Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Review Article on the Coronavirus disease (COVID-19)
Authors: Prof. Amrata D Suryawanshi, Arati R Gite, Anjali S Malge, Dnaneshwar D Dongare, Apurva V Andhare, Bhagwat D Somwanshi, Dipanjali D Raje
DOI Link: https://doi.org/10.22214/ijraset.2023.56107
Certificate: View Certificate
Abstract
Coronaviruses are a group of enveloped viruses with nonsegmented, single-stranded, and positive-sense RNA genomes. Coronaviruses belong to the “Coronaviridae family”, which causes various diseases, from the common cold to SARS and MERS. In March 2020 the World Health Organization declared the SARS-Cov-2 virus a global pandemic. We performed a review to describe existing literature about Corona Virus Disease 2019 (COVID-19) history, Symptoms, Epidemiology, Clinical features, Clinical manifestations, Diagnosis, Treatment, Prevention.
Introduction
I. INTRODUCTION
The world health organization declared an epidemic on january 30, 2020, following the outbreak of the sars- cov-2 virus in wuhan, hubei province, china, and its rapid spread to 25 countries. This happened just 1 month after the announcement of the first case of the disease on december 31, 2019. Coronaviruses are positive single- stranded rna viruses that belong to the coronavirus family and are genetically classified into four genera: α, β, γ and δ coronavirus these viruses often infect animals such as birds and mammals and usually cause mild respiratory infections in humans. Due to the sars- cov-2 rna content and its high potential for emergence, respiratory infections caused by the virus have recently led to deadly endemics in humans, such as sars and mea- sles. The first case of mid- dle east respiratory syndrome (mers) was observed in saudi arabia in 2011–2012, of which 2495 cases have been reported since then, of which 858 cases were associated with death and the death rate was estimated at 34.4%. While no new mers-cov cases have been reported since 2004, the sars-cov-2 outbreak occurred unexpectedly in 2020. Sars-cov-2 is the seventh member of the coronavirus family, which, like mers-cov, sars-cov causes respiratory disease in humans with a genome size of 27–35 kb and belongs to the beta-coronavirus species. Like other coronaviruses, it encodes several structural proteins and non-structural proteins. Spike glycoprotein (s) of and nucleocapsid protein (n), membrane protein (m) and coating protein (e) are among its structural proteins
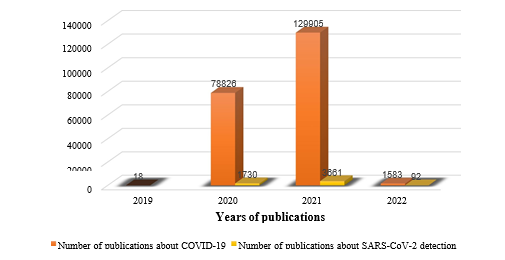
COVID-19 is a rapidly growing pandemic with its first case identified during December 2019 in Wuhan, Hubei Province, China. Due to the rampant rise in the number of cases in China and globally, WHO declared COVID-19 as a pandemic on 11th March 2020. The disease is transmitted via respiratory droplets of infected patients during coughing or sneezing and affects primarily the lung parenchyma. The spectrum of clinical manifestations can be seen in COVID-19 patients ranging from asymptomatic infections to severe disease resulting in mortality
II. HISTORY
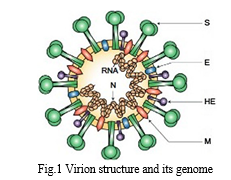
Coronaviruses are enveloped positive sense RNA viruses ranging from 60 nm to 140 nm in diameter with spike like projections on its surface giving it a crown like appearance under the electron microscope; hence the name coronavirus [3]. Four corona viruses namely HKU1, NL63, 229E and OC43 have been in circulation in humans, and generally cause mild respiratory disease. There have been two events in the past two decades wherein crossover of animal betacorona viruses to humans has resulted in severe disease. The first such instance was in 2002–2003 when a new coronavirus of the β genera and with origin in bats crossed over to humans via the intermediary host of palm civet cats in the Guangdong province of China. This virus, designated as severe acute respiratory syndrome coronavirus affected 8422 people mostly in China and Hong Kong and caused 916 deaths (mortality rate 11%) before being contained. Almost a decade later in 2012, the Middle East respiratory syndrome coronavirus (MERS-CoV), also of bat origin, emerged in Saudi Arabia with dromedary camels as the intermediate host and affected 2494 people and caused 858 deaths (fatality rate 34%)
III. SYMPTOMS
A wide range of symptoms are found in COVID-19 patients, ranging from mild/moderate to severe, rapidly progressive, and fulminant disease. Symptoms of COVID-19 are non-specific and disease presentation can range from asymptomatic to severe pneumonia. Incidence of asymptomatic cases ranges from 1.6% to 51.7% and these people do not present typical clinical symptoms or signs and do not present apparent abnormalities in lung computed tomography. The most common symptoms of COVID-19 are fever, cough, myalgia, or fatigue and atypical symptoms include sputum, headache, haemoptysis, vomiting, and diarrhoea. Some patients may present with sore throat, rhinorrhoea, headache, and confusion a few days before the onset of fever, indicating that fever is a critical symptom, but not the initial manifestation of infection. Furthermore, some patients experience loss of smell (hyposmia) or taste (hypogeusia), which are now being considered early warning signs and indications for self-isolation.
