Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Anticancer Activity and Green Synthesized TiO2 Nanoparticles From Calotropis Gigantea Leaves Extract
Authors: P. Arunkumar, P. Ganapathi, J. Gugan, K. Nithya Sri, D. Vijayakumar
DOI Link: https://doi.org/10.22214/ijraset.2024.59719
Certificate: View Certificate
Abstract
Aim & Objective: Research the Anticancer Abilities of TiO2 Nanoparticles Obtain using Calotropis Gigantea by liver cells (HepG2). Materials & Methods: Various plant materials for the synthesis of nanoparticles are considered in green technology. In this present study, reports the synthesis of Tio2 nanoparticles that were synthesized from Calotropis gigantea leaf, and the formation of Tio2 nanoparticles was observed with different time intervals. The characterization was recorded from scanning electron microscope, X-ray diffraction, Fourier transfer infrared spectra, DLS, UV-Vis spectrum. The supporting green synthesis and characterization of Tio2 nanoparticles. Result: The result is recorded from MTT assay and ETBR/AO Staining.
Introduction
I. INTRODUCTION
Cancer are most serious health problems globally. Liver cancer is one of the maximum competitive occurrences of most cancers with a excessive fatality rate global huge[1]. Hepatocellullar carcinoma (HCC) is the maximum common form of number one liver cancer, with cholangiocarcinoma accounting for the remainder. C.gigantea (Apocynaceae, Asclepedaceae) is usually used globally as a traditional medication for the treatment of several elements. C.gigantea plant is extensively grown in many countries in Africa, eastern Asia, and South-east Asia. Extracts from all elements of this plant had been shown to have an expansion of organic activities[2]. The findings of this studies may provide valuable statistics for the improvement of low-chance most cancers remedies based on plant extracts. The blended healing benefits of C.gigantea leaves extracts may be beneficial for destiny anticancer treatment regimens.

A. Plant Profile
Plant name: Calotropis gigantea
Family name: Apocynaceae
Synonyms: Calotropis procera, Calotropis acia
Common name: Giant milkweed, Crown flower
Aakh Parts used: leaves.
B. Classification
Kingdom: Plantea
Subkingdom: Tracheobionata
Superdivision: Spermatophyta
Division: Magnoliopsida
Subclass: Asteridea
Order: Gentianales
Family: Apocynacea
Subfamily: Asclepiadoideae
Genus: Calotropis
Species: gigantea, procera, acia
II. MATERIALS AND METHODS
A. Collection and Authentication of Plant
Calotropis gigantea was collected from Mallasamudram, Namakkal district, TamilNadu, India in the month of August, 2023. The plant material was identified and authenticated by Dr. D.Vijayakumar Assistant Professor, Mahendra Institue of Technology Namakkal, TamilNadu, India.
B. Preparation of Plant Extract
Calotropis gigantea have been washed 3 times with faucet water then double distilled water and dried for 10 days via using a hot air oven at 400C. The dried leaf had been grinded right into a excellent powder[3]. In 100ml of conical flasks, 10 grams of leaf powder were mixed with 100 mL of distilled water. The aggregate become boiled at 200? for 20 min. The extract turned into filtered via muslin cloth .The filtered extract become stored at 4°C within the refrigerator for further use.
C. Bulk Synthesis of Tio2NPs Using Calotropis gigantea leaf
10ml of calotropis gigantea leaf extract was mixed with Tio2 Solution (90ml) in dark condition for 24hours. A change in the color of the solution from white to light yellow indicated the synthesis of Tio2.
