Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Biodegradation of Pharmaceutical Pollutants Using Fungal Enzyme
Authors: Nancy ., Uma Kumari
DOI Link: https://doi.org/10.22214/ijraset.2024.61672
Certificate: View Certificate
Abstract
The potential of filamentous fungi and their enzymes are investigated for bioremediating pharmaceutical pollutants from wastewater thereby addressing the growing concern of emerging pollutants in water resources. Among the various microorganisms, the current abstract discusses the role of fungi in degrading antibiotics and persistent pharmaceutical compounds. These fungi allow the biodegradation via the formation of highly oxidative hydroxyl radicals. It summarizes the role of both intra (cytochrome P450) and extracellular (laccase) enzyme systems for the bioremediation of emerging clinical pollutants. It emphasizes the importance of both biosorption and biodegradation for effective pollutant removal by breaking down pharmaceuticals (e.g. antibiotics) and other hazardous compounds (e.g. endocrine-disrupting compounds, polycyclic aromatic hydrocarbons). Although the enzymes CYP450 are not directly engaged, their metabolic pathways execute mechanisms such as oxidation, the formation of reactive intermediates, and chemical modification, all of which result in the degradation of clinical pollutants. Amongst all enzyme systems in the case of laccase which oxidise a wide spectrum of substrates, converts them to free radicals. Additionally, the role of recombinant enzyme production and transgenic strains for pollutant degradation has also been elaborated. These considerations emphasise the need for ongoing research and biotechnological advancements to address the growing concern of pharmaceuticals and personal care product pollutants in water systems.
Introduction
I. INTRODUCTION
The rise in shortage of water and an increase in wastewater generation has been a global issue (Villarín and Merel 2020). According to previous research, persistent pollutants are frequently not fully eliminated in traditional WWTPs and are released into aquatic ecosystems (Zarei-Baygi, Harb et al. 2019). Wastewater reuse is still limited due to a lack of understanding of the impact of untreated chemicals on the ecosystem. Even though PPCP concentrations in surroundings are modest (ng/L to g/L), even minor amounts may harm aquatic systems and human health (Krah, Ghattas et al. 2016) such as ecotoxicological consequences of various medications, such as estradiol and ethinylestradiol, or diclofenac, have already been observed (Lonappan, Brar et al. 2016). Pharmaceutical removal over wastewater treatment is currently inadequate (Yang, Ok et al. 2017), resulting in the presence of drugs not merely in treated wastewater effluents, but also in surface, ground, and, much less frequently, drinking water (Caban, Bialk-Bielinska et al. 2016). There have been several research investigations on the elimination of pharmaceutical residues. Enzymes catalyse these intra- or extracellular metabolic events, lowering the amount of activating energy needed to perform a given transformation (Gavrilescu, Demnerová et al. 2015). Due to the variety of metabolic processes and degrading enzymatic powers, as well as their capacity to work under a wide range of pH settings, bioremediation with fungus offers benefits over bioremediation with bacteria(Tomasini and León-Santiesteban 2019). Laccases and peroxidases (lignin peroxidase, manganese dependent peroxidase, multifunctional peroxidase) are examples of extracellular enzymes released by fungus (Lu, Zhang et al. 2016). Several studies have demonstrated that filamentous fungus may efficiently remove pharmaceutical chemicals, with clearance rates varying from 78% - 100% in a synthetic wastewater medium.Other critical characteristics include cultivation period, organic and inorganic elements concentration, static or submerged cultures, aeration, inducer concentration, and protease activation(Silva, Delerue-Matos et al. 2019).
Pharmaceuticals and personal care products (PPCPs) frequently include endocrine disruptors and trace organic compounds that can cause bioaccumulation, be toxic to aquatic life, and damage human health which have a negative impact(Malik, Bora et al. 2023) . Before proposing and evaluating any particular system, it is critical to examine the possibility that the solution may cause more significant environmental problems than the problem it is attempting to solve (Spina, Cordero et al. 2015). As a result, PPCP necessitates a unique treatment procedure that differs from standard techniques (Malik, Bora et al. 2023).