The most common symptoms of COVID-19 are
- Fever.
- Dry cough.
- Fatigue.
Other symptoms that are less common and may affect some patients include
- Loss of taste or smell.
- Nasal congestion.
- Conjunctivitis (also known as red eyes).
- Sore throat.
- Headache.
- Muscle or joint pain.
- Different types of skin rash.
- Nausea or vomiting.
- Diarrhea.
- Chills or dizziness.
Symptoms of severe COVID-19 disease include:
- Shortness of breath.
- Loss of appetite.
- Confusion.
- Persistent pain or pressure in the chest.
- High temperature (above 38 °C). Other less common symptoms are
- Irritability.
- Confusion.
- Reduced consciousness (sometimes associated with seizures).
- Anxiety.
- Depression.
- Sleep disorders.
People of all ages who experience fever and/or cough associated with difficulty breathing or shortness of breath, chest pain or pressure, or loss of speech or movement should seek medical care immediately. If possible, call your health care provider, hotline or health facility first, so you can be directed to the right clinicrological complications such as strokes, brain inflammation, delirium and nerve damage.
IV. SAMPLE COLLECTION & ANALYSIS
A. Transmission
Zoonotic transmission initially appeared to be a plausible cause as majority of early cases had a history of exposure to wet markets. However, by the end of January 2020, the number of people who developed the disease without exposure to the market or another person with respiratory symptoms increased. The spread of the disease among persons who did not visit Wuhan and among healthcare workers suggested a person-to-person spread of the virus. The exact mode of transmission of this virus is unknown. But, as with other respiratory viruses, droplet borne infection, either directly or indirectly, through fomites is probably the predominant mode of transmission. At present, there is no evidence for airborne transmission of the virus.12 13 Although virus particles have been detected in stool samples of both symptomatic and convalescing patients, the risk of feco-oral transmission is unclear
V. DURATION OF VIRUS
The duration for which a patient with COVID-19 remains infective is unclear. Viral load in the oropharyngeal secretions is highest during the early symptomatic stage of the disease [13,14]. The patient can continue to shed the virus even after symptom resolution [13]. In a study from China, the median duration of virus shedding was 20 days (interquartile range [IQR] 17.0–24.0) amongst the survivors [15]. A study of viral dynamics in mild and severe cases revealed that mild cases tend to clear the viruses early, while severe cases can have prolonged viral shedding [16]. Data from studies using twin respiratory and fecal sampling have shown viral shedding can persist in stools for more than 4 weeks even when respiratory samples are negative [17]. Xu et al identified male sex, delayed hospitalization after illness, and invasive mechanical ventilation as risk factors for prolonged viral shedding [13]. Transmission during the asymptomatic phase has also been reported. In a study from Singapore, 6.4% of the 157 locally acquired cases of COVID-19 were attributed to transmission during the asymptomatic phase of the disease .Severity of Covid-19 Virus:
A. Spectrum of Infection Severity
The spectrum of symptomatic infection ranges from mild to critical; most infections are not severe [19,39-44]. Specifically, in a report from the Chinese Centre for Disease Control and Prevention that included approximately 44,500 confirmed infections with an estimation of disease severity [45]:
- Mild disease (no or mild pneumonia) was reported in 81 percent.