D. Characterization of synsthesized Tio2 Nanoparticles
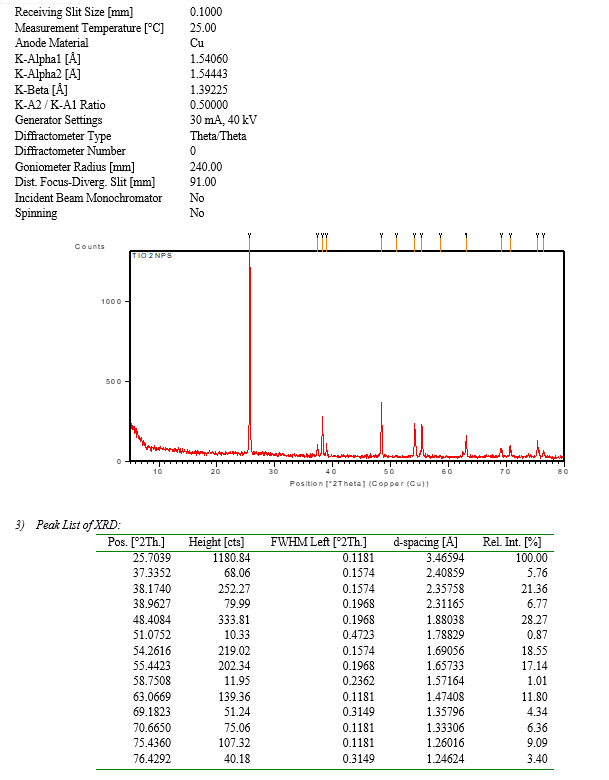
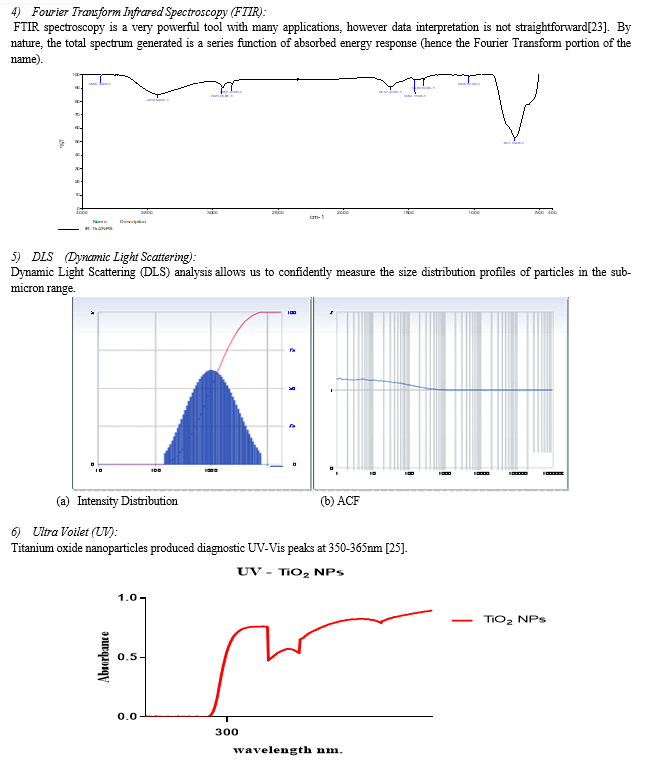
The biosysthesized Tio2NPs have been characterized consistent with the technique. The form and length of Tio2 nanoparticles were determined by using Scanning electron microscopy (SEM)[4]. X-ray diffraction (XRD) is the main method for crystallographic characterization for bulk, nano and thin film materials[5]. Fourier transform infrared spectroscopy (FTIR) turned into used to signify the adjustments and the composition at the floor of the synthesized nanoparticles. The particle size have been measured by means of dynamic light scattering (DLS) the usage of a Zetasizer Nano ZS90[6]. UV-via spectra were measured using a UV-2450.
E. MTT Assay
- Principle: MTT (3-4, 5 dimethylthiazol-2yl-2, 5-diphenyl tetrazolium bromide) assay, is based totally on the capability of a mitochondrial dehydrogenase enzyme of viable cells to cleave the tetrazolium rings of the light yellow MTT and form a dark blue colored formazan crystal which is essentially impermeable to cell membranes, therefore resulting in its accumulation within healthy cells[7]. Solubilization of cells with the addition of detergents (DMSO) results in the liberation of crystals which might be solubilized. The number of surviving cells is directly proportional to the level of formazan product created[8]. The color may be quantified using a multi-well plate reader.
- Materials Required: Fetal Bovine Serum (FBS) and antibiotic solution have been from Gibco (USA), DMSO (Dimethyl sulfoxide) and MTT (3-4,5 dimethylthiazol-2yl-2,5-diphenyl tetrazolium bromide) (5 mg/ml) were from Sigma, (USA), DMEM medium, 1X PBS, (India)[9]. 96 well tissue culture plate and wash beaker have been from Tarson (India)[10].
