of efficient analytical procedures for the selective detection of Mg2+ ions is an important analytical task. Numerous methods have been developed for measuring Mg²? in both fixed and live samples. Each method has its own advantages and limitations. These methods vary in their suitability depending on the specific research or application needs. These techniques can be used with varying levels of confidence to address specific scientific or practical challenges. Due to the limitations of traditional methods [9], it inspires the researchers for creation of new analytical methods to help further our understanding of the distribution, uptake, and trafficking of Mg2+ in living systems. Hence, fluorescent imaging technologies were developed.
II. FLUORESCENCE SIGNALLING MECHANISMS
-
- Intramolecular Charge Transfer (ICT): Movement of charge within the molecule upon excitation. Fluorescent probes based on the ICT mechanism [10] are characterized by the conjugation of an electron-donating unit with an electron-accepting unit within a single molecule. This arrangement creates a "push–pull" π-electron system in the excited state, making ICT-based probes highly effective for cation sensing.
- C=N Isomerization: Structural changes involving double bonds within the probe. A well-known example is Schiff base-based fluorescent sensors where the fluorescence intensity changes when the molecule isomerizes upon binding to a metal ion or analyte [11].
- Photoinduced Electron Transfer (PET): Excited electrons transfer to a site with lower energy, modulating fluorescence. Photoinduced electron transfer (PET) (Figure 2) is a common mechanism responsible for fluorescence quenching, occurring when the PET process is followed by a non-luminescent return to the ground state [12].
- Appropriate Cavities: Structural design that ensures Mg²? binding [13]. Crown ether has been a popular receptor for alkaline or alkaline earth metal ions owing to various sizes of cavities available as well as the effective chelation ability of the oxygen lone pairs for these “hard’’ metal ions.
- Excited-State Intra-/Intermolecular Proton Transfer (ESIPT): Proton movement between molecular states upon excitation. Excited-state intramolecular proton transfer (ESIPT) has emerged as a valuable mechanism [14] for designing fluorescent
III. OPTICAL PROBES FOR MAGNESIUM ION DETECTION
A number of organic molecular probes have been reported for Mg2+ ion monitoring in the literature. However, the rapid industrialization which produces new ionic and metallic pollutants in the ecosystem may affect both plant and human life. Thus, new fluorescent probe having high sensitivity and selectivity towards a particular ion is desired. Some of the reported fluorescent probe for magnesium ion in literature in recent past are summarized below;
A novel fluorescent probe [15], P1 (Figure 1), developed by Ray and coworkers, connects two coumarin derivatives via an imine linkage to create a metal-binding site within a fluorescence signaling system. This molecule exhibits favorable photophysical properties, such as a large Stokes shift, visible excitation/emission wavelengths, and minimal interference from other metal ions.

Figure 1: Structure of P1
P2, designed by Narinder Singh and colleagues [16], is tailored for ratiometric recognition of Mg²? in semi-aqueous solutions at pH 7.0 (Figure 2). It features a Schiff base ligand and undergoes Excited State Intramolecular Proton Transfer (ESIPT), providing dual-channel emission for Mg²? determination. The fluorophore predominantly exists in its enol form, stabilized by intramolecular six-membered ring hydrogen bonding. Upon excitation at 275 nm, the enol form transitions to the excited keto tautomer, emitting fluorescence at 355 nm with a large Stokes shift. This keto form reverts to the enol form via reverse proton transfer upon returning to the ground state. P2 demonstrates excellent selectivity for Mg²?, with minimal interference from Ca²?, and is effective in measuring Mg²? concentrations between 2.0 and 30.0 mM.
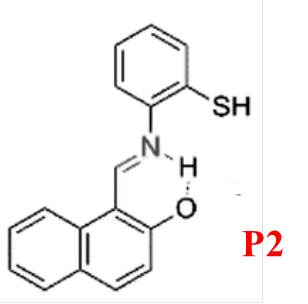
Figure 2: Structure of P2
To address the limited application of Mg²? fluorescent probes in both animal cells and plant tissues, Zhou et al. developed P3 [17]. This probe (Figure 3) exhibits high sensitivity and selectivity for Mg²?, with a fluorescence “turn-on” response in ethanol and negligible interference from Ca²?. P3 provides rapid detection (15–20 seconds), notable photostability, low toxicity, and good cellular permeability. It successfully detects Mg²? in HeLa cells and plant tissues, enabling the tracking of Mg²? transport and fluorescence-based responses to varying Mg²? concentrations.
