Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Characterization of Leachate and Evaluation of Groundwater Pollution in the Vicinity of the Municipal Solid Waste Landfill in Srinagar, Jammu & Kashmir
Authors: Dr. Bashir Ahmad Pandit
DOI Link: https://doi.org/10.22214/ijraset.2024.58183
Certificate: View Certificate
Abstract
The most popular way for disposing of municipal solid waste (MSW) throughout India, including Kashmir, is through landfills. However, the discharge of leachate from these facilities has seriously contaminated groundwater. An evaluation was conducted on the groundwater quality near the Srinagar landfill site (Achan). In order to investigate the potential effects of leachate percolation on groundwater quality, samples of leachate and groundwater were taken from the Srinagar dump site and its surrounding areas. Groundwater and leachate samples were used to measure the concentration of various physio-chemical parameters (pH, EC, TDS, COD, BOD, Ca2+, Mg2+, Na+, K+, Cl-, SO42-, NO3-, NH4+, PO43-, Phenol), as well as microbiological parameters (total coliform and faecal coliform) and heavy metals (Cd, Cr, Cu, Fe, Ni, Pb, Zn, and Hg). The relatively elevated levels of NH4+, EC, TDS, BOD, phenol, Cd, and Mg, Cr were above the limits of Indian standard and WHO for drinking water. In addition to this , these also represent heavy pollutant indicators as per the Single Point Pollution Index as well by the Nemero index, as these elements crossed the upper most limit for both these pollution indeces. Assessing the water contamination in depth and distance wise, closer to landfill and shallow wells were characterized as the most polluted and had some additive contaminants ( Cl- ,SO4, Ca , Ni ). This is an indication of pollution transfer and the leachate movement. The presence of total coliform and faecal coliform although in small counts warns for the groundwater quality and thus renders the associated aquifer unreliable for domestic water supply. There is no natural or other possible reason for high concentration of these pollutants, thus it can be concluded that leachate has significant impact on groundwater quality in the area. In the present study, also an attempt has been made to investigate physico-chemical properties, fertilizing potential and heavy metal polluting potentials of the three types of composts including municipal soild waste compost, green waste compost and mixed waste compost. Each of these types were given a treatment with effective micro organisms (EM) to understand the quality of compost so formed by the composting process by its analysis (Laboratory as well as statistical) and the quality of composts was found out using Quality control Indices such as Fertilizing Index and Clean Index. Parameters like, pH, EC, TOC, total nitrogen, total phosphorous, total potassium, C/N ratio, and heavy metals like zinc, copper, cadmium, nickel, lead, and chromium were analyzed and it was found that all the parameters were within the permissible limits prescribed by FCO-2000. Further Fertility Index and Clean Index were determined for the samples and it was found that proper segregation of the solid waste is important from composting point of view because mixed waste affects the quality of compost. From the calculated values for the compost prepared from untreated MSW, FI (3.40) and CI (2.8), for the treated MSW Compost, FI as 3.47 and CI as 3.0, for the untreated mixed waste FI (3.27) and CI (3.0) and for the treated mixed waste FI (3.47), CI (3.2) The determined values indicates that all these compost types belongs to marketable Class D (medium fertilizing potential and medium heavy metal content).The fertility index value was estimated as 2.0 and the Clean Index value as 3.06 for the untreated green waste while for treated green waste FI(2.73) and CI(3.7), which indicates that both compost types belongs to restricted use Class RU-1 (Should not be allowed to market due to low fertilizing potential. However, these can be used as soil conditioner)
Introduction
I. INTRODUCTION
The population of the earth is increasing and with this increase comes an increased need for food and material goods and an associated increase in wastes from their production and use. These wastes have been labeled as municipal solid waste (MSW) and encompass a large range of material.
The MSW are generated by the routine activities of everyday life, in addition to the unusual activities. The principal sources of MSW are homes, business, and institutions (Agarwal et al., 2015). Landfills are used to store and degrade solid wastes. The oldest and most widely used method for ultimate disposing of solid waste is landfilling. It is the most common method for municipal and industrial solid waste disposal (Nandan et al., 2017). This method is used in many countries around the world. Researchers have shown that between 40 to 80% of municipal solid waste (MSW) is disposed of in developed countries whereas this rate reaches 60 to 90% in developing countries (Pazoki et al., 2014). Landfills generate significant amount of a highly contaminated liquid called leachate. The composition of the leachate varies widely dependably on waste type and waste age (Kumar et al., 2017).