- Procedure
a. Cell Culture: Change liver cellular line have been purchased from NCCS, Pune and have been cultured in liquid medium (DMEM) supplemented 10% Fetal Bovine Serum (FBS), 100 µg/ml penicillin and 100 µg/ml streptomycin, and maintained under an atmosphere of 5% CO2 at 37oC.
b. MTT assay: The Test sample was tested for in vitro cytotoxicity, using Change liver cells by MTT assay. Briefly, the cultured Change liver cells were harvested by trypsinization and pooled in a 15 ml tube[11].
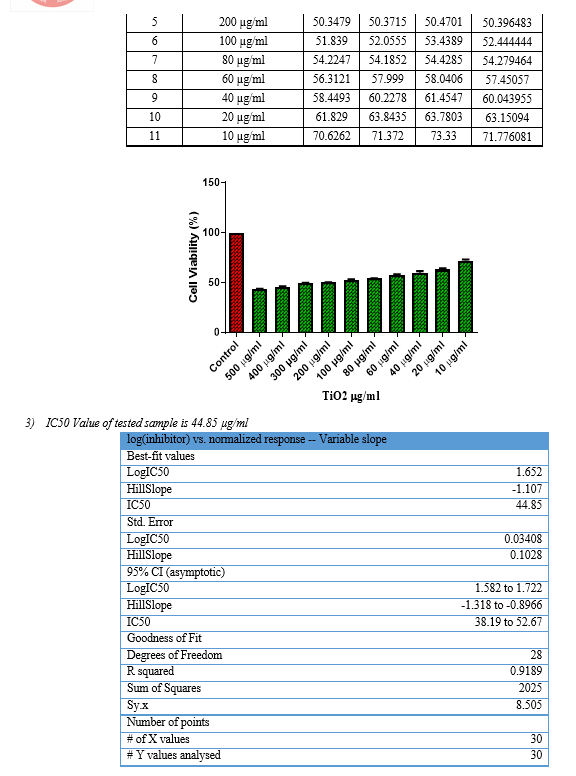
Then, the cells were plated at a density of 1×105 cells/ml cells/well (200 µL) into the 96-well tissue culture plate in DMEM medium containing 10 % FBS and 1% antibiotic solution for 24-48 hour at 37°C. The wells were washed with sterile PBS and treated with the Test sample in a serum-free DMEM medium[12]. Every sample was replicated three times and the cells were incubated at 37°C in a humidified 5% CO2 incubator for 24 h. After incubation, MTT (10 µL of 5 mg/ml) was added to each well and the cells were incubated for another 2-4 h until purple precipitates were clearly visible under an inverted microscope[13]. Later, the medium together with MTT (220 µL) was aspirated off the wells and washed with 1X PBS (200 µl). Furthermore, to dissolve formazan crystals, DMSO (100 µL) was added and the plate changed into shaken for 5 min[14]. The absorbance for each well have measured at 570 nm using a microplate reader (Thermo Fisher Scientific, USA) and IC50 value were calculated using Graph Pad Prism 6.0 software (USA).