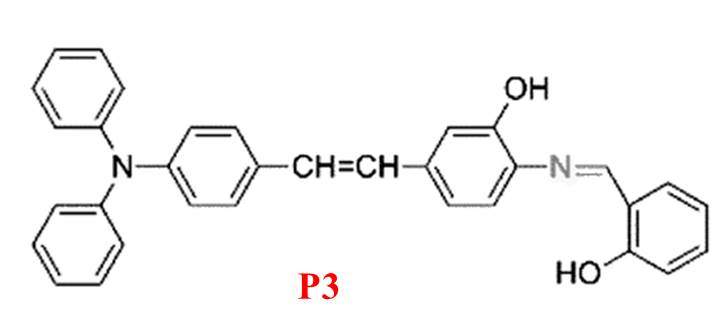
Figure 3: Structure of P3
P4, an aggregation-induced emission (AIE)-active probe (Figure 4) [18], is effective for detecting Mg²? in acetonitrile with no interference from other ions. Upon complexation with Mg²?, the solution changes from colorless to yellow under ultraviolet light, observable with the naked eye. However, its biological application is limited due to suboptimal characteristics.
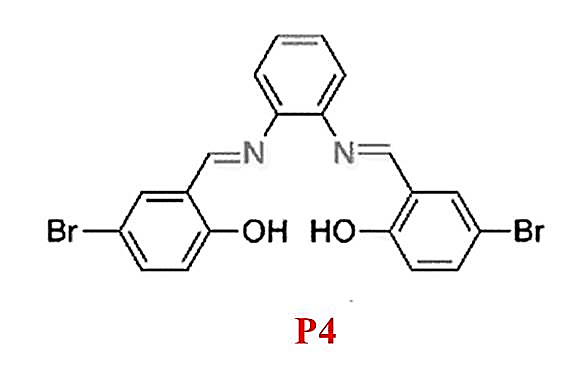
Figure 4: Structure of P4
P5, as reported by Liu and colleagues [19], shows solvent-dependent "turn-on" fluorescence recognition of Mg²? through a combination of C=N isomerization and ESIPT inhibition (Figure 5). Notably, it displays a 148-fold fluorescence enhancement at 458 nm upon binding 20 mM Mg²? in alcohol solvents, accompanied by a color change from colorless to blue. However, the probe's dependence on alcohol solvents restricts its applicability in physiological environments, making it unsuitable for colorimetric or ratiometric sensing in living cells.
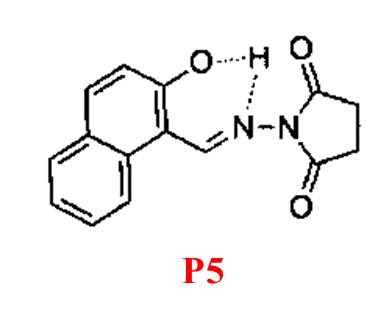
Figure 5: Structure of P5
In 2017, Zhang et al. introduced P6 [20], an “off-on” fluorescent probe based on a 1,8-naphthalimide derivative for detecting Mg²? in ethanol (Figure 6). The o-hydroxyl Schiff base within P6 not only connects the chromophore to the receptor group but also facilitates fluorescence recognition. P6 forms a 1:2 complex with Mg²?, resulting in fluorescence enhancement at 523 nm and a visible color change from nearly colorless to bright yellow-green. It is nearly non-toxic and capable of monitoring intracellular Mg²? levels effectively.
