Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
"Bi-Layer Tablets: Pioneering Novel Drug Delivery Systems"
Authors: Kanika ., Kashish , Mohammad Khalid, Megha Gupta, Shubham Pratap Singh, Dr. Ankit Kumar
DOI Link: https://doi.org/10.22214/ijraset.2024.65979
Certificate: View Certificate
Abstract
In order to ensure regulated administration of various medications with predetermined release profiles, bi-layer tablets have been developed. The pharmaceutical industry has become more interested in creating a bilayer tablet, which combines two or more Active Pharmaceutical Ingredients (API), in a single dose form over the past ten years in an effort to improve patient convenience and compliance. Bi-layer tablets can be used for the sequential release of two medications together, to separate two substances that are incompatible, or for sustained release tablets where the first layer is an immediate release dose and the second layer is a maintenance dose. A bilayer tablet is an enhanced, useful technology that addresses the shortcomings of a single-layered tablet.Currently, a number of pharmaceutical companies are working on creating bi-layer tablets. for a number of reasons, including marketing, therapeutics, and patent extensions. Such tablets are frequently developed and produced using modified but already-existing tablet presses in order to minimize capital investment.
Introduction
I. INTRODUCTION
Chemotherapy, or medication treatment, is the most commonly utilised procedure among the many modern approaches to illness and disease. It is often the suggested therapy approach andhas the widest range of applicability across the widest spectrum of illness conditions (1). For many years, the primary method of treating acute or chronic illnesses has been to administer medications to patients employing a range of dose forms for pharmaceuticals, such as creams,capsules, suppositories, tablet, pills, ointments, aerosols, liquids, and injectables as drug carriers (2,3).Two incompatible substances can be separated, two drugs can be released successively, andtablets with a sustained release that have an instant release dosage in the topmost layer, and bi-layer tablets that can be used for maintenance in the second layer (4).
In order to reach the ageing population, which requires goods that are both affordable and user-friendly, and to prolong the revenue-earning life of their major products, pharmaceutical companies are increasingly adopting medication delivery.The oral delivery medicine business is a $35 billion industry that is expected to increase by up to 10% a year, which is not surprising.For oral delivery drugs, oral delivery offers the most accurate market breakdown (5).
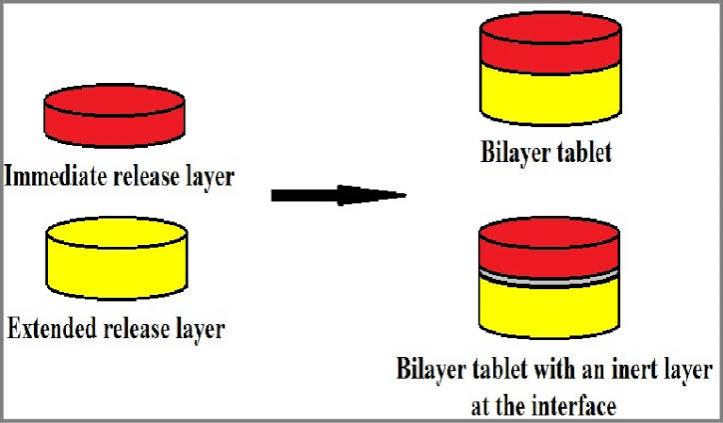
Figure1: Bi-layer tablet
Due of its simplicity in administering dosage, pain avoidance, correct dosage, formulation flexibility andpatient compliance,For systemic effects, 50–60% of all dosage forms are administered orally, which is a well acknowledged method of medication administration (6,7). When designing solid dosage administration, the primary challenge is to comprehend the origins of these problems at both the macro and micro scales and develop methods to solve them (8, 9).
A. Application of bi-layer tablets
- Two medications can be taken together in a bi-layer tablet for sequential release.
- Keep two substances that are incompatible apart.
- A sustained release tablet with a maintenance dose on the second layer and an immediate release initial dose on the first.
- Encouragingpatient convenience and Compliances.
- The upgraded, useful technology of the bilayer tablet addresses the drawbacks of thesingle-layer tablet.
- To provide the loading dosage and sustained dose of the same or different medications, bilayer tablets are utilised.(11)
B. Advantages of bi-layer Tablets
- They serve as a traditional technology's extension.
- The possible application of feed granules for a single entity.
- Distinguishing incompatible parts.
- Better patient adherence results in more effective medication regimens.(11,12)
- Extremely convenient
- Simple to ship and package
- Distinguishing incompatible parts.
- Better patient adherence results in more effective medication regimens.(11,12)
- High level of convenience
- Easy to package and ship
C. Disadvantage of bi-layer tablets
- Bilayer rotary presses are costly and add complexity.
- Reduced yield, layer separation, and inadequate hardness.
- Controlling the weight of individual layers is not precise.