Municipal solid waste (MSW) disposal systems include open dumping, sanitary landfill, composting, and incineration. Comparative studies of the various possible means of eliminating solid urban waste (landfilling, incineration, composting, etc.) have shown that the cheapest, in term of exploitation and capital costs, is landfilling. Besides its economic advantages, landfilling minimizes environmental insults and other inconveniences, and allows waste to decompose under controlled conditions until its eventual transformation into relatively inert, stabilized material (Renou et al., 2008; Aziz et al., 2010; Mojiri et al., 2014). However the production of highly contaminated landfill leachate is a shortcoming of this technique.
Landfill leachate i.e. (a liquid produced principally by the percolation of precipitations or other disposed water/wastewater) through an open landfill or through the cap of a completed site and may contain large quantities of organic pollutants, nitrogen compounds (e.g., ammonia), suspended solids, heavy metals, inorganic salts, phenols, and phosphorus (Renou et al., 2008; Aziz et al., 2014).
The Landfills have been identified as one of the major threats to groundwater resources (Mor et al., 2006) not only in India but throughout the world (United States Environmental Protection Agency, US EPA). More than 90% of the municipal solid waste (MSW) generated in India is directly dumped on land in an unsatisfactory manner (Chatterjee, 2010). The solid waste placed in landfills or open dumps are subjected to either groundwater underflow or infiltration from precipitation or any other possibility of infiltration of water. During rainfall, the dumped solid waste receives water and the by-products of its decomposition move into the water through the waste deposition. The liquid containing innumerable organic and inorganic compounds is called ‘leachate’. This leachate accumulates at the bottom of the landfill and percolates through the soil and reaches the groundwater (Mor et al., 2006). Areas near landfills have a greater possibility of groundwater contamination because of the potential pollution source of leachate originating from the nearby dumping site. Such contamination of groundwater results in a substantial risk to local groundwater resource user and to the natural environment
Traditionally, the main option for the management of waste has been landfilling. The landfilling of biodegradable waste is proven to contribute to environmental degradation, mainly through the production of highly polluting leachate and methane gas. Methane constitutes one of the six greenhouse gases responsible for the global warming, which needs to be reduced, in order to tackle climate change under the Kyoto Protocol (UN, 1998). The methane emissions from landfills constitute about 30% of the global anthropogenic emissions of methane to the atmosphere (COM, 1996). Reducing the amount of methane emitted from landfills is considered to have the greatest potential for reducing the overall climate change impacts of waste management (Smith et al., 2001).
II. Materials and Methods
The present investigation was carried out in Srinagar, Jammu and Kashmir. The details of the sampling sites, techniques followed and materials used during the course of investigation are as follow.
A. Study Area
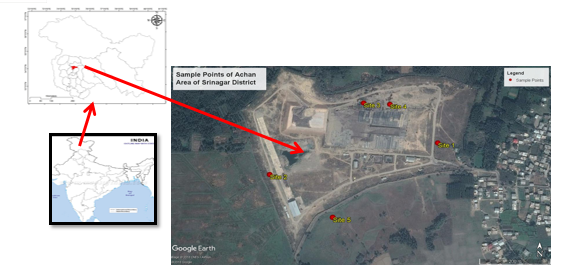
The study area is the municipal solid waste landfill site Achan of Srinagar district, Kashmir. The geographical coordinates of the area are between 34° 5′ 23″ N latitude 74° 47′ 24″ E longitude. It has 1600 m average altitude above sea surface and covers an area of 27 ha. The landfill has been in operation since 1985. The integrated waste management facility at Srinagar is proposed to be set up at present dumping ground located in Achan area. The waste collected from all the generators in the city is transported and disposed off in the landfill site located at Achan. The landfill site is about 7 km. from the city. The land available at this disposal site is about 67.5 acres/ 540 kanals. The site has a boundary wall and has a single entry with a security building. The facility also has a weighbridge, leachate treatment tank, small composting processing pad and a sieving shed. All the incoming waste is directed and is landfilled. No processing or resource recovery from the waste is being undertaken. The first cell at the site was constructed and operated by M/S Ramky Infrastructure Limited. The second cell has been constructed and is under operation by M/s Khilari Enterprises.