- Severe disease (eg, with dyspnea, hypoxia, or >50 percent lung involvement on imaging within 24 to 48 hours) was reported in 14 percent.
- Critical disease (eg, with respiratory failure, shock, or multiorgan dysfunction) was reported in 5 percent.
- The overall case fatality rate was 2.3 percent; no deaths were reported among noncritical cases.
Similarly, in a report of 1.3 million cases reported to the United States Centers for Disease Control and Prevention (CDC) through the end of May 2020, 14 percent were hospitalized, 2 percent were admitted to the intensive care unit (ICU), and 5 percent died [46]. The risk of severe illness varied by age and underlying comorbidities.
B. Infection Fatality Rate
The case fatality rate only indicates the mortality rate among documented cases. Since many severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are asymptomatic and many mild infections do not get diagnosed, the infection fatality rate (i.e., the estimated mortality rate among all individuals with infection) is considerably lower and has been estimated in some analyses to be between 0.15 and 1 percent, with substantial heterogeneity by location and across risk groups [47-50].
C. Fatality Rate Among Patients Hospitalized
Among hospitalized patients, the risk of critical or fatal disease is high. In a study from early in the pandemic that included 2741 patients who were hospitalized for COVID-19 in a New York City health care system, 665 patients (24 percent) died or were discharged to hospice Of the 647 patients who received invasive mechanical ventilation, 60 percent died, 13 percent were still ventilated, and 16 percent were discharged by the end of the study. The in-hospital fatality rate associated with COVID-19 has been higher than that for influenza. As an example, in an analysis of hospital data from the United States Veterans Health Administration, patients with COVID-19 were five times more likely to die during the hospitalization than patients with influenza (21 versus 3.8 percent).
Over the course of the pandemic, declining in-hospital fatality rates have been reported [61-64]. As an example, in a retrospective study of a national surveillance database in England that included over 21,000 critical care patients with COVID-19, ICU survival improved from 58 percent in late March 2020 to 80 percent by June 2020 [61]. The reasons for this observation are uncertain, but potential explanations include improvements in hospital care of COVID-19 and better allocation of resources when hospitals were not overburdened.
In resource-limited settings, in-hospital mortality rates may be higher than those reported elsewhere. As an example, in a study from 10 countries in Africa, where there was a median of two intensive care specialists in each hospital and a minority of facilities did not have pulse oximetry, the in-hospital 30-day mortality rate following critical care admission was 48 percent [65]. Mortality was associated with underlying comorbidities as well as resource shortages.
D. Excess of Death During Pandemic
Neither the case fatality rate nor the infection fatality rate account for the full burden of the pandemic, which includes excess mortality from other conditions because of delayed care, overburdened health care systems, and social determinants of health [66-68].
E. Incubtion Period
The mean or median incubation period of the disease ranges from 5 to 6 days [69,70]. Laueret al estimated that 2.5% of the patients will develop symptoms within 2.2 days (95% CI, 1.8 to 2.9 days) and 97.5% of patients will develop symptoms within 11.5 day(95% CI, 8.2– 15.6 days).
Serial interval refers to the time interval between the onset of symptoms in the primary case and the secondary case. The mean serial interval is estimated to be approximately 4 to 5 days [71,72]. By analysing data from 468 infector–infectee pairs, Du et al noted that 59 secondary cases had symptoms earlier than their primary case. This suggested that there is a possibility that the transmission of the disease occurred during the asymptomatic phase of illness in this group of patients [73].
F. Period of Infectivity
The duration for which a patient with COVID-19 remains infective is unclear. Viral load in the oropharyngeal secretions is highest during the early symptomatic stage of the disease [74].
The patient can continue to shed the virus even after symptom resolution. In a study from China, the median duration of virus shedding was 20 days (interquartile range [IQR] 17.0–24.0) amongst the survivors [75].
A study of viral dynamics in mild and severe cases revealed that mild cases tend to clear the viruses early, while severe cases can have prolonged viral shedding [76].
