Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Innovative Approaches in Biodiesel Production from Waste Cooking Oils and Waste Animal Fats
Authors: Akash Phillip, Nandini Saini
DOI Link: https://doi.org/10.22214/ijraset.2024.64203
Certificate: View Certificate
Abstract
In the past few years, biodiesel has garnered considerable interest from researchers, government bodies, and industries as a sustainable, eco-friendly, and safe alternative fuel. Nonetheless, various feedstocks have been deemed impractical or unviable due to their exorbitant costs, primarily because they are utilized as food sources. Waste cooking oils (WCOs) and waste animal fats (WAFs) stand out as the most viable options for biodiesel feedstocks, despite their challenges, since treating such waste can be quite expensive due to stringent environmental regulations. A portion of these expenses might be mitigated through the production of bioenergy like biodiesel. This review article offers an extensive analysis of the pre-treatment processes and the application of WCOs and WAFs in biodiesel production. The predominant method for generating biodiesel is transesterification. Additionally, this paper emphasizes the purification and examination of the biodiesel produced, along with the latest innovations in biodiesel production from WCOs and WAFs. This review concludes that both WCOs and WAFs hold great potential as feedstocks for the production of biodiesel.
Introduction
I. INTRODUCTION
Current global energy consumption heavily favors fossil fuels, comprising about 84.3% of usage, while renewables like wind and solar account for merely 3.3% despite substantial capacity [1]. This dependency is exacerbated by geopolitical issues, notably the energy crisis stemming from the Russian invasion of Ukraine, which has inflated fuel prices and exposed energy access vulnerabilities in developing countries [1]. With a growing and urbanizing global population, energy demand is expected to rise, necessitating a transition to sustainable energy solutions to address climate change and mitigate greenhouse gas emissions [2]. The COVID-19 pandemic has highlighted the necessity for resilient energy systems, leading to a reassessment of energy policies towards renewable sources [3]. Research demonstrates that integrating renewable energy can substantially reduce fossil fuel use and CO2 emissions, thereby fostering sustainability and economic advantages [4]. hus, the shift to renewable energy is essential for confronting existing consumption patterns and future energy requirements.
Utilizing biofuels like biodiesel offers significant environmental advantages over fossil fuels, particularly in emissions reduction and carbon footprint. Studies show biodiesel can lower harmful emissions, including greenhouse gases, by up to 86% versus petroleum diesel [5]. The use of WCOs and WAFs as biodiesel feedstocks presents notable benefits. These feedstocks are plentiful and readily available, promoting cost-effective production and efficient supply chains [6]. Additionally, sourcing biodiesel from these materials mitigates competition with food resources, enhancing sustainability [5], [7]. Employing WCOs also addresses disposal-related environmental issues, thus contributing to waste management [8]. Biodiesel from these sources is renewable and biodegradable, providing an eco-friendly alternative to fossil fuels while lowering greenhouse gas emissions [9]. Furthermore, biodiesel blends reduce noise emissions and enhance air quality, positively impacting human health [10]. Nonetheless, challenges like elevated nitrogen oxide (NOx) emissions and reduced thermal efficiency must be resolved for wider acceptance [11]. The transesterification process used to convert these oils into biodiesel is adaptable, allowing diverse catalysts to improve production efficiency [12], [13]. Additionally, employing heterogeneous catalysts from waste materials promotes recycling and sustainability in biodiesel production [13].
II. WASTE COOKING OILS (WCOS) AND WASTE ANIMAL FATS (WAFS) AS FEEDSTOCKS
The chemical composition of waste oils and fats critically determines their viability for biodiesel production [14] [15]. Essential parameters encompass triglycerides, free fatty acids (FFA), and total polar matter (TPM) [16]. WCOs exhibits low FFA levels, promoting biodiesel yield, as evidenced by an 85.3% yield from WCOs with an acid value of 1.09 mg KOH/g [17].
The transesterification process converting triglycerides to biodiesel is influenced by the oil's fatty acid composition and impurities like TPM, which may increase viscosity and affect engine performance [18]. Furthermore, heterogeneous catalysts sourced from waste materials have shown potential in improving biodiesel yield, achieving up to 95% yield with innovative catalysts [19]. Likewise, the ideal chemical makeup of WAFs for biodiesel production is contingent upon multiple critical factors, including catalyst type, reaction conditions, and feedstock ratios [20]. Studies indicate that waste animal fat combined with catalysts such as sulfuric acid (H2SO4) and potassium hydroxide (KOH) yields variable results; H2SO4, for instance, attained a biodiesel yield of 65.7% at optimized conditions of 60°C with a 5:1 methanol-to-oil ratio [21]. Additionally, research employing a CaO-based catalyst from agricultural waste achieved a maximum yield of 99.20% under specific conditions of 79.68°C and a catalyst proportion of 5.0% [22].
III. SOURCES OF WASTE COOKING OILS & ANIMAL FAT WASTE
WCOs and WAFs are derived from numerous sectors, each providing distinct categories of byproducts that can be utilized for the production of biodiesel.
A. Waste cooking oils and waste animal fats generated by Households
This category encompasses cooking oils such as sunflower, canola, and olive oils that are employed in domestic culinary practices to fry various food items [23]. Following multiple applications, these oils are frequently discarded. Accumulated grease (Residual Cooking Grease) originating from frying pans or roasting trays, commonly associated with the cooking of meats, can gather over time and is typically disposed of as waste [23]. Waste animal fats that are released during the preparation of meats such as pork, cattle, or poultry (for instance, bacon drippings, poultry fat) have the propensity to accumulate within domestic environments [24].
B. Waste cooking oils and waste animal fats generated by Restaurants
Dining establishments frequently employ substantial volumes of oil for the deep frying of various foods, including but not limited to French fries, poultry, and fish [25]. These oils are routinely replaced and disposed of as waste materials. Oils utilized in the grilling or pan-frying processes for foods such as hamburgers, steaks, or vegetables also contribute to the generation of waste oils [26]. Animal fats such as Tallow, this fats is predominantly derived from bovine or ovine sources, which emerge as a byproduct of culinary practices and food preparation in dining establishments, particularly those that focus on meat-centric dishes. Another example of WAFs from restaurants is Lard, which is a fat from pork, frequently employed in traditional culinary techniques, and has the potential to be classified as waste following the cooking process [27].