Formula Cell viability % = Test OD/Control OD X 100
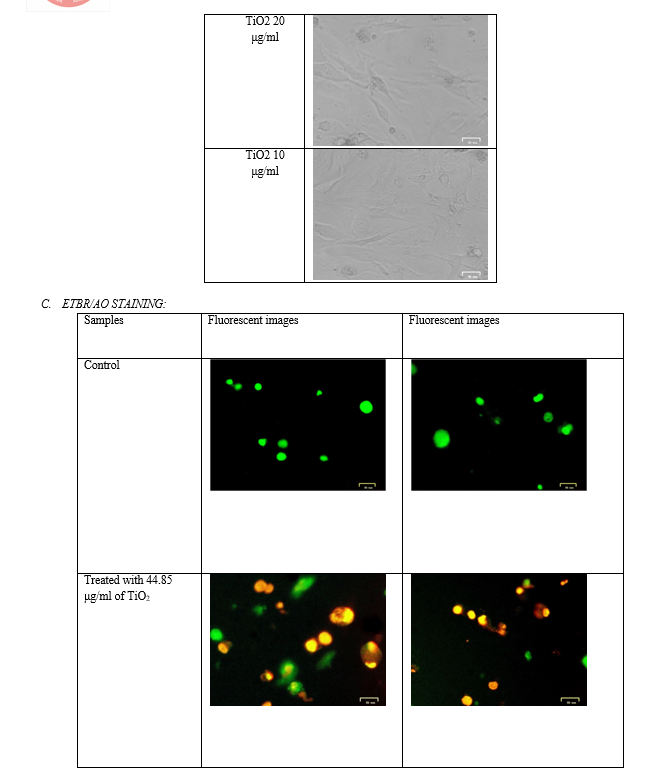
F. ETBR/AO STAINING
- Principle: Fluorescent dyes with aromatic amino or guanidine groups, such as acridine orange (AO), interact with nucleotides to emit fluorescence. EtBr molecules intercalate inside the DNA double helix[15]. AO can molecule intercalate in double-stranded DNA or single-stranded DNA and RNA. One molecule of AO can also interact with one phosphate group of DNA or RNA to form an aggregated, or stacked, structure that emits pink fluorescence with the maximum wavelength at 650 nm. This fluorescent dye is impermeable through the cell membranes of viable cells and can be used as fluorescent indicators of dead cells. Acridine orange is a vital dye and will stain both live and dead cells[16]. Necrotic cells stain orange however have a nuclear morphology such that of viable cells, and not using a condensed chromatin[17]. Ethidium bromide (EtBr) is only taken up by cells when cytoplasmic membrane integrity is lost and stains the nucleus red. EtBr also dominates over AO. For that reason live cells have a normal green nucleus; early apoptotic cells have a bright green nucleus with condensed or fragmented chromatin; late apoptotic cells display condensed and fragmented orange chromatin; cells that have died from direct necrosis have a structurally normal orange nucleus[18]. Ethidium re-emits there energy in the colour of yellow/orange light centered at 590 nm. The fluorescence of ethidium bromide in an aqueous solution is considerably lower than that of the intercalated dye.
- Materials required: DMEM medium, Penicillin/Streptomycin antibiotic solution, Trypsin-EDTA have been purchased from Gibco (USA), EtBr, and Acridine orange also purchased from Sigma Aldrich (USA), Fluorescent Imaging System, (ZOE, Bio-Rad, USA).
- Procedure:
a. Cell culture
Change liver cell line have been purchased from NCCS, Pune and was cultured in liquid medium (DMEM) supplemented for 10% Fetal Bovine Serum (FBS), 100 u/ml penicillin, and 100 µg/ml streptomycin, and maintained under an atmosphere of 5% CO2 at 37oC[19].
b. EtBr/AO staining
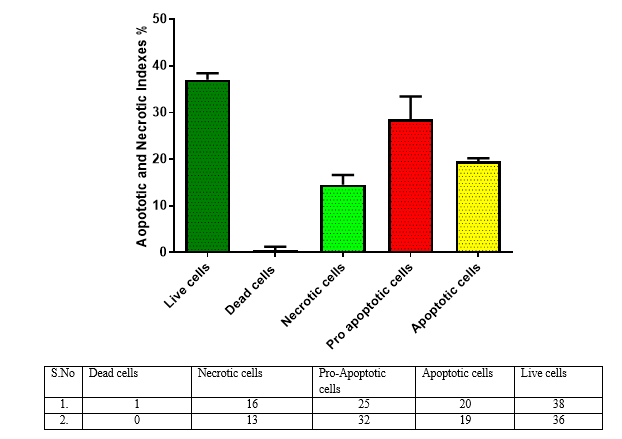
Briefly, 5 x 105 cells/ml of Chang liver cells were plated into a 96 well tissue culture plate and incubated for 24 hr in a DMEM growth medium. After incubation, the cells were treated with 44.85 μg/ml of TiO2 sample in a serum-free DMEM medium[20]. The plate was incubated at 37oC at a 5% Co2 incubator for 24 hours[24]. After incubation, 10 µl of 1 mg/ml acridine orange and ethidium bromide were added to the wells and mixed gently[21]. Finally, the plate was centrifuged at 800 rpm for 2 minutes and evaluated immediately within an hour, and examined at least 100 cells by a Fluorescent Imaging System, (ZOE, Bio-Rad, USA).