Fungi are equipped with non-specific enzymatic machinery and reaction mechanisms that are extremely appropriate for such processes to combat all of these responses (Harms, Schlosser et al. 2011). The hyphal shape is ideal for medicinal drug absorption and immobilisation.
II. KEY ENZYMES INVOLVED IN BIODEGRADATION
Furthermore, extracellular and intracellular fungal enzymes have limited specificity and can degrade medicinal molecules other than their normal substrates extensively (Akhtar and Mannan 2020). Such enzymes can be used to create new compounds, modify existing ones, or destroy harmful substances. In general, these enzymes have the ability to transform pharmaceutical chemicals into less harmful molecules (Arun, Madhavan et al. 2023). Fungi may also make biosurfactants, which are functionally diverse amphiphilic surfactant molecules with hydrophilic and hydrophobic moieties that interact across distinct polarity phases and provide interfacial protection. As a result, tension is decreased and intermolecular interactions are strengthened (Günther, Zibek et al. 2017).
Fungi produce a variety of enzymes, including laccase, lignin peroxidase (LiP) and manganese peroxidase (MnP), which can be used to effectively degrade pesticides, hydrocarbons, polychlorinated biphenyls, chlorinated, and phenolic compounds (Gaur, Narasimhulu et al. 2018). ). These enzymes can oxidise many substrates non-specifically by transfer of electrons to molecular oxygen (laccase) from organic substrates; by redox reactions using H2O2 as electron co-acceptors (type II peroxidase); or by, aromatic peroxidation, epoxidation and sulfoxidation, among others (UPO) (Karich, Ullrich et al. 2017). To boost its efficiency as a bioremediation agent, the enzyme manganese peroxidase has also been recombinantly generated in Aspergillus. A crude enzyme isolated from various fungus is unlikely to exhibit similar behaviour. In terms of removing different pollutants, LiP from Phanerochaete sordida was more efficient than LiP from pharmaceutical compounds.
A. Intracellular Enzyme
As multiple writers have mentioned, an internal mechanism is also required for converting certain xenobiotics (Moktali, Park et al. 2012), notably in fungi with poor LME secretion –Ascomycota (Aranda 2016). The sorption of pollutants inside the cell and the functioning of the intracellular enzyme seem to have increased the effectiveness of breakdown. Despite the fact that intracellular systems play a crucial part in IBP degradation, CYP450 had minimal influence on this process (Marco-Urrea, Pérez-Trujillo et al. 2009). To sum up, extracellular enzymes play no role in the initial stage of breakdown, although intracellular enzymes may accelerate the initial step of PhAC oxidation. As the native and recombinant enzymes have similar physical and biochemical properties, the researchers were able to devise a method for generating the enzyme in Aspergillus through heterologous gene expression. This fungus also possesses a complex internal mechanism for pollutant degradation as well as strong adaptability, resulting in a modified microorganism ideal for PhAC bioremediation. For compounds including CTL, SMX, DCF, IBP, CBZ, SMZ, NPX, and KEP, the role of CYP450 on the biodegradation of PhACs is being widely explored (Nguyen, Hai et al. 2014). T. hirsuta TrOC transformation, we hypothesised, is a multistep process that includes targeted molecule absorption with transformation using intracellular enzymes such as CYP450 (Verlicchi, Al Aukidy et al. 2012). In recent years, intracellular cytochrome P450 has been proposed as a potential TrOCs degrading enzyme(Chen, Wang et al. 2010). A comprehensive examination of the function of the biosorption in addition to biodegradation throughout fungal removal of PhACs, on the other end of the spectrum, would be necessary for the development of effective and stable fungal reactors over micropollutant removal. For example, the removal of DCF using whole-cell Trametes versicolor (TV). DCF revealed high initial sorption (13%), as well as high clearing efficiency (>90%)(Yang, Hai et al. 2013).