C. Waste cooking oils and waste animal fats generated from food processing industries
The production of fried snacks like chips or processed foods often generates large amounts of used oils, which are discarded after multiple batches as well as oils used in preserving or canning foods, such as vegetable oils used in canned fish, can contribute to waste once they are no longer usable [28]. Animal fat trimmings from meat processing plants are rendered into tallow or lard [29]. These fats, once rendered, may not be entirely utilized and are considered waste. Large-scale cooking operations in food processing may generate significant amounts of WAFs from cooking meats, which can be collected and used for biodiesel production [25].
IV. CONVERSION TECHNOLOGIES
A. Process of Transesterification
Transesterification is a chemical process used to convert fats and oils (triglycerides) into biodiesel and glycerol as illustrated in Fig. 1. This process is fundamental in biodiesel production and involves the reaction of triglycerides with an alcohol, typically methanol or ethanol, in the presence of a catalyst [30].
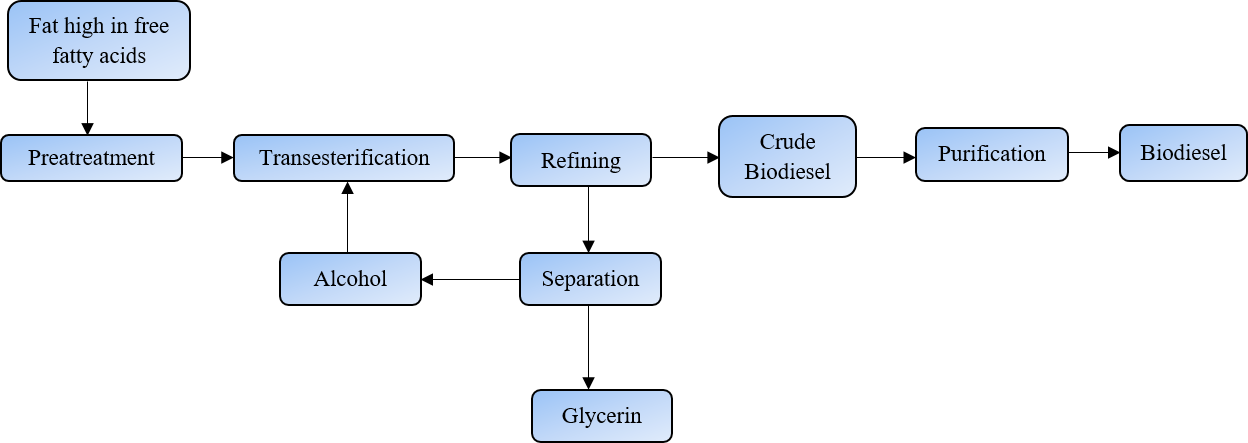
Fig. 1 Steps involved in the production of biodiesel through the process of transesterification.
- Mixing: The triglycerides (fats or oils) are mixed with the alcohol and the catalyst.
- Reaction/Transesterification: The alcohol reacts with the triglycerides, breaking the bonds between the glycerol and the fatty acids. This reaction results in the formation of fatty acid methyl esters (FAME) or fatty acid ethyl esters (FAEE), which are the chemical names for biodiesel, and glycerol as a byproduct. The overall reaction can be simplified as shown in eq (1).
Triglyceride + 3Alcohol → 3Biodiesel + Glycerol (1)
- Refining/Separation: Once the reaction is complete, the mixture is allowed to settle. Because biodiesel and glycerol have different densities, they naturally separate into two layers. Biodiesel, being less dense, forms the upper layer, while glycerol settles at the bottom.
- Purification: The separated biodiesel is then purified to remove any residual catalyst, alcohol, and other impurities. This typically involves washing with water and drying to ensure high-quality fuel.
- Glycerol Utilization: The glycerol byproduct can be further processed and purified for use in various industries, including pharmaceuticals, and cosmetics, and as a feedstock for other chemical processes.
B. Catalyst utilized in the process of transesterification
In the transesterification process used for biodiesel production, both acid and alkaline (base) catalysts play crucial roles. However, their suitability depends on the type of feedstock being used, particularly in terms of the free fatty acid (FFA) content [31].
1) Alkaline Catalysts (e.g., Sodium Hydroxide, Potassium Hydroxide):
Alkaline catalysts are generally faster in catalyzing the transesterification process. They are highly efficient at converting triglycerides to biodiesel under relatively mild conditions (lower temperatures and pressures). These catalysts are most suitable for feedstocks with low levels of free fatty acids (FFA), such as refined vegetable oils or used cooking oils that have been pre-treated to reduce FFA content [32].
Additionally,alkaline-catalyzed transesterification is carried out at lower temperatures (around 50-60°C) and requires less time to complete the reaction. However, they are sensitive to FFAs. When FFAs are present in significant amounts, they react with the alkaline catalyst to form soap, a process known as saponification. This reduces the yield of biodiesel and complicates the separation of biodiesel from glycerol, increasing processing costs and complexity [33].
2) Acid Catalysts (e.g., Sulfuric Acid, Hydrochloric Acid)
Acid catalysts are slower than alkaline catalysts in the transesterification reaction, requiring longer reaction times and higher temperatures (usually above 60°C). These catalysts are particularly effective for feedstocks with high free fatty acid content, such as waste animal fats, greases, or unrefined oils (e.g., crude palm oil). They can catalyze both the esterification of FFAs into esters and the transesterification of triglycerides, making them versatile in handling a broader range of feedstocks [32]. Additionally, acid-catalyzed reactions typically require more stringent conditions, including higher temperatures and longer reaction times, which can lead to higher energy consumption and costs. However, acid catalysts are not affected by the presence of FFAs. Instead of forming soap, they convert FFAs directly into biodiesel through esterification, which makes them ideal for feedstocks with high FFA content [34].
3) Nanocatalysts
Nanomaterial catalysts represent an innovative approach to enhancing the transesterification process in biodiesel production, overcoming traditional catalyst constraints [35]. Their elevated surface area and superior catalytic performance markedly enhance reaction efficiency, facilitating improved triglyceride conversion to fatty acid methyl esters (FAME) [36]. For example, barium oxide (BaO) nanoparticles have achieved a peak yield of 78.38% under optimized conditions, underscoring their efficacy as heterogeneous catalysts [37].
Moreover, advanced materials such as metal-organic frameworks (MOFs) and sulfonated carbon nanoparticles have been investigated, achieving over 90% conversion rates and streamlining industrial processes due to reduced operational demands [38]. Nevertheless, despite the benefits of these nanocatalysts, including reusability and diminished environmental impact, issues related to potential toxicity and the necessity for comprehensive studies on their long-term effects remain pressing. In conclusion, the adoption of nanomaterials in biodiesel production is promising for advancing sustainability and efficiency within the biofuel industry.