- The layers are contaminated with one another.(13)
D. Objective of bi-layer tablets
- To regulate the rate at which one or two distinct active pharmaceutical ingredients are delivered;
- To supervise the release of API from one layer by utilising the functional feature of the outer layer; and to separate incompatible active pharmaceutical ingredients from one another.
- To alter the total surface area available for the API layer, sand wiching with one or two inactive strata can be used to construct erodable or swellable barriers for changed release.
- To develop innovative drug delivery techniques, such as chewing tools, buccal mucoadhesive delivery systems, floating tablets for gastro-retentive drug delivery, and dosage combinations of various active pharmaceutical ingredients.(14 to 19)
E. Bilayer Tablet Press Types
1) Pressing a tablet with one side
A press with a single side and a doublet feeder with two chambers that are separated from one another is the most basic design.With different strengths, each chamber is force-fed or gravity-fed to produce two distinct tablet layers.The die is filled with the first and second layers of powder as it passes under the feeder. The pill is then compacted in a single or double process.(20 and 21).

Figure 2: pressing a tablet with one side
2) Tablet Press with two Sides
Tablet weight is tracked and managed by compression force in the majority of double-sided tablet presses with automated production control. The control system measures the effective peak compression force at the major compression of each tablet or layer. When adjusting the die fill depth, the control system uses this recorded peak compression force as a signal to reject tablets that are not up to grade (23–24).

Figure 3: tablet press with two sides
3) Pressing A Two-Layer Tablet While Tracking Displacement

Figure 4: pressing a two-layer tablet while tracking displacement
There are significant differences between the compressions force-based approach and the displacement pill weight management principle.The appliedforce before compression determines thecontrol system's sensitivity when sensing displacement rather than the tablet weight.(25, 26; 27)
F. Bilayer Tablet Preparation [28–33]
In bilayer pills, one coat of the medication is ready for immediate release. The medicine is supposed to be released later by the second coat, either as a second dose or in an extended release form. Separate layers of each drug can be crushed to reduce the area of contact between two layers in order to create bilayer tablets containing two incompatible drugs. Between them, you can even place another layer of inert material. The intended drug release profile and enough mechanical strength are prerequisites for an appropriate tablet formulation.
G.Assessment of Bi-layer Tablets
- General Appearance: Consumer approval of a tablet depends on its overall "elegance,” general appearance and visual identity. The tablet's dimensions, form texture, colour, taste,physical defects, and this includes the uniformity and legibility of any identifying markers (34) in general.(35, 36)this includes any identifying markings' continuity and legibility (34) as well.(35,36)
- Size and Shape: The tablet's dimensions can be used to describe, track, and regulate its size and shape. (36–40)
- Tablet Thickness: When utilising filling equipment to count and reproduce look, tablet thickness is a crucial factor. Certain filling devices use the tablets' consistent thickness as a counting mechanism (41, 42).Ten tablets were measured for thickness using a micrometre (43–47).
- Variation in Weight: According to the official publications, standard procedures are followed.(48)
- Friability: The most common reasons why tablets break capping, chipping or breaking are friction and shock. (49, 50)The friability test, which assesses the tablet's resistance to abrasion during handling, shipping, and packaging, is closely linked to tablet hardness. % Friability = 1? (weight reduction / initial weight) X 100
- Hardness (Crushing Strength): The tablet hardness determines its resistance to abrasion, capping or breakage during storage, handling, and transit before to use.(51, 52)
- Temperature Dependent Stability Study: The bilayer tablets are packaged appropriately and kept for the duration of the study under the conditions according to ICH Guidelines for Accelerated Studies. (53) After 15 daysthe tablets were removed and examined for physical Flaws, such as dissolution, hardness, friability, and visual defects. (54)
