Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Artificial Intelligence Integration in Biochips: Enhancing Diagnostics and Precision Medicine
Authors: Servesh Gupta, Pratik Tawde
DOI Link: https://doi.org/10.22214/ijraset.2024.65255
Certificate: View Certificate
Abstract
The integration of biochips with AI opened up new possibilities and is expected to revolutionize smart healthcare tools within the next five years. The combination of miniaturized, multi- functional, rapid, high-throughput sample processing and sensing capabilities of biochips, with the computational data processing and predictive power of AI, allows medical professionals to collect and analyze vast amounts of data quickly and efficiently, leading to more accurate and timely diagnoses and prognostic evaluations. Biochips, as smart healthcare devices, offer continuous monitoring of patient symptoms. Integrated virtual assistants have the potential to send predictive feedback to users and healthcare practitioners, paving the way for personalized and predictive medicine. This review explores the current state-of-the-art biochip technologies including gene-chips, organ-on-a-chips, and neural implants, and the diagnostic and therapeutic utility of AI-assisted biochips in medical practices such as cancer, diabetes, infectious diseases and neurological disorders. Choosing the appropriate AI model for a specific biomedical application, and possible solutions to the current challenges are explored. Surveying advances in machine learning models for biochip functionality, this paper offers a review of biochips for the future of biomedicine, an essential guide for keeping up with trends in healthcare, while inspiring cross-disciplinary collaboration among biomedical engineering, medicine, and machine learning fields.
Introduction
I. INTRODUCTION
In clinics, patients commonly seek medical assistance during regular check-ups or when they observe changes and new symptoms related to their well-being. However, seeking timely medical attention upon the onset of symptoms significantly impacts treatment success and recovery rates. Therefore, advances in biochips for highly- sensitive and rapid diagnostics, and novel therapeutic systems such as targeted drug delivery platforms and organ- on-a-chip disease models play a crucial role in the early diagnosis and treatment of diseases, and promise to shape the future of biomedicine Up to date, artificial intelligence (AI) algorithms and machine learning (ML) models have proven to facilitate and enhance single-cell analysis, imaging and image detection, bioprocess design, biomarker discovery, and big data analysis for genomics, metabolomics, proteomics and channel-omics.
In the rapidly evolving field of medicine, the advancements of biochip technology and AI have led to revolutionary advancements in healthcare tools, from virtual medical assistants to AI-powered diagnostics, facilitated disease screening, reduced time-consuming tasks and number of tests, assisted clinical decision-making and potentially enhanced precision and efficiency of the diagnosis and treatment of diseases.
Through the integration of biochip technology with AI, healthcare professionals can gather, integrate, and interpret data efficiently, and address increasing workloads and complexity of clinical data by analyzing patterns that support evidence-based approaches to patient care.
In addition to the AI-assisted diagnosis and treatment strategies, the real-time support by virtual assistants for continuous monitoring and guidance of patients has the potential to significantly improve the healthcare system.
In this paper, we aim to delve into the advances in biochips and AI-driven functionality in significant medical applications, such as cancer, diabetes, infectious diseases. neurological disorders and drug discovery
Through a comprehensive analysis of biochip technology and the transformative impact of AI/ML on novel healthcare tools, our goal is to shed light on further innovation and revitalize cross-disciplinary collaboration in this rapidly evolving field.