4) Homogeneous vs. Heterogeneous Catalysts
Homogeneous catalysts (liquid-based) typically offer faster reaction rates but are difficult to separate and reuse. Heterogeneous catalysts (solid-based), on the other hand, are easier to recover and recycle, but may have slower reaction kinetics. A comparision between homogeneous and heterogeneous catalysts is presented in the Table 1. Understanding these differences helps optimize catalyst selection based on the feedstock and production scale, improving both the economic and environmental performance of biodiesel production.
Table I A comparison between homogeneous and heterogeneous catalysts
|
Aspect |
Homogeneous Catalysts (Liquid) |
Heterogeneous Catalysts (Solid) |
References |
|
Reaction Efficiency |
High, fast reaction rates |
Lower, may require higher temperatures or longer times |
[39], [40] |
|
Separation and Purification |
Difficult, requires additional steps |
Easy, simple separation of solid catalyst |
|
|
Reusability |
Limited, often single-use |
High, can be reused multiple times |
|
|
Environmental Impact |
Higher, requires neutralization or disposal |
Lower, less waste and simpler disposal |
|
|
Corrosion |
High, can cause equipment wear |
Lower, less corrosive to equipment |
|
|
Cost |
Lower initial cost, higher operational cost due to separation |
Higher initial cost, lower operational cost due to reusability |
|
|
Commercial Adoption |
Well-established, widely used |
Emerging, less commercially adopted |
C. Supercritical transesterification
In supercritical transesterification, methanol or ethanol is used in its supercritical state to react with triglycerides in fats or oils, converting them into biodiesel (methyl or ethyl esters) and glycerol [41]. A fluid reaches a supercritical state when it is heated above its critical temperature and compressed above its critical pressure. Methanol becomes supercritical at a temperature of around 240°C and a pressure of about 8 MPa (80 bar) and ethanol becomes supercritical at a slightly higher temperature, around 243°C, and a pressure of about 6.4 MPa (64 bar) [42].
The triglyceride-containing feedstock (e.g., vegetable oil, animal fat) is preheated and mixed with methanol or ethanol, a high alcohol-to-oil ratio is typically used to drive the reaction toward complete conversion [43]. This mixture is then subjected to temperatures and pressures above the critical point of methanol or ethanol. This usually involves temperatures between 240°C and 350°C and pressures ranging from 8 MPa to 20 MPa [44]. Under these supercritical conditions, the methanol or ethanol dissolves the triglycerides, breaking them down into their constituent fatty acids and glycerol. The alcohol molecules then react with the fatty acids to form fatty acid esters (biodiesel) and glycerol. Unlike conventional methods, this process does not require a catalyst [45]. The reaction is typically rapid, often completing within minutes due to the enhanced solubility and reactivity of the supercritical alcohol. After the reaction, the pressure is released, and the reaction mixture is cooled. Biodiesel is separated from glycerol and any unreacted methanol or ethanol, which can be recovered and recycled back into the process [46].
D. Enzymatic transesterification
Enzymatic transesterification uses enzymes, specifically lipases, to catalyze the conversion of triglycerides (fats and oils) into biodiesel (fatty acid methyl esters or FAME) and glycerol [47].
The triglyceride-containing feedstock is mixed with an alcohol (methanol or ethanol) and the lipase enzyme. In some processes, the enzyme is immobilized on a solid support, allowing it to be easily separated and reused [48]. The enzyme catalyzes two main reactions:
- Hydrolysis: The lipase first hydrolyzes the triglycerides into free fatty acids and glycerol.
- Esterification/Transesterification: The free fatty acids react with the alcohol to form biodiesel and water, while the remaining diglycerides and monoglycerides are transesterified to form additional biodiesel and glycerol.
The reaction typically occurs under mild conditions, such as temperatures ranging from 30°C to 60°C, which is significantly lower than the conditions required for chemical catalysis. After the reaction, the mixture is separated. Biodiesel is collected, and the byproducts (glycerol and water) are removed. If an immobilized enzyme is used, it can be filtered out and reused for subsequent batches. The biodiesel may require minimal purification due to the absence of catalysts, reducing the need for extensive washing or neutralization steps [49].
V. TECHNIQUES FOR ENHANCING BIODIESEL PRODUCTION
A. Feedstock pretreatment
Feedstock pretreatment represents an essential phase in the process of biodiesel production, particularly when employing waste oils and fats as feed materials. These feedstocks frequently harbor impurities such as moisture, free fatty acids (FFAs), solid particulates, and various other contaminants that have the potential to detrimentally influence the efficiency and yield of the transesterification reaction [50] as presented in Table 2. Adequate pretreatment serves to ascertain that the feedstock meets the requisite quality standards for biodiesel production, thereby mitigating the likelihood of complications such as soap formation, catalyst deactivation, and suboptimal product quality [51].
Table II Different techniques for the pretreatment of waste oils and fats
|
Impurity |
Pretreatment Technique |
Purpose |
Method |
Applications |
References |
|
Solid Particles |
Filtration |
Remove solid particles and debris |
Passing oil through filters of varying mesh sizes |
Waste cooking oils, waste animal fats with solid impurities |
[51], [52], [53] |
|
Water (Free Water) |
Settling and Decantation |
Separate free water and heavy solids |
Allow oil to settle; decant or siphon off the top layer |
Waste oils and fats containing free water and large particles |
|
|
Fine Solids, Water |
Centrifugation |
Remove fine solids, water, and emulsified impurities |
Spinning oil at high speed to separate impurities |
More thorough separation when higher purification is required |
|
|
Phospholipids (Gums) |
Degumming |
Remove phospholipids that interfere with transesterification |
Adding water or acid to precipitate gums; filtration/centrifugation |
Vegetable oils with high phospholipid content (e.g., soybean) |
|
|
Water-Soluble Impurities |
Water Washing |
Remove water-soluble impurities, salts, and some FFAs |
Mixing oil with warm water; separating oil from water layer |
Reducing polar impurities; must avoid residual water in oil |
|
|
Residual Water |
Drying (Dehydration) |
Remove residual water that can inhibit transesterification |
Heating under vacuum or at moderate temperature; chemical drying agents |
Necessary after water washing or when feedstock has absorbed moisture |
|
|
Free Fatty Acids (FFAs) |
Acid Pretreatment (Esterification) |
Reduce FFAs by converting them into biodiesel precursors |
Treating with acid catalyst and alcohol to esterify FFAs |
Feedstocks with high FFA content (e.g., waste animal fats, waste grease) |
|
|
Residual Water |
Drying (Dehydration) |
Remove residual water that can inhibit transesterification |
Heating under vacuum or at moderate temperature; chemical drying agents |
Necessary after water washing or when feedstock has absorbed moisture |
B. Process Optimization
1) Reaction conditions for the efficient production of biodiesel
In order to maximize yield and quality, certain reaction conditions are necessary for efficient biodiesel manufacturing. The impact of various reaction conditions and the ideal parameters for effective biodiesel generation are explained in Table 3.
