Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Blockchain-Based Approach for Drug Traceability in Healthcare Supply Chain
Authors: Reshma .
DOI Link: https://doi.org/10.22214/ijraset.2023.55609
Certificate: View Certificate
Abstract
Healthcare supply chains are complex structures spanning across multiple organizational and geographical boundaries, providing critical backbone to services vital for everyday life. The inherent complexity of such systems can introduce impurities including inaccurate information, lack of transparency and limited data provenance. Counterfeit drugs is one consequence of such limitations within existing supply chains which not only has serious adverse impact on human health but also causes severe economic loss to the healthcare industry. Consequently, existing studies have emphasized the need for a robust, end-to-end track and trace system for pharmaceutical supply chains. Therein, an end-to-end product tracking system across the pharmaceutical supply chain is paramount to ensuring product safety and eliminating counterfeits. Most existing track and trace systems are centralized leading to data privacy, transparency and authenticity issues in healthcare supply chains. In this article, we present an Ethereum blockchain-based approach leveraging smart contracts and decentralized off-chain storage for efficient product traceability in the healthcare supply chain. The smart contract guarantees data provenance, eliminates the need for intermediaries and provides a secure, immutable history of transactions to all stakeholders. We present the system architecture and detailed algorithms that govern the working principles of our proposed solution. We perform testing and validation, and present cost and security analysis of the system to evaluate its effectiveness to enhance traceability within pharmaceutical supply chains.
Introduction
I. Introduction
Healthcare supply chain is a complex network of several independent entities that include raw material suppliers, manufacturer, distributor, pharmacies, hospitals and patients. Tracking supplies through this network is non-trivial due to several factors including lack of information, centralized control and competing behaviour among stakeholders. Such complexity not only results in in-efficiencies such as those highlighted through COVID-19 pandemic but can also aggravate the challenge of mitigating against counterfeit drugs as these can easily permeate the healthcare supply chain. Counterfeit drugs are products deliberately and fraudulently produced and/or mislabeled with respect to identity and/or source to make it appear to be a genuine product. Such drugs can include medications that contain no active pharmaceutical ingredient (API), an incorrect amount of API, an inferior-quality API, a wrong API, contaminants, or repackaged expired products. Some counterfeit medications may even be incorrectly formulated and produced in substandard conditions.
According to the Health Research Funding Organization, up to 30% of the drugs sold in developing countries are counterfeit. Further, a recent study by World Health Organization (WHO) indicated counterfeit drugs as one of the major causes of deaths in developing countries, and in most cases the victims are children. In addition to the adverse impact on human lives, counterfeit drugs also cause significant economic loss to the pharmaceutical industry. In this respect, the annual economic loss to the US pharmaceutical industry due to counterfeit medicine is estimated around $200 billion.
A typical drug supply chain distribution process is illustrated in Figure 1. An API supplier is responsible for delivering the raw materials to manufacture drugs approved by a regulatory agency such as the US Food and Drug Administration (US FDA). The manufacturer packages the drugs into a Lot or sends it to a re-packager. The primary distributor receives several Lots of the product and is responsible for transferring them to pharmacies based on product demand or secondary distributors (in case the quantity of Lots is very large) who can transfer these Lots to the pharmacies. Finally, a pharmacy will dispense the drug to patients typically based on a doctor’s prescription. Throughout the supply chain, the transfer of drugs is usually facilitated by third party logistic service providers such as UPS or FedEx and in some cases the distributors operate their own fleet of vehicles to transport the products. The primary reason for counterfeit drugs to reach end-user marketplace is due to the complex structure of a healthcare supply chain. Leveraging the complexity of this distribution process, medications can easily pass through with little or no trail of information and verifiable documentation. Consequently, monitoring, effective control and tracking of products in healthcare supply chain is fundamental to combating counterfeits.
The importance of drug traceability (track and trace) is increasingly emphasized and mandated by several countries across the world. For example, the U.S. Drug Supply Chain Security Act (DSCSA) has made it mandatory for the pharmaceutical industry to develop an electronic and interoperable system that identifies and tracks prescription drugs as they are distributed across the United States. Similarly, over the last 8 years, China required all the stakeholders involved in the drugs supply chain to record information of individual pharmaceutical products in a specialized IT system whenever drugs are sent to/from their warehouses. Therefore, drug traceability has become an integral part of the pharmaceutical supply chain as it establishes authenticity, and aims to track and trace chain of custody of the product across drug supply chain.