Data from studies using twin respiratory and fecal sampling have shown viral shedding can persist in stools for more than 4 weeks even when respiratory samples are negative [77]. Transmission during the asymptomatic phase has also been reported. In a study from Singapore, 6.4% of the 157 locally acquired cases of COVID-19 were attributed to transmission during the asymptomatic phase of the disease.
VI. DIGNOSIS
???????A. When is a COVID 19 Diagnostic Test Required?
Diagnostic testing for COVID-19 is conducted to find out whether a person is infected with the SARS-CoV-2 virus, responsible forCOVID-19 infection.
Your healthcare practitioner may recommend you the same if:
- You are experiencing symptoms of COVID 19 such as high fever, cough, shortness of breath, excessive fatigue, etc.
- You have long-term health conditions such as asthma, heart diseases, etc. and experience a sudden worsening of symptoms.
- You have come in contact with someone tested positive for COVID 19 recently.
- You are a healthcare worker working in a hospital environment.
- You require hospitalization for treatment or surgery of existing medical conditions.
B. Different Laboratory Test Available for Dignosis
In general, there are two types of tests for diagnosing COVID-19 namely, Antigen or rapid testing and Molecular or PCR testing. The antigen test is often used as a point- of-care test, less expensive and yields quicker results within minutes. However, there is a higher chance of false-negative results as compared to molecular testing. Molecular testing yields more accurate results but are time-consuming [79].
C. Tretment
Initially, early in the pandemic, the understanding of COVID-19 and its therapeutic management was limited, creating an urgency to mitigate this new viral illness with experimental therapies and drug repurposing. Since then, due to the intense efforts of clinical researchers globally, significant progress has been made which has led to a better understanding of not only COVID-19 and its management but also has resulted in the development of novel therapeutics and vaccine development at an unprecedented speed [80].
D. Prevention
Preventive measures are the current strategy to limit the spread of cases. Early screening, diagnosis, isolation, and treatment are necessary to prevent further spread.
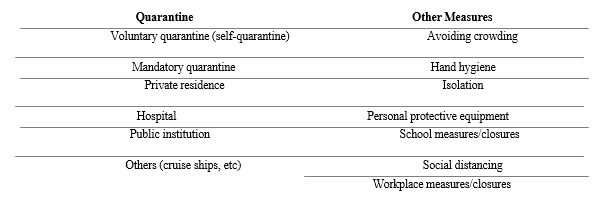
Preventive strategies are focused on the isolation of patients and careful infection control, including appropriate measures to be adopted during the diagnosis and the provision of clinical care to an infected patient [81]. Important COVID-19 prevention and control measures in community are summarized in Table 2.
AuNPs to congregate. The quantity of target RNA is ren- dered into a shift and intensity change of the absorbance peak, a change in the Raman signal of AuNPs coupled with residue DNA probes and a variation in the fluorescence intensity of the supernatant. Each operating mode in the suggested biosensor may detect single-base mismatches in the target gene, reducing false positive/negative readings. The suggested biosensor does not need the extraction and purification of viral RNA. The suggested biosensor provides a relatively easy detection method when compared to PCR- based detection. Due to the long-term stability, the reaction solution conjugated to the DNA probe may be stored in the reaction chamber and ready for diagnosis. The operator only needs to load the detection sample and then centrifuge the supernatant to separate it from the aggregated AuNPs after adding the SSC buffer. A photoluminescence system is used to test the fluorescence intensity, and an automatic, portable microplate reader is used to test the absorption spectrum, while a micro-Raman spectrometer is used to record the Raman spectrum. Using 96 or 384 microplates, the proposed detection method can detect 96 or 384 samples simultane- ously. In all triple modes, the sensor reaches a femtomole level detection limit of 160 fM in absorbance mode, 259 fM in fluorescence mode and 395 fM in SERS mode. The sug- gested sensing platform offers a novel method for detecting COVID-19 and other infections that is rapid, sensitive and selective [123].