- Cytochrome 450
The cytochrome P450 system is a broad family of enzymes, mostly monooxygenases involved in a variety of processes such as hydroxylation, heteroatom oxygenation, dealkylation, C=C bond epoxidation, reduction, and dehalogenation(Díaz-Cruz, Gago-Ferrero et al. 2015). A comparison of fungal CYPomes with recently sequenced fungal genomes shows that fungi contain an abundance of CYPs from various families (Chen, Lee et al. 2014). However, only 12 P450 clans have been discovered in xenobiotic metabolism, including CYP52, CYP53, CYP505, and CYP55, among others (Moktali, Park et al. 2012). Cytochrome P450 monooxygenase has also been studied for its ability to detoxify potentially harmful pharmacological compounds such as antibiotics, -blockers, and anti-inflammatory medications (Olicón-Hernández, González-López et al. 2017). These cytochrome monooxygenases detoxified naproxen, an ecologically harmful pharmaceutical substance that made its way into the drinking water system as a result of its misuse in the treatment of some mammal illnesses.
The breakdown was accomplished by hydroxylation and demethylation (Aracagök, Göker et al. 2017). Various molecular techniques, such as vector expression systems, can be employed to further boost the capacity of monooxygenase enzymes to digest ecologically hazardous contaminants. The intercellular cytochrome P450 that converts ketoprofen into (2-[3-(4-hydroxybenzoyl)phenyl]-propanoic acid) and (2-[3-hydroxy(phenyl)-methyl)phenyl]-propanoic acid) via hydroxylation and reduction of the ketone group, respectively, has been reported to initiate ketoprofen degradation (Marco-Urrea, Pérez-Trujillo et al. 2010). The fungal cytochrome P450 system may be used as a flexible catalyst for the stereospecific and region-specific oxidation of non-activated hydrocarbons.
|
Enzyme |
Strain |
Pollutants |
Specific Pollutants |
Water Solubility(Mg/L) |
Concentration Of Phacs (Mg/L) |
Conversion(%) |
Reference |
|
CYP450 |
T. versicolor |
Anti-inflammatory, analgesic |
Diclofenac |
2.37 |
45 |
94 |
(Marco-Urrea, Pérez-Trujillo et al. 2010) |
|
|
T. versicolor |
Anti-inflammatory, analgesic |
Naproxen |
15.9 |
55 |
>95 |
(Marco-Urrea, Pérez-Trujillo et al. 2010) |
|
|
T. versicolor |
Antibiotic |
ciprofloxacin |
30000 |
2 |
97.7 |
(Prieto, Möder et al. 2011) |
B. Extracellular Enzymes
Because fungi are considered natural decomposers, they are permitted to synthesise a vast number of extracellular enzymes necessary for the bioconversion of a wide range of substrates and complexes (El-Gendi, Saleh et al. 2021). Laccases and peroxidases, such as manganese peroxidase and lignin peroxidase, are two prominent subclasses of extracellular fungal enzymes that have been researched to break xenobiotics and eliminate toxic phenolic components from the environment, wastewater and industrial areas (Berbee, James et al. 2017). Fungal species (such as Alternaria, Aspergillus, and Cladosporium) can produce extracellular enzymes (lignin peroxidase, manganese peroxidase, and laccase) that biodegrade complex synthetic dyes (Congo red, Poly R-478, methyl blue, indigo carmine), which broadens the industrial applications of fungal enzymes towards environmental sustainability (Sosa-Martínez, Balagurusamy et al. 2020).