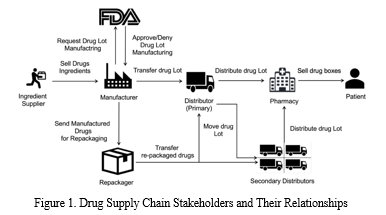
Blockchain technology has introduced a new model of application development primarily based on the successful implementation of the data structure within the Bitcoin application. The fundamental concept of the blockchain data structure is similar to a linked list i.e. it is shared among all the nodes of the network where each node keeps its local copy of all the blocks (associated with the longest chain) starting from its genesis block [15].
Recently, many real-world applications have been developed in diverse domains, such as the Internet of Things [16], e-Government and e-document management. These applications leverage benefits of blockchain technology due to its self-cryptographic validation structure among transactions (through hashes), and public availability of distributed ledger of transaction-records in a peer-to-peer network. Creating a chain of blocks connected by cryptographic constructs (hashes) makes it very difficult to tamper the records, as it would cost the rework from the genesis to the latest transaction in blocks
Within the context of blockchain-based traceability for pharmaceutical supply chain, presents one of the initial efforts. Although our solution has similarities with this effort due to the focus on pharmaceutical supply chain as well as the use of blockchains, we take a holistic view of the pharmaceutical supply chain, presenting an end-to-end solution for drug traceability whereas only focused on a subset of these challenges. Firstly, our approach identifies and engages major stakeholders in the drug supply chain i.e. the FDA, supplier, manufacturer, distributor, pharmacy, and patient, whereas is limited to the supplier, manufacturer, and wholesaler as the stakeholders. Consequently, the pharmacists are represented as an external entity which is not the case in a real drug supply chain. Secondly, we make explicit efforts to identify and define relationships among stakeholders, on-chain resources, smart contracts, and decentralized storage systems which is lacking. Furthermore, in view of the significance of interactions among stakeholders, we have included precise definitions to remove any ambiguity, whereas such interactions have not been defined as part.
Thirdly, we use the smart contracts technology to achieve real-time, seamless traceability with push notifications so as to minimize human intervention and therefore undesired delays. Specifically, each drug Lot is assigned a unique smart contract that generates an event whenever a change in ownership occurs and a list of events is delivered to the DApp user. However, the smart contracts in [20] are programmed for specific roles such as supplier, manufacturer, and wholesaler which requires each participant to manually confirm which drugs are received. Such approach can introduce delays and inaccuracies in the immutable data stored on the ledger. Finally, we have conducted a cost and security analysis to evaluate the performance of the proposed solution including discussion on how the proposed solution can be generalized to other supply chains.
The challenge of achieving traceability to mitigate against counterfeit drugs is well-established and several efforts have been made to address this within pharmaceutical industry.
However, a careful review of literature presents several gaps and opportunities for a comprehensive application of blockchain technology for drug traceability. In this context, the primary contributions of this paper can be summarized as follows:
- We propose a blockchain-based solution for the pharmaceutical supply chain that provides security, traceability, immutability, and accessibility of data provenance for pharmaceutical drugs.
- We design a smart contract capable of handling various transactions among pharmaceutical supply chain stakeholders.
- We present, implement and test the smart contract that defines the working principles of our proposed solution.
- We conduct security and cost analysis to evaluate the performance of the proposed blockchain-based solution.
The reminder of this paper is organized as follows. Section II presents a critical review of existing efforts with respect to traceability in the healthcare supply chain. This is followed by a description of the proposed blockchain-based track & trace system for phamaceutical products in section III. Section IV presents the implementation of the proposed system along with details of the testing and evaluation in section V. Section VI describes the efforts to evaluate the proposed system and analyzes the outcomes of evaluation. Section VII concludes the paper summarizing contributions and highlighting avenues for further work.
II. Literature Survey
- Blockchain Technology in Healthcare: The Revolution Starts Here : In this paper they presented Blockchain technology has shown its considerable adaptability in recent years as a variety of market sectors sought ways of incorporating its abilities into their operations. While so far most of the focus has been on the financial services industry, several Papers in other service related areas such as healthcare show this is beginning to change. Numerous starting points for Blockchain technology in the healthcare industry are the focus of this report. With examples for public healthcare management, user-oriented medical research and drug counterfeiting in the pharmaceutical sector, this report aims to illustrate possible influences, goals and potentials connected to this disruptive technology.