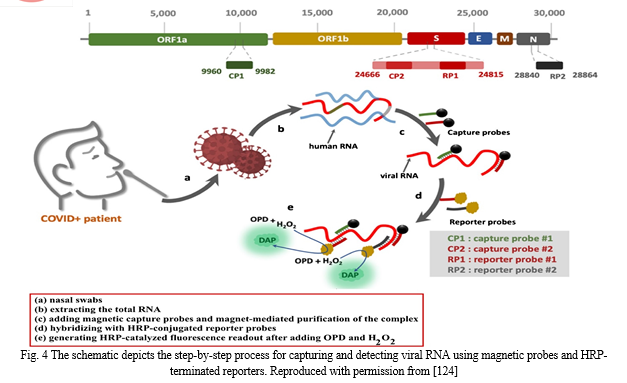
In another work, Zayani et al. described the develop- ment of a magnetofluorescent bioplatform to detect SARS- CoV-2 viral RNA directly in total RNA collected from COVID-19-positive patients’ nasopharyngeal swabs. Two capture probes attached to magnetic beads through a biotin/streptavidin linkage, targeting two particular loca- tions in the ORF1a and S genes, yielded a greater fluo- rescence response (Fig. 12). Through the oxidation of o-phenylenediamine to fluorescent 2,3-diaminophenazine, two horseradish peroxidase (HRP)-conjugated reporter sequences, corresponding to the loci of the S and N genes, were utilized to detect the presence of viral RNA. The bio- platform possesses a linear dynamics range from 0.01 up to 3.0 ng (1 × 103 to 9 × 107 copies/μL) with a low LOD of 0.01 ng of viral RNA (1 × 103 copies/μL) under optimum conditions. This platform is highly selective and sensi- tive that can distinguish SARS-CoV-2 RNA from similar viruses such as West Nile, hepatitis C, measles and non- polio viruses. In addition, 46 clinical samples verified the proposed biosensor (36 COVID-19-positive and 10 COVID-19-negative samples, as assessed with the gold standard RT-qPCR method). The sensitivity and specificity of the proposed technique achieved 100%. Finally, hav- ing such a simple and specific technique available in the field, at a main point of care, can aid in the identification of SARS-CoV-2 infection in resource-limited situations [124].
Pramanik et al. showed the ability to quickly diagnose, within 10 min, specific SARS-CoV-2 spike recombinant antigen or SARS-CoV-2 spike protein pseudo-type bacu- lovirus using Rhodamine 6G (Rh-6G) dye-coupled DNA aptamer-adhering gold nanostar (GNS) spectroscopy. Because the Rh-6G-attached single-stand DNA aptamer enveloped the GNS, the NSET method quenched 99% of the dye’s fluorescence. The fluorescence signal remains in the presence of spike antigen or virus due to aptamer–spike protein binding. In particular, 130 fg/mL for antigen and 8 particles/mL for virus were established as a limit of detec- tion of the NSET test. Finally, it was proven that GNSs with DNA aptamer may terminate the infection by inhibiting the receptor-binding capacity of angiotensin-converting enzyme 2 (ACE2) and dissolving the virus’ lipid membrane [125].
Conclusion
COVID-19 is a serious and dangerous infectious disease with symptoms similar to SARS in the form of fever, cough and fatigue. The disease is mostly transmitted through respiratory droplets and close contact. This dis- ease is a major threat to world health and safety. Bioana- lytical methods designed to diagnose COVID-19 disease are superior to other diagnostic methods due to their lower cost, higher accuracy, better detection limit and lower error. The advantages and disadvantages of various bio- sensing methods used to detect the SARS-CoV-2 virus are given in Table S2. Electrochemical methods can be used in further studies of this disease and similar diseases, and even by simulating the disease using relevant biosen- sors due to their high response speed, which in addition to helping the advancement of science, also offers the pos- sibility of rapid diagnosis and achievement. It provides the appropriate treatment method, so it is recommended to use the simulation and design of appropriate biosen- sors to make the necessary predictions to prevent or even diagnose and treat similar emerging diseases that may occur. Nanotechnology has the potential to accelerate the development of unique diagnostic sensors, the integra- tion of novel devices, improved optimization/validation and improvements in sensing performance at the point of care. Future research should focus on developing novel and next-generation non-invasive, specific, inexpensive and quick biosensing techniques and technologies for diag- nostic applications, particularly in the management of pan- demics and life-threatening infectious illnesses. However, certain difficulties require further investigation and atten- tion. For starters, the majority of these technologies and materials have been studied on a laboratory scale, imply- ing that employing them in real-world circumstances may not be as precise as in the lab [126]. Furthermore, none of these biosensors has yet been developed for detecting the SARS-CoV-2 virus. As a result, the commercialization of numerous efficient biosensors should be hastened. Aside from the approaches and biosensors given, innovative methods such as AI-based technologies, wearable biosen- sors for continuous public monitoring and single-use dis- posable sensors for individual testing should be researched for SARS-CoV-2 mass screening [49]. A. Supplementary Information The online version contains supplemen- tary material available at https://doi.org/10.1007/s00604-022-05167-y. B. Declarations Conflict of interest: The authors declare no competing interests.