References
[1] Banker G S. Drug products: Their role in the treatment of disease, their quality, and their Status and future as drug-delivery systems. In: Banker GS, Rhodes CT, editors. Modern Pharmaceutics. 2nd edition. Marcel Dekker. New York: Madison Avenue; 1990:1-21. [2] Manjula A, Selvam P, Nirmal R and Shakilabanu S. In vitro evaluation studies of crosslinked Chitosan microspheres containing rabeprazole Sodium. Int J Pharm Sci Res 2011; 2(6):1513-7. [3] Chein YW, editor. Novel Drug Delivery Systems. 2nd edition. Marcel Dekker. New York: Madison Avenue; 1992:139-96. [4] Shiyani B, Gattani S, Surana S, Formulation and evaluation of bi-layer tablet of Metoclopramide hydrochloride and Ibuprofen. AAPS Pharm Sci Tech 2008; 9(3):818-27. [5] Sampath Kumar K P, Bhowmik D, Chiranjib, Chadira M and Tripathi K K. Innovations in Sustained release drug delivery system and its market opportunities. J Chem Pharm Res 2010; 2 (1):349-60. [6] Dahiya A, Rohilla A, Rohilla S and Khan MU. Gastroretentive dosage forms: Review on Floating drug delivery systems. Int Res J Pharm 2011; 2(5):72-8. [7] Sharma A, Jain A, Purohit A, Jatav R and Sheorey RV. Formulation and evaluation of Aceclofenac fast dissolving tablets. Int J Pharm & Life Sci 2011;2 (4):681-6. [8] Buri P, Puisicux F, Doelker E, Benoit JP; Formes Pharma centiques Nouvelles, ed. Technique et Documentation, Lavoisier, Paris, 1985. [9] Wilding IR, Coupe AJ, Davis SS; The role of gamma scintigraphy in oral drug delivery. Advance Drug Delivery Review, 2001; 46(1-3)103-124 [10] Sachin SK, Viraj SS, Prajkta LU, Baviskar DT; Bilayer Tablet. International Journal of Pharmaceutical Sciences Review and Research, 2011; 9(1): 654-656. [11] Poddar SS, Abdul S; A flexible technology for modified release of drugs: multilayered tablets. Journal of Control Release, 2004; 97(3): 393– 405. [12] Liu L, Xu X; Preparation of bilayer-core osmotic pump tablet by coating the indented Core tablet. International Journal of Pharmacy, 2008; 352: 225–230. [13] Martindale W, Reynolds JEF; Martindale the Extra Pharmacopoeia. 31st edition, The Pharmaceutical Press, London, 1996: 936–937. [14] Gattani SG, Khabiya SS, Amrutkar JR and KushareSS. Formulation and evaluation of Bilayer tablets of metoclopramide hydrochloride and diclofenac sodium. P DAJ Pharm Scien Technol. 2012;66(2):151-60. [15] Reddy RK and SrinivasN. Formulation and Evaluation of bilayered tablets of losartanPotassium. IP. 2014;2(1):312-320. [16] Prakash VK and Shankar UB. Formulations and evaluation of ibuprofen floating tablets. IJPCBS. 2012;2(4):47-481. [17] Sandeep N and GuptaMM. Immediate drug release dosage form a review. JDDT.2013;3(2):155-161. [18] Pateriya A, Bhowmick M, Pandey GK, Josh A and Dubey BR. Formulation and Evaluation of player tablets of candesartan and hydrochlorothiazide for the treatment of hypertension. JDDT.2013;3(6):21-35. [19] Kumar HG, Jaganathan K, Kumar SR and Peruma lP. Formulation and in Vitroevaluation of bilayer floating tablets of metformin hydrochloride and Sitagliptin phosphate. Intern J Pharma Resea. 2013;5(1):53-5 [20] Available from http://www.elan.com/ [21] Available from http://en.wikipedia.org/wiki/bi- Layer_tablet_press. [22] Formulation and Evaluation of Fixed-Dose combination of Bilayer Gastroretentive Matrix tablet Containing Atorvastatin as Fast-Release and Atenolol as Sustained-Release. Available From http://dx.doi.org/1m 0.1155/2014/396106 . [23] Karudumpala S, Gnanaprakash K, Venkatesh B, Sankar P, Balaji G, Vidya Sagar N; Formulation and Evaluation of Gastro-Retentive Floating Bilayer Tablets of Nifedipine. AJADD, 20131(3): 341-357. [24] Hu L, Hu Q, Kong D; Formulation and in vitro-Evaluation of Aspirin and Isosorbide 5-mono-Nitrate sustained bilayer tablets. IJPSR, 2014; 5(3): 799-804. [25] Sharma SK, Mohan S, Jaimin Mi, Chauhan BS, Chatterjee A; Formulation and In-Vitro Evaluation of Bilayer Tablets containing pioglitazone HCl and Gliclazide for Type II Diabetes. Int J Pharm Tech Res., 2014; 6(2): 607-622. [26] Reddy KR, Srinivas N; Formulation and Evaluation of bilayered tablets of losartan potassium. Innovations in Pharmaceuticals and Pharmacotherapy, 2014; 2(1): 312-320. [27] Saif AA, Alburyhi MM, Noman MA, a Ala`a Almaktari MA; Formulation and evaluation of Trimetazidine hydrochloride and clopidogrel Bisulphate multi-unit solid dosage form. [28] Rudnic EM, Kottke MK; Tablet dosage form. In banker GS, Rhodes CT editors; Modern Pharmaceutics. 