- Benefits and Guidelines for Utilizing Blockchain Technology in Pharmaceutical Supply Chains: Case Bayer Pharmaceuticals In this thesis, I explore how blockchain technology can improve pharmaceutical supply chain operations and discuss how the technology should be implemented. Furthermore, I study how the life science company Bayer's pharmaceutical division can utilize blockchain technology in its supply chain operations. I begin by defining the concepts of blockchain technology, smart contracts and pharmaceutical supply chain. Then I discuss the benefits and implementation in different sections: participating entities and information flow, contracts and payments, logistics, transparency and product security and blockchain infrastructure and governance. Both Bayer and the industry as a whole can benefit from blockchain technology. Blockchain enables for example efficient, safe and private transactions, product transparency and security and open information sharing without exposing trade secrets. For the first time a platform for all stakeholders can be developed that enables transacting information and value simultaneously.
- Drugledger: A Practical Blockchain System for Drug Traceability and Regulation: Drug traceability system is essentially important for public drug security and business of pharmaceutical companies, which aims to track or trace where the drug has been and where it has gone along the drug supply chain. Traditional centralized server-client technical solutions have been far from satisfying for their bad performances in data authenticity, privacy, system resilience and flexibility. In this paper, we propose a scenario-oriented blockchain system for drug traceability and regulation called Drugledger, which reconstructs the whole service architecture by separating service provider into three independent service components and ensures the authenticity and privacy of traceability data. Drugledger is more resilient than traditional solutions with its p2p architecture. Furthermore, Drugledger could efficiently prune its storage, achieving a finally stable and acceptable blockchain storage. Besides, algorithms reflecting the real drug supply chain logic (e.g, package, repackage, unpackage, etc.) are designed based on the expanded UTXO workflow in Drugledger. To our knowledge, it is the first systematic work from both a technical and practical perspective on how blockchain system could be designed for drug traceability and regulation.
- Design of Cold Chain Application Framework (CCAF) based on IOT and Cloud: In this paper, we propose a smart cold chain application framework (SCCAF) based on Cloud and IOT (Internet of Things) techniques. The purpose of SCCAF is to provide PaaS (Platform as a Service) and IaaS (Infra as a Service) to users who want to develop and apply cold chain management systems with low cost and in short time. Also, SCCAF enables users to use any type of IOT devices such as RFID tags, WSN sensor nodes, BLE (Bluetooth Low Energy) sensor nodes and so on. We define common components by generalizing function of existing cold chain management systems, and design SCCAF based on Hadoop and Spark to store the large amount of data stream on salable storage and process stream data to detect events and assess risks in cold chain.
III. Existing System
Existing System In today's world, the healthcare industry relies on extensive supply chains that cross organizational and geographic boundaries. Impurities such as erroneous information, a lack of transparency, and restricted data provenance can be introduced by the intrinsic complexity of such systems. Counterfeit medications are one of the consequences of such constraints in existing supply chains, which not only has a negative impact on human health but also costs the healthcare business a lot of money. A dependable end-to-end track and trace system for pharmaceutical supply chains has thus been emphasized in prior study. An end-to-end pharmaceutical supply chain tracking system is vital to assure product safety and eradicate counterfeits. Most modern track and trace systems in healthcare supply chains are centralized, posing privacy, transparency, and authenticity issues.
IV. Proposed System
We are developing a Drug Traceability System based on Blockchain Technology that will be used to track drugs throughout the healthcare supply chain. There are numerous stakeholders in the healthcare supply chain, including manufacturers, suppliers, distributors, pharmacies, hospitals, and so on. While supplying the drug from the manufacturer to the customer or patient, there is a possibility that one of the stakeholders will mislabel or counterfeit the drug. To avoid this, we propose our solution. Another aspect to consider when examining flaws in the healthcare supplychain is the possibility that one of the stakeholders stocks the drugs for an extended period of time. Waiting for demand to rise before selling the drugs. This drug stockpiling has an impact on the treatment of patients in an emergency. To avoid this, we are gathering information on how many drugs each stakeholder has and when he is supposed to supply the other stakeholder. To achieve all of the above, we will use the blockchain technology which is a distributed ledger used to store transactions between the parties in the form of block
V. System Architecture
- Pharmacy Seller: In this module, the seller has to login by using valid user name and password. After login successful he can do some operations such as View & Authorize Users,Add Categories,Add Drug,View all Drugs, View all Purchased Drugs, Find Total Bill On Purchased Drugs, List All Drugs by Chain Tree, List All reviewed comments on Drugs,List All Search and View Details History,View All User’s Drug Search, View Drugs Rank chart,View Search ratio in chart.