|
enzyme group |
enzyme |
strain |
pollutant |
specific pollutant (conc) |
Water solubility(mg/L) |
Concentration of PhACs (mg/L) |
conversion(%) |
reference |
|
laccase |
Purified laccase |
Trametes versicolor |
Anti-inflammatory, analgesic |
Naproxen |
15.9 |
20 |
10 |
(Marco-Urrea, Pérez Trujillo et al. 2010) |
|
|
Purified laccase |
Trametes versicolor |
Antibacterial |
Sulfanilamide |
7500 |
172.2 |
10 |
(Schwarz, Aust et al. 2010) |
|
|
Crude laccase |
Trametes versicolor |
Anti-inflammatory, analgesic |
Ibuprofen |
21 |
0.01 |
38 |
(Tran, Urase et al. 2010) |
|
|
Purified laccase |
Trametes versicolor |
Antibacterial |
Sulfadimethoxine |
343 |
310.33 |
75.1 |
(Schwarz, Aust et al. 2010) |
Laccase: Multicopper Oxidase
Laccases (benzenediol or oxygen oxidoreductases) among the most abundant members of the multicopper oxidase family, which additionally includes ascorbate oxidase, dioxygenases and monoxygenases (Ye, Li et al. 2010). It is the most common subtype of multi-copper oxidases (MCOs), which have been identified to catalyse the process of oxidation of a wide range of substrate including phenolic substances and aromatic amines. To deconstruct the laccases and MCOs, an appropriate database was developed, concentrating on the species of fungi and other microbes for comparative investigation. All MCOs are made up of several domains, each having a structure similar to the one of cupredoxin (blue Cu protein designated differently from ferredoxin), the -barrel scaffolding. In contrast, tiny laccase16 is composed of two fields, whereas ceruloplasmin is composed of six domains that are indicating the different molecular development of MCO. Laccases, the biggest subclass of MCOs, catalyse the repetitive single-electron oxidation that involves four substrate molecules, includes polyphenols, polyamines, and some inorganic substances, with the concomitant reduction of molecules of oxygen to water in an ordinary reaction cycle. The laccase enzyme found mostly in wood rotting (white rot) fungus, is one of these enzymes. These fungus contain a diverse range of enzymes that help them breakdown lignin and hemicellulose in wood (Becker, Della Giustina et al. 2016). WWTPs based on traditional biological processes are primarily intended to remove nutrients and organic materials, rather than trace contaminants (Kang, Kim et al. 2021). Because fungal and bacterial laccases have different redox potentials, their activity and capacity to oxidise contaminants varies (Margot, Bennati-Granier et al. 2013). Laccases can breakdown phenolic compounds structures, methoxy-substituted phenols, diamines and a variety of inorganic chemicals. Non-phenolic or more complex molecules are not decomposed because they are incompatible with the active site (Becker, Della Giustina et al. 2016) Laccases oxidise a wide range of substrates, most often substituted phenols and aromatic amines, converting them to free radicals (Lassouane, Aït-Amar et al. 2019). Unstable chemical compounds and predominantly produced free radicals frequently initiate domino reactions, leading to complicated chemical changes of biological significance such as lignin production and breakdown (Llevot, Grau et al. 2016).
The entire process of laccase consists of one electron (1e), which successively oxidizes four molecules of the reducing substrates, and also two double electron (2e) reductions of oxygen atoms into their respective H2O molecules. Following this is a catalytic transfer of four H+ equivalents (Polyakov, Gavryushov et al. 2017). Laccase processes are seen physically, mechanistically, and dynamically as two half-reactions connected by an internal electron transfer (IET) step facilitated by catalytic copper ions located at both the T1 Cu and T2 Cu/T3 Cu trinuclear clusters (TNC) sites (Gabdulkhakov, Kostareva et al. 2018).
C. White-rot fungi
Anaerobic treatment is a potential strategy for the treatment of pharmaceutical manufacturing wastewaters that uses an anaerobic microorganism's biogas generation and biodegradation capabilities to adapt to changing environmental circumstances (Aydin 2016). Micropollutants, notably antibiotics, that were insufficiently removed during wastewater treatment operations can be found in significant amounts in sludge (Rodríguez-Rodríguez, Barón et al. 2012). T. versicolor effectively degraded pharmaceuticals discovered in sewage effluent, and the strategy they adopted led in a significant reduction in toxicity. Some PhACs, such as carbamazepine and clofibric acid, have shown resistance to conventional oxidation and photodegradation methods. WRF have emerged as prospective prospects for creating effective bioremediation approaches for PhACs because to their unspecific oxidative enzymatic system, which incorporates lignin-modifier enzymes, including laccase and peroxidises. Because the transformation products formed from the target pollutants during treatment may be more dangerous than the parent molecule, the treated effluents must be ecotoxicologically assessed (Cruz-Morató, Ferrando-Climent et al. 2013).