3rd edition, New York: Marcel Dekker Inc., 1996. [29] Breech AJ, Lucisano LJ, Franz RM; Investigation into substrate cracking of a film Coated bilayered tablet. J Pharm Pharmacol.,1998; 40(4): 282- 283. [30] Kalam MA, Humayun M, Parvez N, Yadav S, Garg A, Amin S et al.; Release kinetics of Modified Pharmaceutical dosage forms: A Review, Continental J. Pharmaceutical Sciences; 2007; 1: 30 – 35. [31] Li SP, Karth MG, Feld KM, Pendharkar CM, Willams RO; Evaluation of Bilayer tablet Machines. A Case study. Drug Dev Ind Pharm.,1995; 21(5): 571-590. [32] Shukla S, PandyaV , Bhardia P, Jonwal N, Bhatt D; Bi-layer Tablet system – An Innovative trend, Asian J Pharm Res., 2013;3(2): 49-56. [33] Park C.R, Munday D.L, Development and Evaluation of a biphasic buccal adhesive Tablet for nicotine replacement therapy, Int. J. Pharm. 2002; 237: 215–226. [34] Sungthongjeen S, Sriamornsak P, Puttipipatkhachorn S, Design and Evaluation of floating multi-layer coated Tablets based on gas formation, Eur. J. Pharm. Biopharm. 2008; 69: 255–263. [35] Deshpande R D, Gowda D V, Mahammed N, Maramwar D N, Bi-Layer Tablets- An Emerging Trend: A Review, IJPSR, 2011; Vol. 2(10): 2534-2544. [36] Lechman L, Liberman H A, Kanig J L, In., The Theory and Practice of Pharmacy, 3rd Ed., Varghese Publishing House, Bombay, 1987, p.430-453. [37] Robinson JR, Lee VH, Controlled Drug Delivery: Fundamentals and Applications 2nd Ed., Marcel Dekker, New York,1987,P.4-36. [38] www.durect.com [39] http://www.port/ technology.com [40] Aulton M. E, Bilayer Tablets In Pharmaceutics, The Science of dosage Form design, Churchill livingstone-2nd ed. 2002, pages 414-418 [41] Kale S.S, Saste V.S, Ughade P.L, Baviskar D.T, Bilayer Tablet, International Journal Of Pharmaceutical Sciences Review and Research , 9, (2011),25-28. [42] www.lifeclinic.com [43] Bhatt, Padmanabh, Osmotic delivery System for poorly soluble drug, The Drug Delivery companies Report Autumn/Winter 2004 ©PharmaVentures .Ltd 2004. [44] Notari R, Biopharmaceutics and Clinical Pharmacokinetics An Introduction, 3rd Ed., Marcel Dekker Inc. New York, 1980, P. 152-154. [45] Schaumann W, Pharmacokinetics of Isosorbide dinitrate and isosorbide-5-Mononitrate, Int. J. Clin. Pharmacology There Toxicology, 1989, 27, p.445–453. [46] Abshagen U, Sporl-Radun S, First data on the effects and pharmacokinetics of Isosorbide-5-mononitrate in normal man, Eur. J. Clin.Pharmacol.,1981, 19, p.423–429. [47] Hutt V, Bonn R, Fritschi E, Jaeger H, Evaluation of the pharmacokinetics and Absolute bioavailability of three Isosorbide- 5-mononitrate preparation in Healthy volunteers, Arzneim.-Forsch./Drug Res., 1995, 19, p.142–145. [48] Reiniger G, Blasini R, Bru ¨gmann U, Rudolph W, Toleranzentwicklung Hinsichtlich der anti ischamischen Wirkung von Isosorbid dinitratbei Regelmassiger, mehrfach taglicher Verabreichung, Herz, 1984,9, p.146–152. [49] Herrmann H, Kuhl A, Maier-Lenz H, Influence of the time of dosage of Isosorbide mononitrate on objective and Subjective angina pectoris parameters,Arzeim.- Forsch./Drug Res., 1988, 38, p.694–698. [50] Raparla D V,, Murthy TE, Formulation And evaluation of oral controlled release Glimepiride matrix tablets. Adv. Phamacol. Toxical. 2007, 8, p.59-62. [51] Indian Pharmacopoeia 1996. The Controller of Publication. Delhi, Vol-2, p-735. [52] Ang X, Cui F, Yonezawa Y and Hisakazu S. Preparation and evaluation of combination tablet containing incompatible active ingredients. Chem Pharm Bull 2003; 51(7):772-8. [53] Ullah I and Jain N B, inventors; Bristol-Mayer Squibb Company, assignee. Pharmaceutical Composition containing a combination of a statin and aspirin and method, US patent 6235311 B1. 2001 May 22. [54] Ozdemir N, Ordu S and Ozkan Y. Studies of Floating dosage forms of furosemide: In vitro and In vivo evaluations of bilayer tablet formulations.Drug Dev Ind Pharm 2000; 26(8):857-66.
Copyright
Copyright © 2024 Kanika ., Kashish , Mohammad Khalid, Megha Gupta, Shubham Pratap Singh, Dr. Ankit Kumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65979
Publish Date : 2024-12-17
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