A. Architecture
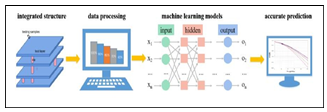
Fig. 1. The working mechanism of the Biochip Platform Integrated System
B. Prototype
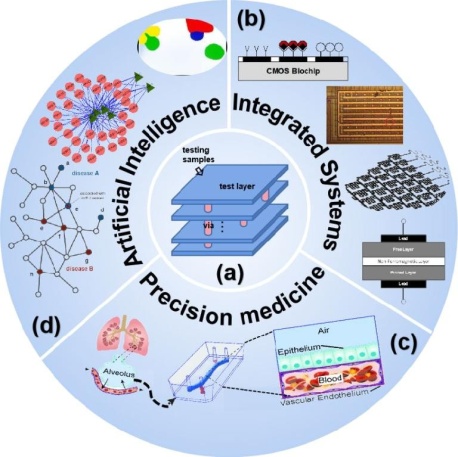
Fig.2.Bio-detectionsystemsolutionsinartificialintelligence and integrated systems
II. UTILITY OF AI-ASSISTED BIOCHIPS IN MEDICAL APPLICATIONS
In the 21st century, there has been remarkable advancement in bio-sensing technology. Advances in micro-fabrication and miniaturization of devices, enable the integration of multiple smart & small-scale systems to enhance bio-sensing capabilities, such as sample processing, rapid and sensitive-detection, and also instant-feedback with wireless communication (Fig. 3). Although lab-on-a-chip platforms contribute to biomedicine with point-of-care testing and high-throughput analyses, one of the challenges is the complexity of the data for the analysis, which makes it difficult for practitioners to interpret and utilize it effectively. In addition, real-time processing of the data obtained from continuous monitoring of vital signs and biomarkers could be important for assisting disease diagnosis in clinics. AI algorithms can rapidly analyze a vast amount of datasets obtained using biochips, discerning patterns and anomalies indicative of various diseases, enabling efficient and accurate testing for diseases such as cancer and infectious diseases, and also ensuring microbial safety. In addition, AI/ML integrated biochips can enable real-time monitoring and analysis of biological processes, paving the way for personalized medicine and improved patient outcomes. Moreover, the AI/ML integrated lab-on-a-chip platform scan reduce the dependence on skilled data analysts, making it more suitable for implementation in resource-limited areas
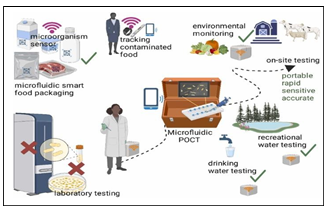
Fig.3.Biochiptechnologyformicrobialpoint-of-caretesting (POCT)
Biochips, particularly organ-on-a-chip, offer many advantages for disease modeling and drug testing, where they can reduce the number of tests and provide an alternative to animal testing . For tackling challenges in data processing and analysis, researchers can utilize AI and machine learning (ML) algorithms, which can greatly improve the automation of data analysis, pattern recognition, and prediction of outcomes, that paves the way for the simplicity, efficiency and accuracy for medical applications.
To date, for medical applications including medical imaging such as MRI, ultrasound, computer tomography and biological big data analysis, deep learning (DL) has demonstrated superior performance with high-quality video- rate imaging, high-throughput image acquisition from multiple chambers of the biochips, rapid image analysis and facilitating predictive medicine. Nevertheless, the limited availability of clinical samples remains a significant obstacle for training a model. In the future, AI-assisted imaging chips with data processing capability and analytical precision can be useful for the early detection of diseases, understanding of individual patient profiles, and the development of personalized therapies
A. Cancer Diagnosis
Cancer continues to be a major global health concern, and early cancer diagnosis plays a significant role in ensuring effective treatment. For the early diagnosis, DNA microarrays and gene chips can be useful to analyze thousands of genes at once, revealing insights into tumor gene expression patterns. However, cross-hybridization between similar sequences, or a mutation near the binding site, may cause false-positive results and decrease the accuracy. Using predictive AI/ML models can improve target-specific DNA probes and the sensitivity of assays. Furthermore, AI-assisted biochips can facilitate high- throughput testing, collecting vast amounts of genomic data to identify unique genetic mutations in cancer cells
The utility of AI has proven to be particularly impactful in biomedicine and adopted by important research and pharmaceutical companies to predict biological behaviors and to improve the diagnosis and treatment of cancer. While biochips can analyze genetic mutations or protein expressions in cancer cells, AI algorithms are then used to interpret this data, identifying atterns and classifying tumors more accurately, ultimately leading to a more precise diagnosis. Recently, Joshi et al. developed an ML-assisted biochip for single-cell analysis in cancer research, focusing on cellular heterogeneity within tumors. The developed method combines ML with inkjet printing and microfluidics technology to provide a compact, cost-effective platform for high-throughput single-cell analysis based on their electrical properties
B. Diabetes Monitoring And Drug Delivery