B. Climate
The climate of Srinagar city is temperate and characterized by mild summers and chilling winters having normal annual maximum temperature of 19.53°C and minimum of 6.80°C, with normal annual rainfall of 786.2 mm and average monthly rainfall of 60 mm.
C. Wells Location
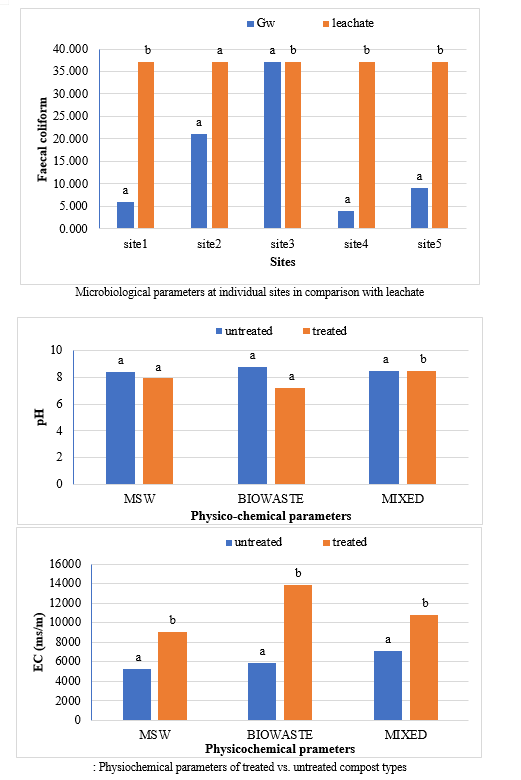
In order to assess the state of quality of the groundwater in the vicinity of the Achan landfill, five testing wells surrounding Achan- Srinagar landfills were choosen, in addition to this the leachate samples were collected from a channelizing path formed at the base of heaps, the filitrate samples were collected from the outlet discharge of filtration unit. The exact location of the wells are presented in above Fig. The wells were located near the entrance of landfill in the east, at the demarcated end of landfill in the west side, two in the northward and one from the south of the landfills. The depth of the wells vary from 30 feet to 60 feet to study the pollutant transport.
D. Collection and preparation of water samples
Groundwater sampling from the existing five testing wells, leachate samples from the source heaps for comparative study and filtrate samples to check the efficiency of filtration unit were collected in the month of June 2017 from the Achan landfill site. After the sampling, the samples were immediately transferred to the lab and were stored in a refrigerator below 4ºC. The analysis was started in lab based on the priority to analyse parameters as prescribed by APHA (2005) methods. All the samples were analysed for selected relevant physio-chemical, heavy metals and microbiological parameters according to the internationally accepted procedures and standard methods APHA (2005)
E. Tested parameters for ground water, leachate and filtrate analysis
Ground water, Leachate and Filtrate samples were analysed for the following parameters:
- Physiochemical parameters
- Soil reaction (pH)
Soil reaction (pH) was determined in 1:2.5 soil : water suspension with a digital glass electrode pH meter. (Jackson, 1973).
- Electrical conductivity (EC)
It was estimated by using solubridge conductivity meter. (Jackson, 1973).
2. Available macro-nutrients
- Available potassium (K)
Available potassium was extracted with Neutral Normal Ammonium Acetate determined by flamephotometer (APHA, 2005).
- Available calcium (Ca) and Magnesium (Mg)
These were determined by Atomic Absorption Spectrophotometer. APHA (2005).
- Available micronutrients and toxic elements
Zinc (Zn), Iron (Fe), Copper (Cu), Manganese (Mn), Nickel (Ni), Lead (Pb), Cadmium (Cd), Chromium (Cr), and Mercury (Hg)
Available micronutrients and toxic elements were extracted by using DTPA extraction method and determined on Atomic Absorption Spectrophotometer (AAS) [APHA, 2005].
- Biochemical Oxygen Demand (BOD)
BOD was measured with OxiTop measuring system using Winkler titration method. The samples discharged into OxiTop bottles followed by placing a magnetic stirring rod. Rubber quiver inserted in the neck of the bottle. Three sodium hydroxide tablets were placed into the rubber quiver with a tweezers. OxiTop bottle was directly tightly closed and pressed on S and M buttons simultaneously for two second until the display shows 00. The bottles were placed in the stirring tray and incubated for 5 days at 20 ºC. Readings of stored values was registered after 5 days by pressing on M until values displayed for 1second (APHA, 2005).