Table III The influence of reaction conditions on biodiesel production efficiency
|
Parameter |
Impact on Biodiesel Yield and Quality |
Optimal Range/Considerations |
References |
|
Temperature |
Higher temperatures increase the reaction rate by enhancing molecular interactions, leading to faster transesterification. Optimal temperatures can maximize biodiesel yield by driving the reaction to completion. Excessively high temperatures can cause unwanted side reactions, such as the formation of soap (especially with alkaline catalysts), or thermal degradation of biodiesel, reducing its quality.
|
Typically between 50°C and 65°C, depending on the feedstock and catalyst used. Temperatures above 65°C may lead to evaporation of alcohol (methanol or ethanol) and increased soap formation in alkaline-catalyzed processes. |
[54], [55], [56] |
|
Pressure |
In conventional transesterification, atmospheric pressure is usually sufficient, but in supercritical transesterification, high pressures are necessary to maintain the alcohol in a supercritical state. High pressure, particularly in supercritical processes, enhances the solubility of alcohol in oil, improving yield. High pressure can help prevent the formation of soap and other side reactions by maintaining the alcohol in a supercritical state, leading to a cleaner reaction and higher biodiesel purity.
|
For conventional processes: Atmospheric pressure. High pressure is particularly important in supercritical methanol or ethanol transesterification, where it helps achieve high yield and quality without the need for a catalyst. |
|
|
Catalyst Concentration |
Sufficient catalyst concentration is needed to initiate and sustain the transesterification reaction. Too little catalyst may result in incomplete conversion, lowering biodiesel yield, while too much can lead to excessive soap formation (in alkaline-catalyzed reactions), complicating separation and purification. Optimal catalyst concentration ensures high conversion of triglycerides to biodiesel while minimizing side reactions that can degrade quality. |
Typically, 0.5% to 1% weight of the oil for alkaline catalysts (e.g., sodium hydroxide, potassium hydroxide). Acid catalysts (e.g., sulfuric acid) may require different concentrations, particularly in pretreatment steps. Careful balance is needed to maximize yield without compromising biodiesel quality.
|
C. Emerging Technologies
1) Utilizing Biochar as a Catalyst for Biodiesel Production
Biochar, which is rich in carbon and is a byproduct of pyrolysis, is being investigated as a catalyst for producing biodiesel. It is highly appreciated for its extensive surface area, excellent porosity, and capacity to absorb impurities [57]. Scientists have created catalysts supported by biochar, such as biochar infused with metal oxides (e.g., CaO-biochar), which have demonstrated potential in catalyzing the conversion of waste oils into biodiesel [58]. These catalysts can be used again and are eco-friendly, which helps decrease the total expenses and waste linked to the procedure. Using biochar not only reuses biomass waste but also improves biodiesel production efficiency by supplying a steady, inexpensive catalyst [59].
2) Genetically Engineered Microorganisms for Biodiesel Production
Researchers are investigating genetically modified microorganisms for their capacity to effectively transform waste oils into biodiesel using enzymatic transesterification methods [60]. Modified strains of E. coli have been developed by researchers to produce lipase enzymes that can convert waste oils into biodiesel without any intermediate steps [61]. These genetically modified bacteria can carry out the complete transformation at low temperatures, minimizing the reliance on outside chemical catalysts and significant energy requirements [62]. Microbial catalysis provides a specialized and effective method of converting substances, which could reduce production expenses and energy needs. These systems have the potential to be expanded for use in industrial settings, providing a sustainable and renewable method for producing biodiesel [63].
VI. CHALLENGES IN BIODIESEL PRODUCTION
Various technical obstacles can affect the efficiency, yield, and overall sustainability of the biodiesel production process. Important concerns include variations in feedstock, loss of activity in the catalyst, and increasing the size of the process [64].
A. Feedstock variability
This variation may occur because of the utilization of various feedstocks (such as vegetable oils, WAFs, and WCOs) and variations in purity, free fatty acid (FFA) levels, moisture content, and impurity presence. Various raw materials have different amounts of FFAs, water content, and impurities, all of which can impact how well the transesterification process works. Elevated FFA levels, for instance, may result in the production of soap, which can decrease biodiesel output and make product separation more difficult. Differences in raw materials can result in variations in the ultimate quality of biodiesel, impacting characteristics like viscosity, cetane number, and cloud point [65].
B. Catalyst Deactivation
Catalyst deactivation happens when the effectiveness of a catalyst diminishes with time, decreasing its capability to aid the transesterification reaction. This might result from physical, chemical, or thermal causes. Impurities in feedstock like water, free fatty acids, and metal ions can render the catalyst inactive or harmful. Extended exposure to elevated temperatures can result in catalyst degradation, particularly in ongoing processes. A build-up of waste materials or unused raw materials on the catalyst's surface can obstruct active sites, leading to decreased catalyst effectiveness [65].
Deactivation causes a reduction in the efficiency of triglyceride conversion to biodiesel, leading to decreased yields and more waste. Additional catalysts might be necessary to reach the desired speed of the reaction, which could raise operating expenses and possibly result in more instances of side reactions such as the creation of soap. Regularly changing catalysts, particularly costly options such as heterogeneous or enzymatic ones, can lead to higher overall expenses in production [66].
C. Process Scaling
Process scaling involves overcoming difficulties that come with expanding the output capability of a biodiesel plant, and transitioning from smaller-scale testing to large-scale manufacturing. Enlarging the scale can result in challenges in upholding consistent reaction conditions (such as temperature, mixing, and catalyst concentration), impacting the uniformity of the product and its yield. Inadequate heat and mass transfer in bigger reactors can cause incomplete reactions or hot spots, resulting in decreased efficiency and potential safety problems. Expanding typically involves investing in bigger or more sophisticated machinery and making changes to infrastructure like storage and handling systems, which can be expensive and complicated [66].