Recent advances in wearable or implantable biochips may enable continuous monitoring of glucose levels in interstitial fluid, providing real-time data to the user for timely adjustments in insulin dosages or dietary interventions based on real-time data. For instance, Olivo et al. developed an Iron IC Patch that utilizes a class-E power amplifier to wirelessly transmit power to implanted sensors by driving the transmitting coil . The system, with centimeter-scale dimensions, is intended to be integrated into a skin patch and positioned on the surface of the implantation site. It is capable of transferringupto15mWwithina6mmrangeinair. Powered by two rechargeable lithium-ion polymer batteries, the system offers a standby autonomy of 10hours and is found suitable for powering implantable biosensors intended for glucose and lactate monitoring AI can aid in diabetes monitoring by analyzing the complex data generated by biosensors, identifying patterns and predicting glucose levels based on multiple factors such as diet, exercise, and medication. Such integration of AI with biochips may enable personalized and precise diabetes management, allowing patients to make informed decisions about their diet, medication dosage, and lifestyle choices to maintain optimal glucose levels. Furthermore, AI can also assist in identifying trends and patterns in glucose data over time, helping healthcare providers tailor treatment plans for patients individually
C. Pathogen Detection And Drug Susceptibility Testing
Rapid and accurate detection of microorganisms is critical for containing outbreaks of infectious diseases and preventing their spread. Micro fluidic detection and drug testing platforms are poised to revolutionize conventional microbiology techniques by offering the capability to capture and monitor bacteria at single-cell resolution within a controlled micro environment. These platforms present numerous advantages, including hydrodynamic trapping of individual bacteria, concentration of microbial samples for sensitive detection and drug screening Lab-on-a-chip (LOC) systems, enables minimal manual interventions by micro fluidic operational steps, facilitate rapid detection of pathogenic bacteria, and wide-spectrum drug screening, making them valuable for environmental and clinical applications. For example, Wangetal.reporteda microfluidic method incorporating mmuno magnetic separation, fluorescent labeling, microfluidic chip technology, and smartphone-based analysis with detection limits as low as 58CFU/ML Salmonellaty phimurium.
Current limitations of the imaging-based methods are speed of image/video processing and image resolution. To enhance the sensitivity of bacteria detection, advances in computational power and imaging technology can be utilized. Alternatively, without necessitating high-resolution image input, AI-assisted single-bacteria imaging and analysis may also provide accurate diagnostic results, potentially providing similar advances in AI-assisted medical imaging Biochips and AI-assisted biochips offer significant advantages for pathogen detection in blood, serum, saliva, or sweat, and phenotypic and genotypic antibiotic susceptibility testing to aid decision making in clinics. AI algorithms can analyze the biochip data or individual- bacterium images, for identifying and sorting bacteria, leading to faster and more accurate diagnoses compared to traditional methods. This early detection allows starting an efficient drug therapy, and better management of infectious diseases.
D. Drug Development
AI can complement microfluidics by enhancing its analytic capabilities such as high-throughput testing of drug candidates, assessing toxicity, and optimization of experimental processes. AI-assisted biochips hold promise for expediting drug development timelines, improving efficacy, and enabling personalized medicine by accelerating the identification of effective drug candidates tailored to individual patient responses. For drug testing and development, the Quantitative Structure-Activity Relationship (QSAR) modeling plays a critical role in establishing mathematical relationships between chemical structures and biological activities. This approach enables the identification of therapeutic compounds from molecular databases using Gaussian processes (GPs). Recent advancements in drug development include using classification models such as intrinsic GP classification methods and a combination of GP regression and probit analysis. These models aid in cytotoxicity testing, environmental risk-benefit analyses, and selecting promising compounds for drug development. The effectiveness of a Quantitative Structure-Activity Relationship model heavily relies on the chosen learning algorithms and the hyperparameter configurations tailored to the specific training data. Nonetheless, no single algorithm generally outperforms others across all datasets, as stated by the No- Free-Lunch (NFL) theorem. For instance, Gedeck et al. suggested algorithmic applications, while Marchese Robinson et al. compared random forest with linear models like Support Vector Machines and Partial Least Squares Regression on benchmark datasets, favoring RF for its predictive accuracy and interpretability. Li et al. further extended this comparison, revealing that multitask models outperform single-task deep neural networks and traditional machine learning algorithms