References
[1] Almutairi MA. The coronavirus disease 2019 (COVID-19) outbreak: Challenges for pediatric dentistry. J Res Med Dent Sci 2021, 9:116-121 [2] https://www.who.int/emergencies/diseases/ novel-coronavirus-2019/question-and-answers- hub/q-a-detail/coronavirus-disease-covid-19 [3] Nagargoje B, Palod A, Dixi J, et al. Seroprevalence of COVID-19 in a city in India: A community-based cross-sectional study. J Res Med Dent Sci 2021; 9:48-53 [4] Singh DK, Garg A, Bagri S, et al. COVID-19 presentation and effect of associated co- morbidities on severity of illness at a dedicated COVID hospital in North India. J Res Med Dent Sci 2021; 9:49-54. [5] Tanu Singhal. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr 2020; 87:281–286. [6] Dane S, Akyuz M. Symptom spectrum and the evaluation of severity and duration of symptoms in patients with COVID-19. J Res Med Dent Sci 2021; 9:262-266. [7] Milibari AA. Current situation of coronavirus disease: (COVID-19) Review Article. Health Sci J 2020; 1-4. [8] https://covid19.who.int/ [9] Https://Www.Mohfw.Gov.In/ [10] Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–1207. [11] Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The lancet 2020; 395:514-23. [12] Faridi S, Niazi S, Sadeghi K. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ 2020; 725:138401–138401. [13] Chowdhury SD, Oommen AM. Epidemiology of COVID-19. J Dig Endosc 2020; 11:3–7. [14] To KKW, Tsang OTY, Leung WS.Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect Dis 2020; S1473-30992030196–1. [15] Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study Lancet 20203; 95:1054–1062. [16] Liu Y, Yan LM, Wan L.Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; S1473-30992030232–2. [17] Wu Y, Guo C, Tang L. Prolonged presence of SARS- CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–435. [18] Tong ZD, Tang A, Li KF, et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang province, China, 2020. Emerging Infect Dis 2020; 26:1052. [19] Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020; 395:514. [20] Liu YC, Liao CH, Chang CF, et al. A locally transmitted case of SARS-CoV-2 Infection in Taiwan. N Engl J Med 2020; 382:1070. [21] Chen D, Xu W, Lei Z et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: A case report. Int J Infect Dis 2020; 93:297-9. [22] Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan. Euro Surveill 2020; 25. [23] Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility-King County, Washington. Morb Mortal Wkly Rep 2020; 69:377. [24] Wang Y, Liu Y, Liu L, et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis 2020; 221:1770. [25] Sutton D, Fuchs K, D\'Alton M et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382:2163. [26] Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the icelandic population. N Engl J Med 2020; 382:2302. [27] Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : A narrative review. Ann Intern Med 2020; 173:362. [28] Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: A systematic review. Ann Intern Med 2021; 174:655. [29] https://www.niid.go.jp/niid/en/2019-ncov-e/ 9407-covid-dp-fe-01.html [30] Sakurai A, Sasaki T, Kato S, et al. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med 2020; 383:885. [31] Tabata S, Imai K, Kawano S, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: A retrospective analysis. Lancet Infect Dis 2020; 20:1043. [32] Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081. [33] Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA 2020; 323:2191. [34] Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in southern connecticut. JAMA 2020; 323:2520. [35] Louie JK, Scott HM, DuBois A, et al. Lessons from mass-testing for coronavirus disease 2019 in long-term care facilities for the elderly in San Francisco. Clin Infect Dis 2021; 72:2018. [36] Kasper MR, Geibe JR, Sears CL, et al. An outbreak of covid-19 on an aircraft carrier. N Engl J Med 2020; 383:2417. [37] Letizia AG, Ramos I, Obla A et al. SARS-CoV-2 Transmission among marine recruits during quarantine. N Engl J Med 2020; 383:2407. [38] Hu Z, Song C, Xu C, et al. Clinical characteristics of [39] 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020; 63:706. [40] Bajema KL, Oster AM, McGovern OL, et al. Persons evaluated for 2019 novel coronavirus United States, January. MMWR Morb Mortal Wkly Rep 2020; 69:166. [41] Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497. [42] Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395:507. [43] Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061. [44] Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J 2020; 133:1025. [45] Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475. [46] Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 2020; 323:1239. [47] Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance- United States. MMWR Morb Mortal Wkly Rep 2020; 69:759. [48] Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: Reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med 2020; 35:3063-6. [49] World Health Organization. Estimating mortality from COVID-19: Scientific brief. World Health Organization 2020. [50] Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis 2020; 101:138. [51] Ioannidis JPA. Reconciling estimates of global spread and infection fatality rates of COVID-19: An overview of systematic evaluations. Eur J Clin Invest 2021; 51:e13554. [52] Levin AT, Hanage WP, Owusu-Boaitey N, et al. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta- analysis, and public policy implications. Eur J Epidemiol 2020; 35:1123. [53] Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052-2059. [54] Myers LC, Parodi SM, Escobar GJ, et al. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 2020; 32:2195-8. [55] Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020; 395:1763. [56] Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. Br Med J 2020; 369. [57] Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: Prospective cohort study. Br Med J 2020; 369. [58] Drake TM, Docherty AB, Harrison EM, et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respiratory Critical Care Med 2020; 202:1656-65. [59] Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Network Open 2020; 3:e2012270. [60] Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for in-hospital complications associated with COVID-19 and Influenza-veterans health administration, United States. Morb Morta Week Rep 2020; 69:1528. [61] Xie Y, Bowe B, Maddukuri G, et al. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: Cohort study. Br Med J 2020; 371. [62] Verma AA, Hora T, Jung HY, et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ 2021; 193:E410-8. [63] Dennis JM, McGovern AP, Vollmer SJ, et al. Improving survival of critical care patients with coronavirus disease 2019 in England: A national cohort study. Crit Care Med 2021; 49:209. [64] Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med 2021; 16:90. [65] Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, outcomes, and trends of patients with COVID-19 related critical illness at a learning health system in the United States. Ann Intern Med 2021; 174:613. [66] Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Network Open 2021; 4:e210417-. [67] African COVID-19 critical care outcomes study (ACCCOS) investigators. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): A multicentre, prospective, observational cohort study. Lancet 2021; 397:1885. [68] Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths from COVID-19 and other causes in the US. JAMA 2021. [69] Islam N, Shkolnikov VM, Acosta RJ, et al. Excess deaths associated with covid-19 pandemic in 2020: Age and sex disaggregated time series analysis in 29 high income countries. Br Med J 2021; 373. [70] Linton N M, Kobayashi T, Yang Y. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J Clin Med 2020; 9:E538– E538. [71] Jantien AB, Don K, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China. Eurosurveillance 2020; 25:2000062. [72] Nishiura H, Linton N M, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis 2020; 93:284–286. [73] Zhang J, Litvinova M, Wang W. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: A descriptive and modelling study Lancet Infect Dis 2020; S1473-30992030230–9. [74] Du Z, Xu X, Wu Y, et al. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis 2020; 26:1341. [75] To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lanc Infect Dis 2020; 20:565-574. [76] Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. The Lanc 2020; 395:1054-62. [77] Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lanc. Infec Dis 2020; 20:656-657. [78] Wu Y, Guo C, Tang L. Prolonged presence of SARS- CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–435. [79] Wei WE, Li Z, Chiew C J, et al. Presymptomatic transmission of SARS-CoV-2-Singapore. Morb Mortal Wkly Rep 2020; 69:411–415. [80] https://neubergdiagnostics.com/blog/all-you- need-to-know-about-covid-19-diagnosis/ [81] Cascella M, Rajnik M, Aleem A, et al. Features, evaluation, and treatment of coronavirus (COVID-19). Stat Pearls 2021. [82] Gu¨ ner R, Hasanog? lu I, Akta? F. COVID-19: Prevention and control measures in community. Turk J Med Sci 2020; 50: 571–577. [83] Who-China-Joint-Mission-on-Covid-19-Final-Report.pdf. Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint- mission-on-covid-19-final-report.pdf (accessed June 1, 2020). [84] Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. (2020) 12:372. doi: 10.3390/v12040372 [85] Naming the Coronavirus Disease (COVID-19) and the Virus That Causes it. (2020). Available online at: https://www.who.int/emergencies/diseases/novel- coronavirus-2019/technical-guidance/naming-the-coronavirus-disease- (covid-2019)-and-the-virus-that-causes-it (accessed June 7, 2020). [86] Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. (2020) 13:667–73. doi: 10.1016/j.jiph.2020. 03.019 [87] WHO Director-General’s opening remarks at the media briefing on COVID-19. (2020). Available online at: https://www.who.int/dg/speeches/detail/who- director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-$-$11-march-2020 (accessed June 7, 2020). [88] Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433 [89] Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. (2020). doi: 10.1016/j.ajog.2020.07.049. [Epub ahead of print]. [90] Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. doi: 10.1016/j.jinf.2020.02.018 [91] Cave E. COVID-19 super-spreaders: definitional quandaries and implications. Asian Bioeth Rev. (2020) 12:235–42. doi: 10.1007/s41649-020-00118-2 [92] Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. (2020) 12:e7560. doi: 10.7759/cureus.7560 [93] Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID- 19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8 [94] Veerdonk F van de, Netea MG, Deuren M van, Meer JWM van der, Mast Q de, Bruggemann RJ, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. eLife. (2020) 9:e57555. doi: 10.7554/eLife.57555 [95] Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037 [96] Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220, 1–13. doi: 10.1016/j.trsl.2020.04.007 [97] Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV- 2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199 [98] Ottestad W, Seim M, Mæhlen JO. COVID-19 with silent hypoxemia. Tidsskr Den NorLegeforening. (2020) 140. doi: 10.4045/tidsskr.20.0299 [99] Couzin-Frankel J. The mystery of the pandemic’s “happy hypoxia.” Science. (2020) 368:455–6. doi: 10.1126/science.368.6490.455 [100] Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032 [101] Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. (2020) 30:4381–9. doi: 10.1007/s00330-020-06801-0 [102] Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Neck Surg. (2020) 163:3–11. doi: 10.1177/0194599820926473 [103] Krajewska J, Krajewski W, Zub K, Zaton´ski T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol. (2020) 277:1885–97. doi: 10.1007/s00405-020-05968-y [104] Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x [105] Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. (2020) 126:1443– 55. doi: 10.1161/CIRCRESAHA.120.317055 [106] Clerkin Kevin J, Fried Justin A, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. (2020) 141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941 [107] Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. (2020) 14:247–50. doi: 10.1016/j.dsx.2020.03.013 [108] Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950 [109] Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. (2020) 31:1003–8. doi: 10.1111/jce.14479 [110] Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585 [111] Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5 [112] Patients Taking ACE-i and ARBs who Contract COVID-19 Should Continue Treatment, Unless Otherwise Advised by Their Physician. American Heart Association (2020). Available online at: https://newsroom.heart.org/ news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should- continue-treatment-unless-otherwise-advised-by-their-physician (accessed June 27, 2020). [113] Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID-19 patients receiving renin- angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020). doi: 10.22541/au.158880148.8425 0526. [Epub ahead of print]. [114] Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. (2020) 51:843–51. doi: 10.1111/apt.15731 [115] An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, et al. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset. Rochester, NY: Social Science Research Network (2020). Available online at: https://papers.ssrn.com/abstract=3532530 (accessed June 27, 2020). doi: 10.2139/ssrn.3532530 [116] Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of covid-19 patients with digestive symptoms in hubei, china: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. (2020) 115, 766–73. doi: 10.14309/ajg.0000000000000620
Copyright
Copyright © 2023 Prof. Amrata D Suryawanshi, Arati R Gite, Anjali S Malge, Dnaneshwar D Dongare, Apurva V Andhare, Bhagwat D Somwanshi, Dipanjali D Raje. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET56107
Publish Date : 2023-10-11
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