VII. ENVIRONMENTAL AND ECONOMIC CONSIDERATIONS
Using WCOs and WAFs for biodiesel production offers significant environmental benefits, particularly in reducing waste and greenhouse gas emissions compared to conventional fossil fuels [67].
A. Waste Reduction
Redirecting waste oils and fats away from landfills decreases the space required for waste disposal, thereby reducing the environmental impact of waste management. Avoiding dumping oils and fats in sewage systems prevents blockages and decreases the chance of water pollution, which can negatively impact aquatic animals and disrupt water treatment procedures. Biodiesel production reduces the need for new feedstocks by repurposing waste materials, supporting the principles of a circular economy and efficient resource utilization [68].
B. Greenhouse Gas Emissions Reduction
The combustion of biodiesel leads to reduced net CO2 emissions due to the use of feedstocks from biological sources that absorb CO2 as they grow, balancing out the emissions released when the fuel is combusted. If not used, waste oils and fats can break down without oxygen in landfills, producing methane, a strong greenhouse gas. Transforming these resources into biodiesel rather than letting them decompose greatly decreases the amount of methane released into the environment. Biodiesel made from waste feedstocks plays a role in reducing carbon emissions to aid in achieving global and national goals in combating climate change [69].
C. Reducing our dependence on fossil fuels
Adding biodiesel to the energy mix decreases dependence on fossil fuels and helps create a more sustainable and robust energy system. A decline in the need for fossil fuels can result in lower environmental effects linked to their extraction, like habitat loss, water pollution, and air pollution caused by drilling and mining operations [70].
D. Cost analysis of using waste oils and fats versus virgin feedstocks
Recycled oils and fats are generally priced between $0.10 and $0.30 per liter, much more affordable than new oils such as soybean or palm oil, which are priced at around $0.70 to $1.00 per liter. The cost fluctuates depending on worldwide market factors, sometimes resulting in higher prices and reduced economic viability for large-scale biodiesel manufacturing [71].
Additional processing such as filtration and degumming is often necessary for waste oils to eliminate impurities such as free fatty acids and water content. Yet, the increased processing expenses are frequently balanced out by the inexpensive waste feedstocks. The cost of the transesterification process is consistent whether utilizing waste or virgin feedstocks, as triglycerides in oils react with methanol or ethanol [72]. Nevertheless, the higher FFA content in waste oils may require acid catalysts, leading to a slight increase in expenses.
Numerous governments offer financial assistance or tax breaks for producing biodiesel, especially when using leftover feedstocks. The Biodiesel Mixture Excise Tax Credit in the United States provides $1.00 for every gallon of biodiesel mixed with petroleum diesel. Producers who use waste oils may take advantage of carbon credits or other incentives that are designed to lower greenhouse gas emissions, thus enhancing the economic viability [73].
Research indicates that producing biodiesel from waste oils can cost 30-50% less than using virgin oils. Biodiesel derived from waste oils costs approximately $0.40 to $0.70 per liter, whereas virgin oil biodiesel is priced between $1.00 and $1.20 per liter. Current data shows that waste oils make up around 30-40% of the feedstock used for global biodiesel production, thanks to their cost-effectiveness and environmental advantages [74].
Conclusion
Overall, the chemical characteristics of waste oils, including their triglyceride content and FFA levels, are critical in determining their viability as biodiesel feedstocks. Overall, the optimal composition appears to be a blend of animal fat with effective catalysts and carefully controlled reaction parameters to maximize yield and efficiency. Furthermore, the use of WCO and WAF blends has shown promising results, with biodiesel yields reaching up to 92.34% when optimized for methanol-to-oil ratios and catalyst weight. Additionally, using waste oils and animal fat for biodiesel production offers substantial environmental benefits, including waste reduction, lower greenhouse gas emissions, reduced reliance on fossil fuels, and improved air quality. Collectively, these elements highlight the potential of WCOs and WAFs in propelling biodiesel as a feasible energy source.
References
[1] M. H. J. Bollen and A. Moreno-Muñoz, “The power grid as part of a sustainable energy system,” in Large Scale Grid Integration of Renewable Energy Sources: Solutions and technologies, IET Digital Library, 2024, pp. 1–29. doi: 10.1049/PBPO222E_ch1. [2] S. Pathak, T. Asthana, D. Singh Rataul, and N. Kaur, “Sustainable Energy Consumption Analysis through Data Driven Insights,” Int. J. Innov. Sci. Res. Technol. IJISRT, pp. 2386–2401, May 2024, doi: 10.38124/ijisrt/IJISRT24APR2218. [3] M. Shaheen, M. Anjum, F. Ahmad, and A. Anum, “Computational Data Analysis on Global Energy and COVID-19 Pandemic,” Int. J. Inf. Eng. Electron. Bus., vol. 15, pp. 1–17, Dec. 2023, doi: 10.5815/ijieeb.2023.06.01. [4] E. Soko?owska and J. W. Wi?niewski, “Sustainable energy consumption – Empirical evidence of a household in Poland,” Energy Strategy Rev., vol. 53, p. 101398, May 2024, doi: 10.1016/j.esr.2024.101398. [5] S. Dubey, E. Godara, E. Dar, E. Sharma, and