- View and Authorize Users: In this module, the seller can view the list of users who all registered. In this, the admin can view the user’s details such as, user name, email, address and admin authorizes the users.
- View Chart Results: In this, the seller can view all charts related to View Search ratio in chart, View Drugs rank in Chart.
- User: In this module, there are n numbers of users are present. User should register before doing any operations. Once user registers, their details will be stored to the database. After registration successful, he has to login by using authorized user name and password. Once Login is successful user will do some operations like My Profile,Account Management,Search Drugs and Purchase,View my search History,View Drugs by Chain Tree,View Other Patient Comments On Drugs,View Top K Drugs Purchase,View Top K Query Details.
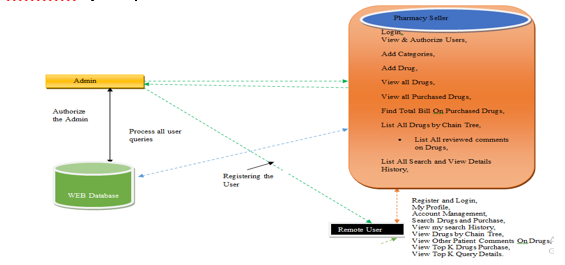
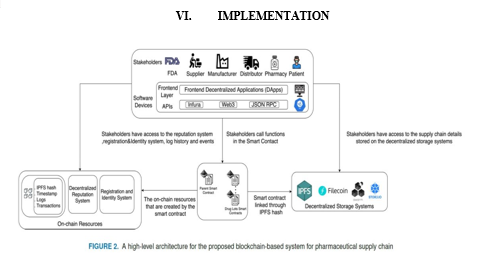
Figure 2 presents a high-level architecture for the proposed drug traceability system together with the stakeholder and their interactions with the smart contract. The stakeholders are envisioned to access the smart contract, decentralized storage system and on-chain resources through software devices that have front-end layer denoted by a DApp (Decentralized Application) which is connected to the smart contract, on-chain resources, and decentralized storage system by an application program interface (API) such as Infura, Web3, and JSON RPC. The stakeholders will interact with the smart contract to initiate pre-authorized function calls and with the decentralized storage systems to access data files. Finally, their interaction with the on-chain resources will be for obtaining information such as logs, IPFS hashes, and transactions. More details on the system components are presented below.
- Stakeholders include regulatory agencies such as FDA, manufacturers, distributors, pharmacies, and patients. These stakeholders act as participants in the smart contract and are assigned specific functions based on their role in the supply chain. They are also given access to the on-chain resources such as history and log information to track transactions in supply chain. Further, they are authorized to access information stored on the IPFS such as the drug Lot images, and information leaflets.
- Decentralized Storage System (IPFS [42] )provides a low-cost off-chain storage to store supply chain transactions data to ensure reliability, accessibility, and integrity of the stored data. The integrity of data is maintained by generating a unique hash for every uploaded file on its server, and the different hashes for the different uploaded files are then stored on the blockchain and accessed through the smart contract, and any change that occurs to any of the uploaded file is reflected in the associated hash.
- Ethereum Smart Contract is used to handle the deployment of the supply chain. The smart contract is central and essential for tracking the history of transactions and manages the hashes from the decentralized storage server which allows the participants to access the supply chain information. Moreover, the functions of the different stakeholders in the supply chain are defined within the smart contract and access to these functions is given to the authorized participants by using modifiers. A modifier is basically a way to decorate a function by adding additional features to it or to apply some restrictions. The smart contract also handles the transactions, such as selling drug Lots or boxes.
- On-chain Resources are used to store the logs and events that are created by the smart contract allowing track and trace. Moreover, a registration and identity system is used as an on-chain resource to associate the Ethereum address of the different participants to a human readable text which is stored in a decentralized way.