III. COMPUTATIONAL MODELS IN ENHANCING BIOCHIP FUNCTIONALITY
Machine learning (ML) models can be trained on large datasets to recognize patterns, uncover meaningful associations that may not be easily apparent to specialists, identify specific biomarkers, predict outcomes (such as nanoparticle, immune cell, or microorganism behavior, drug release kinetics, and potential toxic based on biochip data, improves accuracy and efficiency of testing, and facilitates the feedback mechanism such as drug-response, patient- treatment plan and patient-healthcare practitioner communication in clinics ML methods include (1) linear regression; showing the relationship of input features and the target variable, (2) support vector machines; analyzing the data used for classification and regression, (3) random forests; multiple decision trees for regression and classification tasks, (4) Partial Least Squares Regression(PLSR);modeling relationship between two data matrices, (5) autoencoder networks, encode data into a compressed representation, then decoding it back to the original form and (6) neural networks. These models can be trained using extensive datasets produced by biochips. Using these models is particularly valuable for bio-sensing in several ways: categorization, detection of regularities, associations, and irregularities/anomalies within the data, and noise reduction of raw data, ultimately leading to enhanced accuracy in diagnosis and customized treatment recommendations
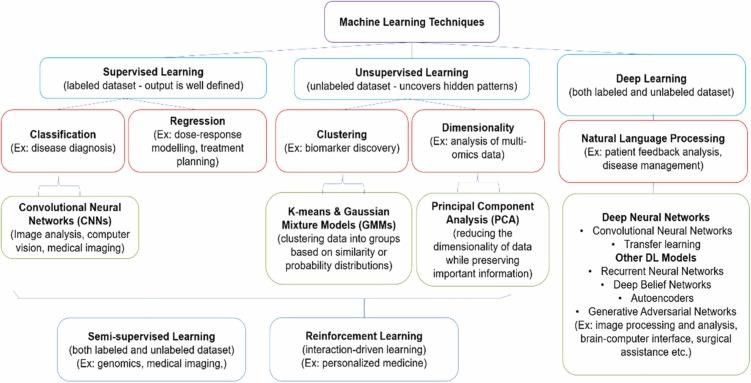
Fig 4. A simplified mind-map showing the classification of ML models for biomedical applications.
Advanced deep learning approaches such as convolutional neural networks (CNN) and recurrent neural networks (RNN) have the capacity to process large and complex biological data originating from biochips - for instance object identification, pattern recognition for gene expression profiles or protein interactions. ML provides direct, automatic, accurate, rapid support for biosensor readouts – useful for point-of-care detection or diagnosis. For developing an AI/ML models, there are several popular software tools and libraries including; Python, R (modules such as mixOmics hclust, prcomp, and muma), MATLAB, Roboflow, TensorFlow, OpenCV, scikit-learn, Keras, PyTorch, Microsoft Azure, Google Cloud AI Platform and IBM Watson. They offer a wide range of services such as image-preprocessing, auto labelling, and image detection, and application programming interface (API)s for building and deploying AI solutions When employing multiple algorithms to construct a model, it's important to carefully select a conceptually simple and easy-to-apply model that can be easily comprehended and validated by domain experts or end-users, fostering trust, accountability, and confidence in the model's reliability
The common AI models including Convolutional Neural Network (CNN), Long Short-Term Memory network (LSTMN), Support Vector Machine (SVM), and Multilayer Perceptron (MLP), which can enhance the effectiveness of diagnostics and the rape tics are summarized in the
Table1.CommonAIconceptswhichcanbe useful for biochip functionality
|
AI concept |
Feature |
Biochipapplications |
|
1. Supervised Learning |
Learning from labeled data |
Disease diagnosis, biomarker identification, drug screening |
|
2. Reinforcement Learning(RL) |
Learning through interaction with an environment |
Optimization of biochip experimental protocols |
|
3. Convolutional Neural Networks (CNN) |
Utilizes deep neural networks with convolutional layers |
Image segmentation, analysis, cell classification, detection |
|
4. Deep Learning(DL) |
Utilizes deep neural networks with fully connected layers |
Feature extraction, pattern recognition, data modeling, biomarker detection |
|
5. Bayesian Networks |
Probabilistic graphical models representing uncertain relationships |
Predictive modeling of disease outcomes, risk assessment |
|
6. Computer Vision(CV) |
Utilizes machine learning a lgorithms for image analysis and recognition |
Cell imaging, quality control,object tracking, detection |
|
7. Natural Language Processing (NLP) |
Processing and analyzing human language data using AI techniques |
Data interpretation, literature mining, knowledge extraction |
|
8. Transfer Learning |
Utilizes knowledge gained from one task to improve learning in another task |
Feature extraction, model initialization in biochip analysis |
|
9. Random Forest Model |
Ensemble learning method using decision trees and random feature subsets |
Classification, regression, feature selection |
A. Challenges and solutions of AI-based biochip implementation in clinics
It is important to follow the established standards and guidelines in maintaining consistency across biochip manufacturing processes and AI algorithms prior to testing patient cohort. Following this, the applicability of the developed AI-based biochip technology should undergo rigorous testing using a diverse and extensive patient data.