E. Kawal, “A Study of Biodiesel as Opportunities for Environmental Improvement,” Int. J. Adv. Res. Sci. Commun. Technol., pp. 465–470, Apr. 2023, doi: 10.48175/IJARSCT-9238. [6] K. V. Yatish, M. Mounesh, C. R. Manjunatha, K. S. Sharath Kumar, and H. S. Lalithamba, “Overview of Feedstocks for Biodiesel Production”, Accessed: Aug. 16, 2024. [Online]. Available: https://books.rsc.org/books/edited-volume/2199/chapter/8035323/Overview-of-Feedstocks-for-Biodiesel-Production [7] M. Manzanera, M. Molina-Muñoz, and J. Gonzalez-Lopez, “Biodiesel: An Alternative Fuel,” Recent Pat. Biotechnol., vol. 2, pp. 25–34, Feb. 2008, doi: 10.2174/187220808783330929. [8] E. Rahadianti, Y. Yerizam, and M. Martha, “Biodiesel Production from Waste Cooking Oil,” Indones. J. Fundam. Appl. Chem., vol. 3, pp. 77–82, Oct. 2018, doi: 10.24845/ijfac.v3.i3.77. [9] D. Debnath, J. Whistance, P. Westhoff, and M. Helmar, “Chapter 9 - Consequences of US and EU biodiesel policies on global food security,” in Biofuels, Bioenergy and Food Security, D. Debnath and S. C. Babu, Eds., Academic Press, 2019, pp. 165–178. doi: 10.1016/B978-0-12-803954-0.00009-7. [10] A. R. Bhutto, T. Hussain, L. Kumar, S. Bhangwar, and A. H. Shah, “Comparative Investigation of Performance Analysis & Carbon Emission of Biodiesel and Conventional Fuel,” J. Appl. Eng. Technol. JAET, vol. 7, no. 2, Art. no. 2, Dec. 2023, doi: 10.55447/jaet.07.02.118. [11] K. Lee and H. M. Cho, “Effects of Castor and Corn Biodiesel on Engine Performance and Emissions under Low-Load Conditions,” Energies, vol. 17, no. 13, Art. no. 13, Jan. 2024, doi: 10.3390/en17133349. [12] S. S. Al-Khalasi, A. S. Al-Ghafri, S. N. Al-Saqri, and J. H. Al-Habsi, “Biodiesel Production from Waste Cooking Oil Using Ethanol Produced from Sugar or Dates Syrup,” IOP Conf. Ser. Earth Environ. Sci., vol. 1365, no. 1, p. 012004, Jun. 2024, doi: 10.1088/1755-1315/1365/1/012004. [13] P. Puspitasari, D. Dwi Pramono, D. Nur Fiansyah, A. Ayu Permanasari, N. Mufti, and J. Abd Razak, “Biodiesel production from waste cooking oil using calcium oxide derived from scallop shell waste,” Clean Energy, vol. 8, no. 2, pp. 113–126, Apr. 2024, doi: 10.1093/ce/zkae005. [14] M. U. H. Suzihaque, H. Alwi, U. Kalthum Ibrahim, S. Abdullah, and N. Haron, “Biodiesel production from waste cooking oil: A brief review,” Mater. Today Proc., vol. 63, pp. S490–S495, Jan. 2022, doi: 10.1016/j.matpr.2022.04.527. [15] F. Toldrá-Reig, L. Mora, and F. Toldrá, “Trends in Biodiesel Production from Animal Fat Waste,” Appl. Sci., vol. 10, no. 10, Art. no. 10, Jan. 2020, doi: 10.3390/app10103644. [16] F. Toldrá-Reig, L. Mora, and F. Toldrá, “Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste,” Appl. Sci., vol. 10, no. 15, Art. no. 15, Jan. 2020, doi: 10.3390/app10155085. [17] Z. Hu et al., “Waste cooking oil biodiesel and petroleum diesel soot from diesel bus: A comparison of morphology, nanostructure, functional group composition and oxidation reactivity,” Fuel, vol. 321, p. 124019, Aug. 2022, doi: 10.1016/j.fuel.2022.124019. [18] J. Mercy Nisha Pauline, R. Sivaramakrishnan, A. Pugazhendhi, T. Anbarasan, and A. Achary, “Transesterification kinetics of waste cooking oil and its diesel engine performance,” Fuel, vol. 285, p. 119108, Feb. 2021, doi: 10.1016/j.fuel.2020.119108. [19] Monika, S. Banga, and V. V. Pathak, “Biodiesel production from waste cooking oil: A comprehensive review on the application of heterogenous catalysts,” Energy Nexus, vol. 10, p. 100209, Jun. 2023, doi: 10.1016/j.nexus.2023.100209. [20] G. R. Srinivasan et al., “Influence of fatty acid composition on process optimization and characteristics assessment of biodiesel produced from waste animal fat,” Energy Sources Part Recovery Util. Environ. Eff., vol. 0, no. 0, pp. 1–19, doi: 10.1080/15567036.2020.1771477. [21] N. Hasan and M. V. Ratnam, “Biodiesel Production from Waste Animal Fat by Transesterification Using H2SO4 and KOH Catalysts: A Study of Physiochemical Properties,” Int. J. Chem. Eng., vol. 2022, no. 1, p. 6932320, 2022, doi: 10.1155/2022/6932320. [22] E. O. Ajala, A. B. Ehinmowo, M. A. Ajala, O. A. Ohiro, F. A. Aderibigbe, and A. O. Ajao, “Optimisation of CaO-Al2O3-SiO2-CaSO4-based catalysts performance for methanolysis of waste lard for biodiesel production using response surface methodology and meta-heuristic algorithms,” Fuel Process. Technol., vol. 226, p. 107066, Feb. 2022, doi: 10.1016/j.fuproc.2021.107066. [23] J. M. Fonseca, J. G. Teleken, V. de Cinque Almeida, and C. da Silva, “Biodiesel from waste frying oils: Methods of production and purification,” Energy Convers. Manag., vol. 184, pp. 205–218, Mar. 2019, doi: 10.1016/j.enconman.2019.01.061. [24] E. Rosson, P. Sgarbossa, F. Pedrielli, M. Mozzon, and R. Bertani, “Bioliquids from raw waste animal fats: an alternative renewable energy source,” Biomass Convers. Biorefinery, vol. 11, no. 5, pp. 1475–1490, Oct. 2021, doi: 10.1007/s13399-020-00634-z. [25] P. Baladincz and J. Hancsók, “Fuel from waste animal fats,” Chem. Eng. J., vol. 282, pp. 152–160, Dec. 2015, doi: 10.1016/j.cej.2015.04.003. [26] M. Ndiaye, A. Arhaliass, J. Legrand, G. Roelens, and A. Kerihuel, “Reuse of waste animal fat in biodiesel: Biorefining heavily-degraded contaminant-rich waste animal fat and formulation as diesel fuel additive,” Renew. Energy, vol. 145, pp. 1073–1079, Jan. 2020, doi: 10.1016/j.renene.2019.06.030. [27] E. Alptekin, M. Canakci, A. N. Ozsezen, A. Turkcan, and H. Sanli, “Using waste animal fat based biodiesels–bioethanol–diesel fuel blends in a DI diesel engine,” Fuel, vol. 