The system components are envisaged to function in an integrated manner to track the history of the drug under consideration to verify its authenticity, and no real-time tracking will be required because the DApp user will only need to use the proposed solution to verify that the drug under consideration is not counterfeit and it came from a trusted manufacturer. If real-time location of a drug Lot is to be tracked, a number of technologies can be implemented to accomplish this task. For example, IoT-enabled smart containers is equipped with sensors that continuously monitor and track the TRU from its starting point to its destination. The IoT sensor includes Global Positioning System (GPS) receiver to locate where the TRU is at, temperature sensor to keep track of the temperature, and pressure sensor to measure the pressure differences that detect any opening or closing of the container [43]
Figure 3 illustrates interaction among different participants of the supply chain within proposed system and can be loosely divided into three phases explained below.
a. Manufacturing: Typically, a manufacturer will send a request for approval from the FDA to initiate the manufacturing process of a drug Lot. Once the FDA approves the request, the manufacturer initiates the manufacturing process and an event is declared to all participants. The manufacturer will upload images of the drug Lot to the IPFS, and the IPFS will send a hash to the smart contract so that the images can be accessed later by authorized participants. The drug Lot will be delivered to the distributor for packaging concluding the manufacturing process.
b. Distribution: The next step is the initiation of the distribution process, the distributor will pack the drug Lot, and an image of the package will be uploaded to the IPFS which will send a hash to the smart contract. Once this step is completed, the drug Lot packages will be delivered to pharmacies, and this ends the distribution phase.
c. Sale/Consumption: The last step in the sequence diagram is related to the interaction between the pharmacy and the patients. Here, the pharmacy will initiate the sale of drug Lot box and it will be declared to the participants of the supply chain. Then, an image of the sold drug package will be uploaded to the IPFS, and a hash will be sent by the IPFS to the smart contract. The drug Lot box will be sold to the patient, and this concludes the drug Lot selling phase. This process will ensure that all the transactions are stored and can be accessed later by all the supply chain participants to check the authenticity and validity of the products in the supply chain in the form of a sequence of events.
Conclusion
In this Paper, we have investigated the challenge of drug traceability within pharmaceutical supply chains highlighting its significance specially to protect against counterfeit drugs. We have developed and evaluated a blockchain-based solution for the pharmaceutical supply chain to track and trace drugs in a decentralized manner. Specifically, our proposed solution leverages cryptographic fundamentals underlying block chain technology to achieve tamper-proof logs of events within the supply chain and utilizes smart contracts within Ethereum block chain to achieve automated recording of events that are accessible to all participating stakeholders. We have demonstrated that our proposed solution is cost efficient in terms of the amount of gas spent in executing the different functions that are triggered within the smart contract. Moreover, the conducted security analysis has shown that our proposed solution achieves protection against malicious attempts targeting is integrity, availability and nonrepudiation of transaction data which is critical in a complex multi-party settings such as the pharmaceutical supply chain. We continue our efforts to enhance the efficiency of pharmaceutical supply chains and envision to focus on extending the proposed system to achieve end to end transparency and verifiability of drugs use as future work.