One important aspect is the protecting the data privacy and confidential information against unauthorized access or breaches in biochip-AI implementation. In addition, scalability- handling different amounts of samples without losing the performance and efficiency, is important for an AI-driven biochip application in healthcare.
Although AI enables use-friendly data-analysis, user training initiatives are essential to empower healthcare professionals with the necessary skills for the proficient utilization of biochip-AI technologies in clinical settings. More importantly, addressing cost barriers and ensuring equitable access to biochip-AI diagnostics is of great importance, and can be achieved with miniaturization of the biochips and possibly the integrated sample processing, culturing, or image acquisition platforms.
Conclusion
Advances in bio-microfluidic chips allow controlling and manipulating the fluid flow thereby the separation, concentration, and co-culture of cells in small-volumes, that provides benefits such as faster sample processing,improved sensitivity, reduction in the reagent consumption, and better mimicking the extracellular microenvironment . The integration of AI and lab-on-a-chip platforms further improves the automation and analysis of large datasets, enhancing efficiency in medical decision-making and advancements in healthcare AI-assisted biochip technology has the potential to revolutionize medicine by enabling rapid and accurate diagnosis, predictive medicine, personalized treatment, anda better understanding of disease pathogenesis . The integration allows for the automation and analysis of large datasets, enhancing efficiency in medical decision-making and advancements in healthcare. AI is also revolutionizing healthcare by automating administrative tasks analyzing health records for decision-making and assisting in clinical tasks like imaging interpretation and treatment planning. In addition, it is crucial to prioritize education and awareness about the benefits and challenges of biochip-AI technologies, thereby increasing acceptance and adoption from healthcare providers, patients, and young generations.
References
[1] Artificial intelligence in medical sensors for clinical decisions ACS Nano, 15 (3) (2021), pp. 3557-3567 [2] Microfluidic Diagnostics and Drug-Delivery Platforms for the Early Diagnosis and Treatment of Bacterial Diseases [3] T.S. Santra, A.U.S. Shinde (Eds.), Advanced Drug Delivery: Methods and Applications, Springer Nature, Singapore, Singapore(2023), pp. 201-228 [4] Fully integrated biochip platforms for advanced healthcare Sensors, 12 (8) (2012), pp. 11013-11060. [5] .Hydrogel sensors for biomedical electronics Chem. Eng. J., 481 (2024), Article 148317 [6] Current state of the art and future directions for implant ablesensors in medical technology: clinical needs and engineering challengesAPLBioeng.,7(3)(2023) [7] An integrated brain-machine interface platform with thousands of channels J. Med. Internet Res., 21 (10) (2019), Article e16194 [8] An integrated brain-machine interface platform with thousands of channels J. Med. Internet Res., 21 (10) (2019), Article e16194 [9] A machine learning-assisted nanoparticle-printed biochip for real-time single cancer cell analysis Adv. Biosyst., 4 (11) (2020), p.2000160 [10] SoftbioelectronicsbasedonnanomaterialsChem.Rev.,122(5)(2022),pp.5068-5143 [11] Deep learning and single-cell phenotyping for rapid antimicrobial susceptibility detection in Escherichia coli Commun.Biol., 6 (1) (2023), p. 1164 [12] AI-based detection, classification and prediction/prognosis in medical imaging::towardsradiophenomicsPETClin.,17(1)(2022),pp.183-212 [13] Review of Artificial Intelligence Techniques in Imaging Data Acquisition, Segmentation, and Diagnosis for COVID-19 IEEE Rev.Biomed. Eng., 14 (2021), pp. 4-15 [14] Artificial intelligence technologies in bioprocess: opportunities and challenges Bioresource. Technol., 369 (2023), Article 128451 [15] ArtificialintelligenceidentifiesnewcancerbiomarkersNat.Rev.Bioeng., 1 (5) (2023)
Copyright
Copyright © 2024 Servesh Gupta, Pratik Tawde. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65255
Publish Date : 2024-11-14
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