157, pp. 245–254, Oct. 2015, doi: 10.1016/j.fuel.2015.04.067. [28] D. Singh et al., “A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock,” J. Clean. Prod., vol. 307, p. 127299, Jul. 2021, doi: 10.1016/j.jclepro.2021.127299. [29] B. Riazi, J. M. Mosby, B. Millet, and S. Spatari, “Renewable diesel from oils and animal fat waste: implications of feedstock, technology, co-products and ILUC on life cycle GWP,” Resour. Conserv. Recycl., vol. 161, p. 104944, Oct. 2020, doi: 10.1016/j.resconrec.2020.104944. [30] M. Salaheldeen, A. A. Mariod, M. K. Aroua, S. M. A. Rahman, M. E. M. Soudagar, and I. M. R. Fattah, “Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production,” Catalysts, vol. 11, no. 9, Art. no. 9, Sep. 2021, doi: 10.3390/catal11091121. [31] B. Wang, B. Wang, S. K. Shukla, and R. Wang, “Enabling Catalysts for Biodiesel Production via Transesterification,” Catalysts, vol. 13, no. 4, Art. no. 4, Apr. 2023, doi: 10.3390/catal13040740. [32] B. Maleki, S. S. Ashraf Talesh, and M. Mansouri, “Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: a review study,” Mater. Today Sustain., vol. 18, p. 100157, Jun. 2022, doi: 10.1016/j.mtsust.2022.100157 [33] A. O. Etim, P. Musonge, and A. C. Eloka-Eboka, “Process optimization of bio-alkaline catalysed transesterification of flax seed oil methyl ester,” Sci. Afr., vol. 16, p. e01275, Jul. 2022, doi: 10.1016/j.sciaf.2022.e01275. [34] K. Vasi?, G. Hojnik Podrepšek, Ž. Knez, and M. Leitgeb, “Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides,” Catalysts, vol. 10, no. 2, Art. no. 2, Feb. 2020, doi: 10.3390/catal10020237. [35] H. Kazemi Shariat Panahi et al., “Nanotechnology applications in biodiesel processing and production: A comprehensive review,” Renew. Sustain. Energy Rev., vol. 192, p. 114219, Mar. 2024, doi: 10.1016/j.rser.2023.114219. [36] M. Mofijur et al., “Effect of nanocatalysts on the transesterification reaction of first, second and third generation biodiesel sources- A mini-review,” Chemosphere, vol. 270, p. 128642, May 2021, doi: 10.1016/j.chemosphere.2020.128642. [37] A. Al-Abbasi et al., “BaO as a heterogeneous nanoparticle catalyst in oil transesterification for the production of FAME fuel,” Inorg. Chem. Commun., vol. 158, p. 111620, Dec. 2023, doi: 10.1016/j.inoche.2023.111620. [38] V. Mishra et al., “Advances in Nanocatalysts Mediated Biodiesel Production,” in Agricultural Biomass Nanocatalysts for Green Energy Applications, M. Srivastava and A. K. Rai, Eds., Singapore: Springer Nature, 2024, pp. 205–235. doi: 10.1007/978-981-97-1623-4_9. [39] V. Mandari and S. K. Devarai, “Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: a Critical Review,” BioEnergy Res., vol. 15, no. 2, pp. 935–961, Jun. 2022, doi: 10.1007/s12155-021-10333-w. [40] M. E. Kibar, L. Hilal, B. T. Çapa, B. Bahç?vanlar, and B. B. Abdeljelil, “Assessment of Homogeneous and Heterogeneous Catalysts in Transesterification Reaction: A Mini Review,” ChemBioEng Rev., vol. 10, no. 4, pp. 412–422, 2023, doi: 10.1002/cben.202200021. [41] C. S. Singh, N. Kumar, and R. Gautam, “Supercritical transesterification route for biodiesel production: Effect of parameters on yield and future perspectives,” Environ. Prog. Sustain. Energy, vol. 40, no. 6, p. e13685, 2021, doi: 10.1002/ep.13685. [42] A. Ahmed, A. Ali, M. Mubashir, H. R. Lim, K. S. Khoo, and P. L. Show, “Process optimization and simulation of biodiesel synthesis from waste cooking oil through supercritical transesterification reaction without catalyst,” J. Phys. Energy, vol. 5, no. 2, p. 024003, Feb. 2023, doi: 10.1088/2515-7655/acb6b3. [43] M. U. Qadeer et al., “Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process,” J. Clean. Prod., vol. 309, p. 127388, Aug. 2021, doi: 10.1016/j.jclepro.2021.127388. [44] D. Bose, A. Bangal, A. Hens, and S. Barma, “Design and optimization of reactive distillation for enhancing supercritical transesterification process to produce biodiesel,” Chem. Eng. Process. - Process Intensif., vol. 203, p. 109877, Sep. 2024, doi: 10.1016/j.cep.2024.109877. [45] W. Sakdasri, C. Komintarachat, R. Sawangkeaw, and S. Ngamprasertsith, “A Review of Supercritical Technologies for Lipid-Based Biofuels Production: The Glycerol-free Processes,” Eng. J., vol. 25, no. 2, pp. 1–14, Feb. 2021, doi: 10.4186/ej.2021.25.2.1. [46] P. Andreo-Martínez, V. M. Ortiz-Martínez, N. García-Martínez, A. P. de los Ríos, F. J. Hernández-Fernández, and J. Quesada-Medina, “Production of biodiesel under supercritical conditions: State of the art and bibliometric analysis,” Appl. Energy, vol. 264, p. 114753, Apr. 2020, doi: 10.1016/j.apenergy.2020.114753. [47] M. K. Pasha, L. Dai, D. Liu, W. Du, and M. Guo, “Biodiesel production with enzymatic technology: progress and perspectives,” Biofuels Bioprod. Biorefining, vol. 15, no. 5, pp. 1526–1548, 2021, doi: 10.1002/bbb.2236. [48] P. Kalita, B. Basumatary, P. Saikia, B. Das, and S. Basumatary, “Biodiesel as renewable biofuel produced via enzyme-based catalyzed transesterification,” Energy Nexus, vol. 6, p. 100087, Jun. 2022, doi: 10.1016/j.nexus.2022.100087. [49] L. Lv, L. Dai, W. Du, and D. Liu, “Progress in Enzymatic Biodiesel Production and Commercialization,” Processes, vol. 9, no. 2, Art. no. 2, Feb. 2021, doi: 10.3390/pr9020355. [50] P. Sharma, M. Usman, E.