References
[1] \\\"Shortage of personal protective equipment endangering health worker worldwide\\\" https://tinyurl.com/v5qauvp. [Accessed on: 3-June-2020] [2] Chambliss W, Carroll W, Yelvigi M, el al. \\\"Role of the pharmacist in preventing distribution of counterfeit medications\\\". J Am pharm Assoc. 2012;52(2): 195-199. [3] Ziance RJ. \\\"Roles for pharmacy in combating counterfeit drugs\\\". J Am pharm Assoc. 2008;48: e71-e88. [4] Toscan P. \\\"The dangerous world of counterfeit prescription drugs\\\". Available: http://usatoday30.usatoday.com/money/industries/health/ drugs/story/2011-10-09/cnbc-drugs/50690880/1.[Accessed on: 3-June-2020] [5] T.Adhanom, \\\"Health is a fundamental human right\\\",2017. Available:https://www.who.int/mediacentre/news/statements/ fundamental-human-right/en/. [Accessed: 26- May- 2020]. [6] World Health Organization, \\\"Growing threat from counterfeit medicines\\\", 2010. [7] Daniela Bagozzi, C.L. \\\"1 in 10 Medical Products in Developing Countries Is Substandard or Falsified\\\". 2017. Available: https://www. who.int/news-room/detail/28-11-2017-1-in-10-medical-products-indeveloping- countries-is-substandard-or-falsified.[Accessed: 3-June- 2020]. [8] The Guardian. \\\"10% of Drugs in Poor Countries Are Fake, Says WHO\\\". 2017. Available: https://www.theguardian.com/globaldevelopment/ 2017/nov/28/10-of-drugs-in-poor-countries-are-fakesay who.[Accessed: 3-June-2020]. [9] Funding, H.R. \\\"20 Shocking Counterfeit Drugs Statistics\\\". 2017. Available: https://healthresearchfunding.org/20-shocking-counterfeit-drugsstatistics. [Accessed: 3-June-2020]. [10] Blackstone, E.A.; Fuhr, J.P., Jr.; Pociask, S. \\\"The health and economic effects of counterfeit drugs\\\". Am. Health Drug Benefits 2014, 7, 216–224 [11] U.S. Food and Drug Administration, \\\"A drug supply chain example\\\". Available: https://www.fda.gov/drugs/drug-shortages/graphicdrugsupply-chain example}.Accessed: 3-June-2020]. [12] Marucheck, Ann; Greis, Noel; Mena, Carlos; Cai, Linning (November 2011). \\\"Product safety and security in the global supply chain: Issues, challenges and research opportunities\\\". Journal of Operations Management. 29 (7–8): 707–720 [13] U.S. Food and Drug Administration, \\\"Drug Supply Chain Security Act\\\". Available: https://fda.gov. [Accessed: 3-June-2020]. [14] State Food and Drug Administration of China, \\\"On suspension of drug electronic supervision system\\\", Feb. 2016. Available: http://www.sda. gov.cn/WS01/CL0051/144782.html.[Accessed: 3-June-2020]. [15] M. Andrychowicz, S. Dziembowski, D. Malinowski, L. Mazurek, \\\"On the Malleability of Bitcoin Transactions\\\" in the proceedings of Financial Cryptography and Data Security, Pages 1—18 , 2015. [16] A. Suliman, Z. Husain, M. Abououf,M. Alblooshi, and Khaled Salah, \\\"Monetization of IoT data using smart contracts\\\", IET Networks, issue 1, volume 8, pages 32-37, 2019. [17] K. M. Khan, J. Arshad and M. M. Khan,\\\"Simulation of transaction malleability attack for blockchain-based e-Voting\\\", Computers & Electrical Engineering, volume 83, pages 106583, 2020, [18] N. Nizamuddin, K. Salah, M. A. Azad, J. Arshad and M.H. Rehman, \\\"Decentralized document version control using ethereum blockchain and IPFS\\\", Computers & Electrical Engineering, volume 76, pages 183 - 197,2019. [19] S. Nakamoto, \\\"Bitcoin: A Peer-to-Peer Electronic Cash System\\\", Cryptography Mailing list at https://metzdowd.com, 2009 [20] Muniandy, M., & Ong Tze Ern, G. (2019). \\\"Implementation of Pharmaceutical Drug Traceability Using Blockchain Technology\\\". Inti Journal. Vol. 2019:035. eISSN:2600-7920 [21] Olsen, P., Borit, M., \\\"The components of a food traceability system\\\", Trends in Food Science & Technology (2018), doi: 10.1016/j.tifs.2018.05.004. [22] Bougdira, A., Ahaitouf, A. and Akharraz, I. (2019), \\\"Conceptual framework for general traceability solution: description and bases\\\", Journal of Modelling in Management, Vol. 15 No. 2, pp. 509-530. [23] K. Al Huraimel, R. Jenkins, \\\"Smart Track\\\", 2020. Available: https: //smarttrack.ae/. [Accessed: 26-May-2020] [24] \\\"GS1 DataMatrix: A tool to improve patient safety through visibility in the supply chain\\\". Available: https://www.gs1.org/docs/healthcare/ MC07\\\\_GS1\\\\_Datamatrix.pdf. [Accessed: 26-May-2020] [25] Cameron Faulkner. \\\"What is NFC? Everything you need to know\\\". Available: https://techradar.com. [Accessed: 3-June-2020].
Copyright
Copyright © 2023 Reshma .. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55609
Publish Date : 2023-09-02
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