- Chemical Oxygen Demand (COD)
The closed dichromate reflux method (colorimetric method) was used to determine COD. Two ml of the sample is refluxed in strongly acid solution vessel. After digestion in COD reactor at 160oC for 2 hrs, oxygen consumed was measured against standard at 620 nm with a spectrophotometer (APHA, 2005).
- Chloride (Cl-)
10 ml of sample diluted to 100 ml was placed into an Erlenmeyer flask and 1ml potassium chromate solution was added. The mixture was then titrated against a white back ground with silver nitrate solution until the color changes from greenish yellow to reddish brown. Blank sample with distilled water was treated in the same way as the sample (Mohr’s titration) [APHA, 2005].
- Ammonia (NH4+)
Ammonia was tested by using distillation method which was followed by titration step to determine the concentration of ammonia. Ammonia was distilled into a solution of boric acid and the ammonia in the distillate was determined titrimetrically with standard HCl (APHA, 2005).
- Nitrate (NO3-)
Nitrate in water samples were determined by using Salicylate method, using Nitrate solution prepared from KNO3 as standard and measuring the absorbance at 410 nm (APHA, 2005).
- Sulfate (SO42-)
Sulfate was measured using Turbidimetric Method. Sulfate ion (SO4-2) is precipitated in an acetic acid medium with barium chloride (BaCl2) so as to form barium sulfate (BaSO4) crystals of uniform size. Light absorbance of the BaSO4 suspension is measured by a turbidimeter and the SO4-2 concentration is determined by comparison of the reading with a standard curve. APHA (2005)
- Phosphate (PO4-2)
Phosphate in water samples was estimated by chlorimetric method (spectrometry) [APHA, 2005].
- Carbonates and bicarbonates
Carbonates and bicarbonates in water samples were determined by titrating a known volume of water against standard H2SO4 using phenolphthalein and methyl orange indicators respectively (APHA, 2005].
- Phenol
Total phenolic content was determined by modified method reported by APHA (2005). 500µl of sample was combined with 2.5ml of double distillation water and immediately 0.5ml of folin-cioucalteau reagent was added. After 3 minutes of incubation period, 20% sodium carbonate was added to each sample, vortexed and boiled in a water bath for exactly one minute. The absorbance of each sample was measured at 650 nm against reagent blank.A standard curve was established using catechol as standard and concentration of phenol in sample was determined accordingly.
- Total dissolved solids
TDS in the water samples were calculated by using the following formula:
TDS=0.67*EC
Where, TDS is in (mg/L) and EC is in (µS/cm)
3. Biological pollutant indicators
- Sterilization
Glasswares used were thoroughly washed in detergent water, running tap water followed by rinsing in distilled water. Glasswares were sterilized in hot air oven at 180oC temperature for 30 minutes. All the media, water blanks etc., were sterilized in autoclave at 15 lbs per square inch pressure of pure steam for 20 minutes, unless mentioned otherwise. Laminar airflow chamber was sterilized by disinfectant followed by ultra violet (UV) irradiation for 30 minutes before start of the work.
4. Preparation of Media
The estimation of E. coli microorganisms were done by serial dilution technique (Aneja, 2001) using a specific media. Following media were used for isolation of different group of microorganisms:-
Nutrient specific media for E. coli
|
Constituent |
Quantity/litre |
|
Agar |
12 |
|
Crystal violet |
0.002 |
|
Lactose |
10 |
|
Neutral red |
0.03 |
|
Peptone(vegetable) |
7.0 |
|
Nacl |
5 |
|
Synthetic detergent |
1.5 |
|
Yeast extract |
3 |
5. Isolation And Enumeration Of Polluting Microorganisms
- Isolation of bacteria
One milliliter of water sample was placed in 9 ml of sterilized distilled water under aseptic conditions. Serial dilution of 102, 103, 104, 105, 106, 107 were prepared. One ml of aliquot from specific dilution was added over cooled and solidified nutrient media (NA) in petriplates. The plates were rotated for uniform distribution. The plates were incubated at 28±2oC for 3-4 days. The bacterial colonies were identified on the basis of colony features and morphological characters of cells. Three replications were taken for each sample. The bacterial counts were expressed as colony forming unit per milliliter of water (Cfu/ml water).