Advanced oxidation processes (AOPs) are technologies that produce highly explosive species such as radicals of hydroxyl (HO) which this radical is very oxidative and non-selective. Currently, HO is created chemically, photochemically, or electrochemically. However, it has recently been demonstrated that radicals of HO may be produced in biological processes using a BFenton-like mechanism that does not require the presence of hydrogen peroxide as an oxidant (Del Álamo, Pariente et al. 2018). The WRF's enhanced bio-oxidation method for the breakdown of the aforementioned medications via physiologically generated oxidising species (Marco-Urrea, Radjenovi? et al. 2010). This ABOP is based on the white-rot fungus (WRF) being incubated with chelated ferric ions and lignin-derived quinones. Under these circumstances, fungi might encourage the oxidation of DBQH to semiquinone radicals (DBQ) in the extracellular media by either of the WRF's lignin-modifying enzymes (laccase and peroxidases), as well as the conversion of quinone into hydroquinone (DBQH2) by intracellular quinone reductase. Then, by DBQ autoxidation catalysed 3+2+ by Fe, Fenton's reagent (Fe /Fe /H2O2) is formed in which Fe and superoxide anion radicals (O2) are produced (reactions 1 and 2) followed by O2 dismutation (reaction 3) (Del Álamo, Pariente et al. 2018).
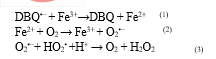
III. FACTORS INFLUENCING FUNGAL ENZYME-MEDIATED BIODEGRADATION
The temperature of sewage significantly influences biocatalytic systems, impacting their stability and reaction rates. As temperatures rise, reaction rates generally increase, but enzyme denaturation can occur above 40°C, depending on the fungal strain(Zhang and Geißen 2010). The efficacy of enzymes breaking down lignin in wastewater depends on various factors including temperature, pH, salinity, and metal content, which collectively affect microbial culture viability and enzyme activity (Yang, Hai et al. 2013). A preliminary investigation focused on diclofenac, a prevalent NSAID in wastewater, to determine optimal removal settings. Despite chitosan beads showing no adsorption impact, both free and immobilized laccase systems demonstrated a high drug degradation rate of 90% (Apriceno, Astolfi et al. 2019). Notable benefits of immobilized laccases include reusability, long preservation time, and resistance to temperature and pH variations. Additionally, a newly designed immobilized biocatalyst consistently catalyzed the breakdown of chlortetracycline at various influx rates(Wang, Cui et al. 2014).
Laccases derived from mesophilic proteins in animals inhabiting natural environments, such as T. versicolor, A. bisporus, P. ostreatus, and Rhus vernicifera, are economically viable. Seeking less expensive laccase sources, native edible mushrooms have shown potential(Patel, Shahane et al. 2019). These enzymes, found in fungi with diverse applications from pharmaceuticals to paper production, often require lower pH for optimal performance. While acidic pH levels suit most fungal laccases, certain applications necessitate neutral or alkaline conditions. Optimal enzyme activity is observed as pH and temperature increase until reaching an ideal level. Thermostable laccase isoforms, like those from Steccherinum ochraceum strain 1833, exhibit highest activity in the 75-80°C range (Camarero, Pardo et al. 2012). However, the reduced durability of immobilized laccase at 45°C compared to the free form remains challenging to explain. The immobilization technique employed, CLEAs, demonstrated effectiveness in decomposing tetracycline and bisphenol A, highlighting the potential of immobilized biocatalytic systems in pollutant degradation. Verification of the catalytic action of laccase-mediator systems on target substrates is critical due to varying environmental conditions in actual applications. Multiple studies have showcased the capacity of immobilized biocatalytic systems to effectively break down various pollutants of rising concern.
Conclusion
Over the past two decades, researchers have explored various techniques for removing pharmaceuticals and personal care products (PhACs) from wastewater. Enzymatic systems, particularly those associated with WRF-based treatment, offer an environmentally friendly approach to eliminating these contaminants. Factors such as temperature, pH, aeration, and dissolved components influence the efficacy of enzymatic removal. T. versicolor demonstrates promise in removing PhACs in fluid-bed bioreactors, achieving significant removal percentages, even under non-sterile conditions. Peroxidases show potential in degrading organic contaminants, although some chemicals remain resistant. Further studies on microbial physiology and genomics are needed to enhance drug and product removal in wastewater treatment. Genetically modified fungi hold potential in this field, but suitable microbial strains for bioremediation require identification through screening contaminated sites. Advanced biotechnological approaches, including molecular biology and bioinformatics, can enhance understanding and application of fungal bioremediation for organic pollutant degradation. Overall, enzymatic approaches offer a promising avenue for addressing PhAC contamination in wastewater, complementing chemical and physical methods.