-S. Salama, M. Redina, N. Thakur, and X. Li, “Evaluation of various waste cooking oils for biodiesel production: A comprehensive analysis of feedstock,” Waste Manag., vol. 136, pp. 219–229, Dec. 2021, doi: 10.1016/j.wasman.2021.10.022. [51] J. Matušinec et al., “Cooking oil and fat waste management: A review of the current state,” 2020, doi: 10.3303/CET2081128. [52] J. M. Encinar, S. Nogales-Delgado, and N. Sánchez, “Pre-esterification of high acidity animal fats to produce biodiesel: A kinetic study,” Arab. J. Chem., vol. 14, no. 4, p. 103048, Apr. 2021, doi: 10.1016/j.arabjc.2021.103048. [53] S. M. Y. Kosuru, Y. Delhiwala, P. B. Koorla, and M. Mekala, “A review on the biodiesel production: Selection of catalyst, Pre-treatment, Post treatment methods,” Green Technol. Sustain., vol. 2, no. 1, p. 100061, Jan. 2024, doi: 10.1016/j.grets.2023.100061. [54] T. Eevera, K. Rajendran, and S. Saradha, “Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions,” Renew. Energy, vol. 34, no. 3, pp. 762–765, Mar. 2009, doi: 10.1016/j.renene.2008.04.006. [55] S. Ishak and A. Kamari, “A review of optimum conditions of transesterification process for biodiesel production from various feedstocks,” Int. J. Environ. Sci. Technol., vol. 16, no. 5, pp. 2481–2502, May 2019, doi: 10.1007/s13762-019-02279-6. [56] I. Raheem et al., “A review on influence of reactor technologies and kinetic studies for biodiesel application,” J. Ind. Eng. Chem., vol. 91, pp. 54–68, Nov. 2020, doi: 10.1016/j.jiec.2020.08.024. [57] K. Shikhaliyev, B. H. Hameed, and P. U. Okoye, “Utilization of biochars as sustainable catalysts for upgrading of glycerol from biodiesel production,” J. Environ. Chem. Eng., vol. 9, no. 2, p. 104768, Apr. 2021, doi: 10.1016/j.jece.2020.104768. [58] R. Foroutan, R. Mohammadi, J. Razeghi, and B. Ramavandi, “Biodiesel production from edible oils using algal biochar/CaO/K2CO3 as a heterogeneous and recyclable catalyst,” Renew. Energy, vol. 168, pp. 1207–1216, May 2021, doi: 10.1016/j.renene.2020.12.094. [59] K. Velusamy et al., “A review on nano-catalysts and biochar-based catalysts for biofuel production,” Fuel, vol. 306, p. 121632, Dec. 2021, doi: 10.1016/j.fuel.2021.121632. [60] H. Wang, X. Peng, H. Zhang, S. Yang, and H. Li, “Microorganisms-promoted biodiesel production from biomass: A review,” Energy Convers. Manag. X, vol. 12, p. 100137, Dec. 2021, doi: 10.1016/j.ecmx.2021.100137. [61] C. Bhan and J. Singh, “Role of microbial lipases in transesterification process for biodiesel production,” Environ. Sustain., vol. 3, no. 3, pp. 257–266, Sep. 2020, doi: 10.1007/s42398-020-00119-9. [62] G. Pant, D. Garlapati, U. Agrawal, R. G. Prasuna, T. Mathimani, and A. Pugazhendhi, “Biological approaches practised using genetically engineered microbes for a sustainable environment: A review,” J. Hazard. Mater., vol. 405, p. 124631, Mar. 2021, doi: 10.1016/j.jhazmat.2020.124631. [63] F. T. T. Cavalcante et al., “Opportunities for improving biodiesel production via lipase catalysis,” Fuel, vol. 288, p. 119577, Mar. 2021, doi: 10.1016/j.fuel.2020.119577. [64] G. M. Mathew et al., “Recent advances in biodiesel production: Challenges and solutions,” Sci. Total Environ., vol. 794, p. 148751, Nov. 2021, doi: 10.1016/j.scitotenv.2021.148751. [65] A. G. Alsultan et al., “A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production,” Catalysts, vol. 11, no. 11, Art. no. 11, Nov. 2021, doi: 10.3390/catal11111261. [66] A. Syafiuddin, J. H. Chong, A. Yuniarto, and T. Hadibarata, “The current scenario and challenges of biodiesel production in Asian countries: A review,” Bioresour. Technol. Rep., vol. 12, p. 100608, Dec. 2020, doi: 10.1016/j.biteb.2020.100608. [67] N. S. Topare, R. I. Jogdand, H. P. Shinde, R. S. More, A. Khan, and A. M. Asiri, “A short review on approach for biodiesel production: Feedstock’s, properties, process parameters and environmental sustainability,” Mater. Today Proc., vol. 57, pp. 1605–1612, Jan. 2022, doi: 10.1016/j.matpr.2021.12.216. [68] H. K. Jeswani, A. Chilvers, and A. Azapagic, “Environmental sustainability of biofuels: a review,” Proc. R. Soc. Math. Phys. Eng. Sci., vol. 476, no. 2243, p. 20200351, Nov. 2020, doi: 10.1098/rspa.2020.0351. [69] O. Ogunkunle and N. A. Ahmed, “Overview of Biodiesel Combustion in Mitigating the Adverse Impacts of Engine Emissions on the Sustainable Human–Environment Scenario,” Sustainability, vol. 13, no. 10, Art. no. 10, Jan. 2021, doi: 10.3390/su13105465. [70] H. Hosseinzadeh-Bandbafha et al., “Environmental life cycle assessment of biodiesel production from waste cooking oil: A systematic review,” Renew. Sustain. Energy Rev., vol. 161, p. 112411, Jun. 2022, doi: 10.1016/j.rser.2022.112411. [71] L. Chen, D. Debnath, J. Zhong, K. Ferin, A. VanLoocke, and M. Khanna, “The economic and environmental costs and benefits of the renewable fuel standard,” Environ. Res. Lett., vol. 16, no. 3, p. 034021, Feb. 2021, doi: 10.1088/1748-9326/abd7af. [72] T. Mizik and G. Gyarmati, “Economic and Sustainability of Biodiesel Production—A Systematic Literature Review,” Clean Technol., vol. 3, no. 1, Art. no. 1, Mar. 2021, doi: 10.3390/cleantechnol3010002. [73] R. Johansson, S. Meyer, J. Whistance, W. Thompson, and D. Debnath, “Greenhouse gas emission reduction and cost from the United States biofuels mandate,” Renew. Sustain. Energy Rev., vol. 119, p. 109513, Mar. 2020, doi: 10.1016/j.rser.2019.109513. [74] Y. Zhao, C. Wang, L. Zhang, Y. Chang, and Y. Hao, “Converting waste cooking oil to biodiesel in China: Environmental impacts and economic feasibility,” Renew. Sustain. Energy Rev., vol. 140, p. 110661, Apr. 2021, doi: 10.1016/j.rser.2020.110661.
Copyright
Copyright © 2024 Akash Phillip, Nandini Saini. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64203
Publish Date : 2024-09-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