- Isolation of fungi
One milliliter of the water sample was placed in 9 ml of sterilized distilled water under aseptic conditions. Serial dilution of 102, 103, 104, 105, 106, 107 were prepared. One ml of aliquot from specific dilution was added over cooled and solidified nutrient media in petriplates. The plates were rotated for uniform distribution. The plates were incubated at 28±2oC for 3-4 days. The fungal colonies were identified on the basis of colony features and morphological characters of cells. Three replications were taken for each sample. The fungal counts were expressed as colony forming unit per milliliter of water (Cfu/ml water).
6. Statistical analysis
- Exploratory statistical analysis
The statistical analyses were conducted using two software SPSS (Version 17.0) and OP-STAT. Difference between the parameters of leachate and the groundwater samples at each sampling site were tested using one-way analysis of variance (ANOVA) (P<0.05). The mean, minimum, maximum, standard deviation (SD), were calculated in SPSS for considered variables to describe the spread of properties.
7. Pollution assessment of ground water
- Single factor pollution index (pi)
The single factor pollution index method (Liang and Zheng, 2009) was used to assess groundwater quality based on data cited from published reference. The standard for parameters was referenced to the level III water quality category which cited in “Quality Standard for Ground Water” (GB/T14848-93) (AQSIQ, 1993). The default in the above standard was cited at level III water quality categories of “Environmental Quality Standards for Surface Water” (GB3838-2002) (EPA and AQSIQ, 2002). The single factor pollution index method is formulated as:
Pi=Ci/Si (Eq. 1)
Where,
Pi the pollution index for ith parameter;
Ci the monitoring value of ith parameter in each groundwater sample;
Si the standard value for ith parameter in each groundwater sample.
- Nemerow index (PI)
Water quality is a complex issue that involves many different kinds of contaminants, the Nemerow index (Liang and Zheng, 2009) can be essential for scientifically reflecting the kinds and levels of main pollutants, according to water pollution standards. The Nemerow index method is formulated as ;
Where
PI =√ Pi2avg+ Pi2max/2 (Eq. 2)
PI the Nemerow index for ith pollutant;
Pi avg the mean value of Pi for all samples;
Pi max the maximum value of Pi for all samples.
- Collection of compost samples