III. RESULTS & DISCUSSION
A. Characterization of synsthesized Tio2 Nanoparticles:
1) Scanning Electron Microscopy ( SEM ): In the SEM Pictures, a microscope is used to qualitatively identify the microstructural developments within the matrix of the stabilized soil specimens[21]. The SEM photos of clays are shown (Unique magnifications); thus, the microstructure is easily located because the pictures can be enlarged[22].
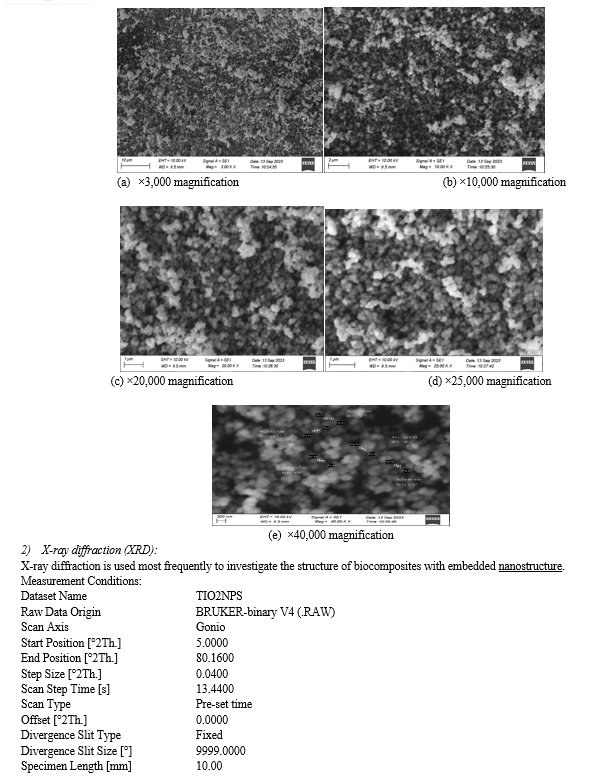


IV. ACKNOWLEDGEMENT
We acknowledge TRI-BIOTECH ( Trichy Research Center) for characterization studies.
V. CONFLICT OF INTEREST
The authors declare that they have“ No Conflict of Interest”.
Conclusion
The present study discover that the extracts of Calotropis gigantea exhibited anticancer activity on HepG2 cellular lines. Thus the result further studies are needed to investigate and isolate its energetic compounds and also to assess its anticancer potential on other cellular lines.
References
[1] Basnakian, A. G. & James, S. J. 1994. A rapid and sensitive assay for the detection of DNA fragmentation during early phases of apoptosis. Nucleic acids research, 22, 2714-2715. [2] Deepa, S., Kanimozhi, K. and Panneerselvam, A. (2013). Antimicrobial activity of extracellularly synthesized silver nanoparticles from marine derived actinomycetes. Int J Curr Microbiol App Sci 2(2), 223-230. [3] Devi, L.S. and Joshi, S. (2015). Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. Journal of Microscopy Ultrastructure 3(1), 29- 37. [4] Escarcega-Gonzalez,C.E,GarzaCervantes,J.A,VazquezRodriguez,A.; MontelongoPeralta, L.Z.; Trevino-Gonzalez, M.T.; Diaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chavez, M.R.M.; Regalado Soto, D.I.; Trevino Gonzalez, F.M.; Carrazco Rosales, J.L., Cruz ?R.V. and Morones-Ramirez, J.R. (2018). In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomedicine, 13:2349-2363. [5] Hackley V A and Clogston D J 2007 Measuring the size of Nanoparticles in Aqueous Media Using DLS Gaithersburg: NIST,22p. [6] Henderson, J. M. et al. Multiple liver insults synergize to accelerate experimental hepatocellular carcinoma. Sci. Rep. 8, 1–12 (2018). [7] James, S. J. 1994. A rapid and sensitive assay for the detection of DNA fragmentation during early phases of apoptosis. Nucleic acids research, 22, 2714-2715. [8] Jayshree, B., Kar, S. S., Anandam, A., Thomas, S., Jain, P., Rai, A. & Rao, C. 2012. Elucidation of Structure?activity Relationship of 2-Quinolone Derivatives and Exploration of Their Antitumor Potential Through Bax?induced Apoptotic Pathway. Chemical Biology & Drug Design, 80, 291- 299. [9] Karthik, L. & Bhaskara Rao, K. 2010. In vitro antiCandida activity of Calotropis gigantea against clinical isolates of Candida. Journal of Pharmacy research, 3, 539-542. [10] Karthik N., Raj, V. P., Jayshree, B., Kar, S. S., Anandam, A., Thomas, S., Jain, P., Rai, A. & Rao, C. 2012. Elucidation of Structure?activity Relationship of 2-Quinolone Derivatives and Exploration of Their Antitumor Potential Through Bax?induced