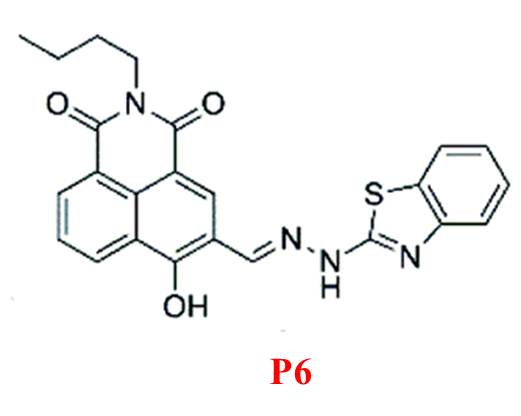
Figure 6: Structure of P6
Li et al. developed P7, a fluorescein-derived Schiff base probe [21] linked to a chromone moiety (Figure 7). This probe selectively binds Mg²?, with fluorescence emission intensity at 504 nm increasing approximately 50-fold upon complexation. The enhanced fluorescence is attributed to the fluorescein ring-opening mechanism triggered by Mg²? binding. P7 responds rapidly (within 3 minutes) and is suitable for real-time Mg²? detection.
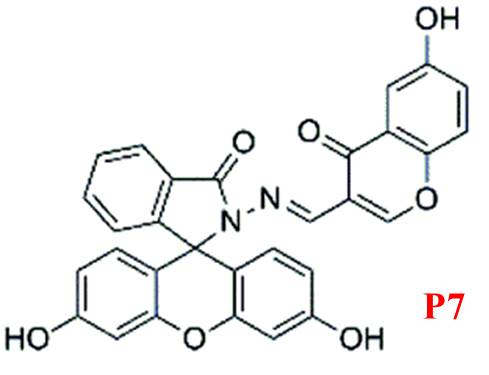
Figure 7: Structure of P7
Finally, P8, developed by Maity and colleagues [22], serves as a dual-analyte probe for quantifying Mg²? and Zn²? (Figure 8). It uses a coumarin derivative conjugated via a C=N bond, with binding sites potentially including the carbonyl oxygen, imine nitrogen, and phenolic oxygen. However, its lack of selectivity for Mg²? limits its broader applicability.
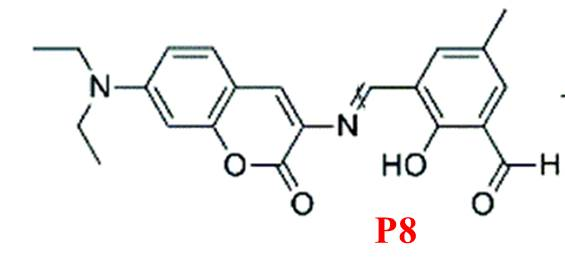
Figure 8: Structure of P8
Conclusion
Fluorescent probes have proven to be powerful tools for Mg²? detection due to their high sensitivity, selectivity, and real-time response. While many probes exhibit promising properties, challenges such as solvent dependency, biocompatibility, and selectivity remain. Future research should focus on developing probes with enhanced stability, broader applicability in biological systems, and improved environmental compatibility. Most probes exhibit high sensitivity, selectivity, and photophysical performance. Limitations such as solvent dependence or lack of selectivity restrict some probes\' applications. Advances like rapid response, biocompatibility, and intracellular utility make specific probes suitable for real-world applications in biological systems.
References
[1] (a) H. Rubin, Magnesium: The missing element in molecular views of cell proliferation control, BioEssays, 27, 2005, 311–320. (b) A. M. Alexandra, A ribosomal strategy for magnesium deficiency, Sci. Signaling, 9, 2016, ec269. [2] M. H. Pontes, J. Yeom and E. A. Groisman, Reducing Ribosome Biosynthesis Promotes Translation during Low Mg2+ Stress, Mol. Cell, 64, 2016, 480–492. [3] B. O\'Rourke, P. H. Backx and E. Marban, Phosphorylation-independent modulation of L-type calcium channels by magnesium-nucleotide complexes, Science, 257, 1992, 245–248. [4] A. Romani, Regulation of magnesium homeostasis and transport in mammalian cells, Arch. Biochem. Biophys., , 458, 2007, 90–102. [5] B.J.Y. Yam, D.K. Le, N. H. Do , P.T.T. Nguyen, Q.B. Thai, N.P.-Thien, H. M. Duong, Recycling of magnesium waste into magnesium hydroxide aerogels, Journal of Environmental Chemical Engineering, Journal of Environmental Chemical Engineering, 8, 2020, 104101. [6] S. S. Pathak, S. K. Mendon, M. D. Blanton and J.W. Rawlins, Magnesium-Based Sacrificial Anode Cathodic Protection Coatings (Mg-Rich Primers) for Aluminum Alloys Metals, 2, 2012, 353-376. [7] L. Chen, M. Liu, Y. Cai, L. Wu, Q. Zhang, Magnesium deficiency differentiated effects on the roots growth and shoot metabolism by regulating the distribution of photosynthetic