References
[1] Akhtar, N. and M. A.-u. Mannan (2020). \"Mycoremediation: expunging environmental pollutants.\" Biotechnology reports 26: e00452. [2] Aracagök, Y. D., et al. (2017). \"Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites.\" Zeitschrift für Naturforschung C 72(5-6): 173-179. [3] Aranda, E. (2016). \"Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi.\" Current opinion in biotechnology 38: 1-8. [4] Arun, K., et al. (2023). \"Filamentous fungi for pharmaceutical compounds degradation in the environment: A sustainable approach.\" Environmental Technology & Innovation 31: 103182. [5] Aydin, S. (2016). \"Enhanced biodegradation of antibiotic combinations via the sequential treatment of the sludge resulting from pharmaceutical wastewater treatment using white-rot fungi Trametes versicolor and Bjerkandera adusta.\" Applied microbiology and biotechnology 100: 6491-6499. [6] Becker, D., et al. (2016). \"Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase–degradation of compounds does not always eliminate toxicity.\" Bioresource technology 219: 500-509. [7] Berbee, M. L., et al. (2017). \"Early diverging fungi: diversity and impact at the dawn of terrestrial life.\" Annual Review of Microbiology 71: 41-60. [8] Caban, M., et al. (2016). \"Current issues in pharmaceutical residues in drinking water.\" Current Analytical Chemistry 12(3): 249-257. [9] Chen, B., et al. (2010). \"Biosorption and biodegradation of polycyclic aromatic hydrocarbons in aqueous solutions by a consortium of white-rot fungi.\" Journal of Hazardous Materials 179(1-3): 845-851. [10] Chen, W., et al. (2014). \"Fungal cytochrome p450 monooxygenases: their distribution, structure, functions, family expansion, and evolutionary origin.\" Genome biology and evolution 6(7): 1620-1634. [11] Cruz-Morató, C., et al. (2013). \"Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor.\" Water research 47(14): 5200-5210. [12] Del Álamo, A. C., et al. (2018). \"Removal of pharmaceutical compounds from urban wastewater by an advanced bio-oxidation process based on fungi Trametes versicolor immobilized in a continuous RBC system.\" Environmental Science and Pollution Research 25: 34884-34892. [13] Díaz-Cruz, M. S., et al. (2015). \"Fungal-mediated biodegradation of ingredients in personal care products.\" Personal Care Products in the Aquatic Environment: 295-317. [14] El-Gendi, H., et al. (2021). \"A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges.\" Journal of Fungi 8(1): 23. [15] Gabdulkhakov, A., et al. (2018). \"Incorporation of copper ions into T2/T3 centers of two-domain laccases.\" Molecular Biology 52: 23-29 [16] Gaur, N., et al. (2018). \"Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment.\" Journal of Cleaner Production 198: 1602-1631. [17] Günther, M., et al. (2017). \"Fungal glycolipids as biosurfactants.\" Current Biotechnology 6(3): 205-218. [18] Gavrilescu, M., et al. (2015). \"Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation.\" New biotechnology 32(1): 147-156. [19] Harms, H., et al. (2011). \"Untapped potential: exploiting fungi in bioremediation of hazardous chemicals.\" Nature Reviews Microbiology 9(3): 177-192. [20] Kang, B. R., et al. (2021). \"Removal of pharmaceuticals and personal care products using native fungal enzymes extracted during the ligninolytic process.\" Environmental Research 195: 110878. [21] Karich, A., et al. (2017). \"Fungal unspecific peroxygenases oxidize the majority of organic EPA priority pollutants.\" Frontiers in microbiology 8: 1463. [22] Krah, D., et al. (2016). \"Micropollutant degradation via extracted native enzymes from activated sludge.\" Water research 95: 348-360. [23] Lassouane, F., et al. (2019). \"A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions.\" Bioresource technology 271: 360-367. [24] Llevot, A., et al. (2016). \"Selective laccase-catalyzed dimerization of phenolic compounds derived from lignin: Towards original symmetrical bio-based (bis) aromatic monomers.