Apoptotic Pathway. Chemical Biology & Drug Design, 80, 291- 299. [11] Meenashi Vanathi.B, Vishnu Priya., Prateek Jain., C.gigantea induces Apoptosis in Human Hepatocellular Carcinoma by Altering Bax/Bcl-2 Expression Science and Education publishing 2015 [12] Moreira DRM ,2007 ,Synthesis and anti tumour evaluation of peptidyl-like derivatives containing the 1,3-benzodioxole system.Eur J Med Chem 42 [13] Oliveira JS, Bezerra DP, 2007,In vitro cytotoxicity against different human cancer cell lines of laticifer proteins of Calotropis procera(Ait) R.Br. Toxicol in vitro 21. [14] Organization, W. H. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All (WHO, 2020). [15] Prateek Jain, B.Meenashi Vanathi, Vishnu Priya., C.gigantea induces Apoptosis in Human Hepatocellular Carcinoma by Altering Bax/Bcl-2 Expression Science and Education publishing 2015 [16] Rajakaruna, N., Harris, C. S. & Towers, G. 2002. Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka. Pharmaceutical Biology, 40, 235-244 [17] Saravana Kumar, P.; Balachandran, C.; Duraipandiyan, V.; Ramasamy, D.; Ignacimuthu, S .and Al-Dhabi, N.A. (2015). Extracellular biosynthesis of silver nanoparticle using Streptomyces sp. 09 PBT 005 and its antibacterial and cytotoxic properties. Appl. Nanosci., 5(2):169- 180. [18] Schulien, I. et al. Extracellular ATP and purinergic P2Y2 receptor signaling promote liver tumorigenesis in mice by exacerbating DNA damage. Cancer Res. 80, 699–708 (2020). [19] Singh, D.; Rathod, V.; Fatima, L.; Kausar, A.; Vidyashree, N.A. and Priyanka, B.J. (2014). Biologically reduced silver nanoparticles from Streptomyces sp. VDP-5 and its Antibacterial Efficacy, 4(2): 31-36. [20] Thomas, S., Jain, P., Rai, A. & Rao, C. 2012. Elucidation of Structure?activity Relationship of 2-Quinolone Derivatives and Exploration of Their Antitumor Potential Through Bax?induced Apoptotic Pathway. Chemical Biology & Drug Design, 80, 291- 299. [21] Tripathi, P. K., Awasthi, S., Kanojiya, S., Tripathi, V. & Mishra, D. K. Callus culture and in vitro biosynthesis of cardiac glycosides from Calotropis gigantea (L.) Ait. In Vitro Cell. Dev. Biol. 49, 455–460 (2013). [22] Venkanna A,Siva B,Poornima B,Phytochemical investication of sesquiterpenes from the fruits of schisandra chinensis and their cytotoxic activity,Elseveir BV,2014. [23] Vishnu Priya., Prateek Jain., C.gigantea induces Apoptosis in Human Hepatocellular Carcinoma by Altering Bax/Bcl-2 Expression Science and Education publishing 2015 [24] Wong, S. K., Lim, Y. Y., Abdullah, N. R. & Nordin, F. J. 2011. Antiproliferative and phytochemical analyses of leaf extracts of ten Apocynaceae species. Pharmacognosy research, 3, 100. [25] Xu, F., Jin, T., Zhu, Y. & Dai, C. Immune checkpoint therapy in liver cancer. J. Exp. Clin. Cancer Res. 37, 1–12 (2018).
Copyright
Copyright © 2024 P. Arunkumar, P. Ganapathi, J. Gugan, K. Nithya Sri, D. Vijayakumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59719
Publish Date : 2024-04-01
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