products in tea plants, Scientia Horticulturae, 338, 2024, 113748. [8] U. Okereafor, Mamookho M. Lukhanyo Mekuto, N. Uche-Okereafor, T. Sebola, V. Mavumengwana, Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health, Int J Environ Res Public Health, 17, 2020, 2204 [9] (a) A. Walsh, Spectrochim. Acta, 7, 1955, 108–117. (b) C. Feillet-Coudray, A. Trzeciakiewicz, C. Coudray, M. Rambeau, A. Chanson, Y. Rayssiguier, A. Opolski, F. I. Wolf and A. Mazur, Eur. J. Nutr., 45, 2006, 171–177. [10] B. Valeur and I. Leray, Molecular fluorescence: Some aspects of the photophysical behaviour and applications, Coord. Chem. Rev., 205, 2000, 3–40. [11] A. Tamrakar, M. Ahmad Wani, G. Mishra, A. Srivastava,. R. Pandey and M. D. Pandey, Advancements in the development of fluorescent chemosensors based on C=double bond isomerization/modulation mechanistic approaches, Anal. Methods, 16, 2024, 2198-2228 [12] G. G. Guilbault, Practical Fluorescence, 2nd edn, Marcel Dekker Inc, New York, 1990, ISBN 0824783506. [13] H. Hama, T. Morozumi and H. Nakamur, Novel Mg2+-responsive fluorescent chemosensor based on benzo-15-crown-5 possessing 1-naphthaleneacetamide moiety, Tetrahedron Lett., 48, 2007,1859–1861. [14] Y. M. Yang, Q. Zhao, W. Feng and F. Y. Li, Luminescent Chemodosimeters for Bioimaging, Chem. Rev., 113, 2013, 192–270. [15] D. Ray and P. K. Bharadwaj, A Coumarin-Derived Fluorescence Probe Selective for Magnesium, Inorg. Chem., 47, 2008, 2252–2254. [16] N. Singh, N. Kaur, R. C. Mulrooney and J. F. Callan, A ratiometric fluorescent probe for magnesium employing excited state intramolecular proton transfer. Tetrahedron Lett., 49, 2008, 6690–6692. [17] T. T. Yu, P. Sun, Y. J. Hu, Y. G. Ji, H. P. Zhou, B. W. Zhang, Y. P. Tian and J. Y. Wu, , A novel and simple fluorescence probe for detecting main group magnesium ion in Hela cells and Arabidopsis Biosens. Bioelectron., 86, 2016, 677–682. [18] Y. J. Bian, L. Q. Wang, F. X. Cao and L. J. Tang, A Simple Fluorescence Probe Based on Aggregation-Induced Emission (AIE) Property for the Detection of Mg2+ Ions, J. Fluoresc., 26, 2016, 53–57. [19] Z. D. Liu, H. J. Xu, S. S. Chen, L. Q. Sheng, H. Zhang, F. Y. Hao, P. F. Su and W. L. Wang, Solvent-dependent “turn-on” fluorescence chemosensor for Mg2+ based on combination of CN isomerization and inhibition of ESIPT mechanisms, Spectrochim. Acta, Part A, 149, 2015, 83–89. [20] H. F. Zhang, C. X. Yin, T. Liu, J. B. Chao, Y. B. Zhang and F. J. Huo, Selective “off-on” detection of magnesium (II) ions using a naphthalimide-derived fluorescent probe, Dyes Pigm., 146, 2017, 344–351. [21] C. R. Li, S. L. Li, G. Q. Wang and Z. Y. Yang, Spectroscopic properties of a chromone-fluorescein conjugate as Mg2+ “turn on” fluorescent probe, J. Photochem. Photobiol. A: Chem., 356, 2018, 700–707. [22] S. B. Maity and P. K. Bharadwaj, A molecular dual fluorescence-ON probe for Mg2+ and Zn2+: Higher selectivity towards Mg2+ ove
Copyright
Copyright © 2025 Irfana Nahid, Ajit Kumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66807
Publish Date : 2025-02-02
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