\" Journal of Molecular Catalysis B: Enzymatic 125: 34-41. [25] Lonappan, L., et al. (2016). \"Diclofenac and its transformation products: environmental occurrence and toxicity-a review.\" Environment International 96: 127-138. [26] Malik, S., et al. (2023). \"Fungal-Based Remediation in the Treatment of Anthropogenic Activities and Pharmaceutical-Pollutant-Contaminated Wastewater.\" Water 15(12): 2262. [27] Moktali, V., et al. (2012). \"Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes.\" BMC genomics 13(1): 1-13. [28] Marco-Urrea, E., et al. (2009). \"Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor.\" Chemosphere 74(6): 765-772. [29] Marco-Urrea, E., et al. (2010). \"Oxidation of atenolol, propranolol, carbamazepine and clofibric acid by a biological Fenton-like system mediated by the white-rot fungus Trametes versicolor.\" Water research 44(2): 521-532. [30] Margot, J., et al. (2013). \"Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase.\" New biotechnology 30(6): 803-813. [31] Nguyen, L. N., et al. (2014). \"Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor.\" International Biodeterioration & Biodegradation 95: 25-32. [32] Olicón-Hernández, D. R., et al. (2017). \"Overview on the biochemical potential of filamentous fungi to degrade pharmaceutical compounds.\" Frontiers in microbiology 8: 1792. [33] Polyakov, K., et al. (2017). \"Structural study of the X-ray-induced enzymatic reduction of molecular oxygen to water by Steccherinum murashkinskyi laccase: Insights into the reaction mechanism.\" Acta Crystallographica Section D: Structural Biology 73(5): 388-401. [34] Prenafeta-Boldú, F. X., et al. (2018). \"Fungal communities in hydrocarbon degradation.\" Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; McGenity, TJ, Ed: 1-36. [35] Rodríguez-Rodríguez, C. E., et al. (2012). \"Removal of pharmaceuticals, polybrominated flame retardants and UV-filters from sludge by the fungus Trametes versicolor in bioslurry reactor.\" Journal of hazardous materials 233: 235-243. [36] Silva, A., et al. (2019). \"The use of algae and fungi for removal of pharmaceuticals by bioremediation and biosorption processes: a review.\" Water 11(8): 1555. [37] Sosa-Martínez, J. D., et al. (2020). \"Synthetic dyes biodegradation by fungal ligninolytic enzymes: Process optimization, metabolites evaluation and toxicity assessment.\" Journal of hazardous materials 400: 123254. [38] Spina, F., et al. (2015). \"Removal of micropollutants by fungal laccases in model solution and municipal wastewater: evaluation of estrogenic activity and ecotoxicity.\" Journal of Cleaner Production 100: 185-194. [39] Tomasini, A. and H. H. León-Santiesteban (2019). \"The role of the filamentous fungi in bioremediation.\" Fungal Bioremediation: Fundamentals and Applications: 3-21. [40] Villarín, M. C. and S. Merel (2020). \"Paradigm shifts and current challenges in wastewater management.\" Journal of Hazardous Materials 390: 122139. [41] Yang, S., et al. (2013). \"Removal of trace organic contaminants by nitrifying activated sludge and whole-cell and crude enzyme extract of Trametes versicolor.\" Water Science and Technology 67(6): 1216-1223. [42] Yang, Y., et al. (2017). \"Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review.\" Science of the Total Environment 596: 303-320. [43] Ye, M., et al. (2010). \"Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and highly soluble expression.\" Applied microbiology and biotechnology 87: 1023-1031. [44] Zarei-Baygi, A., et al. (2019). \"Evaluating antibiotic resistance gene correlations with antibiotic exposure conditions in anaerobic membrane bioreactors.\" Environmental Science & Technology 53(7): 3599-3609.
Copyright
Copyright © 2024 Nancy ., Uma Kumari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET61672
Publish Date : 2024-05-06
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