Srinagar Muncipal Corporation (SMC) is responsible for the collection and disposal of all the municipal solid waste (MSW) and green waste (GW) produced within the premises of Srinagar city to the Achan landfill Srinagar Kashmir. The samples of municipal solid waste as well as green waste collected by SMC Srinagar coming to the landfill for its disposal were taken for the preparation of compost. Municipal solid waste sources for composting were taken from newly established segregation unit in the landfill. The green waste (rotten fruits, vegetables and straw) were taken from the same site. The mixed waste (MSW +GW) type was prepared by taking both the waste in the ratio of 1:1.
- Composting procedure
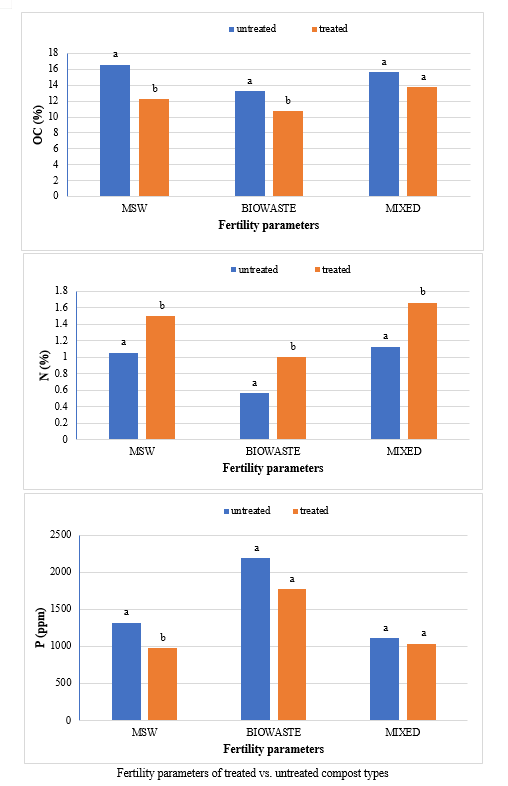
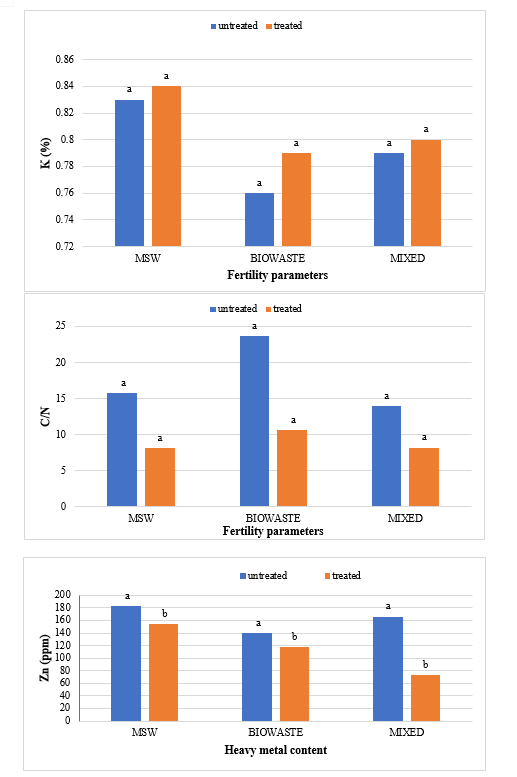
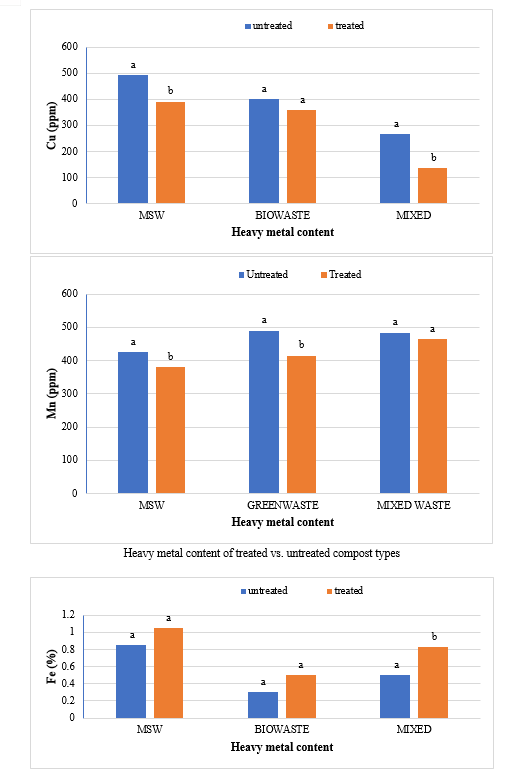
The waste for composting was taken to a shaded area that was covered at the top to prevent the area from rain and direct exposure of sun. The composting was carried out by heap method. In this experiment there were two types of treatment- compost heap (treated) with Bioagents and compost heap (untreated) without bioagent. About 1m3 heap for each type of composting sources viz, untreated and treated municipal solid waste, untreated and treated green waste, untreated and treated mixed waste. The particulars for the treated composting are mentioned in Table . Water was added until the moisture content reached 60% (wet basis) in each compost heap. To retain the moisture and prevent excessive loss of heat, the heaps of composting material were then covered using plastic sheets. The moisture content was maintained at 50-60% by the addition of water throughout the active composting period by frequent checking. The mixtures were turned at 3-day intervals initially to maintain porosity. The temperature was measured daily with a digital thermometer at random depths. Compost samples were taken from untreated heaps after 90 days and from treated heaps after 75 days of the composting and were analysed for their physical and chemical properties The representative samples were collected from the piles in air-tight polythene bags after proper mixing and then labelled carefully The samples were carried to laboratory and stored in a cold room at a temperature of 4°C for further analysis. Samples were dried at room temperature, homogenised and sub-sampled by quartering and ground to pass through 2 mm sieve. These processed samples were sub sampled for further analysis.
Composition of particulars in treated case
|
S. No. |
Particulars |
|
1 |
Molasses |
|
2 |
Black polythene (200micron) |
|
3 |
Trichoderma spp (1 litre) |
|
4 |
Biofertilizers (azatobacter+PSB 1 litre) |
|
5 |
Shalimar microbes (1 litre) |
|
6 |
Pseudomonas spp (1 litre) |
|
7 |
Lime (5 kg) |




Conclusion
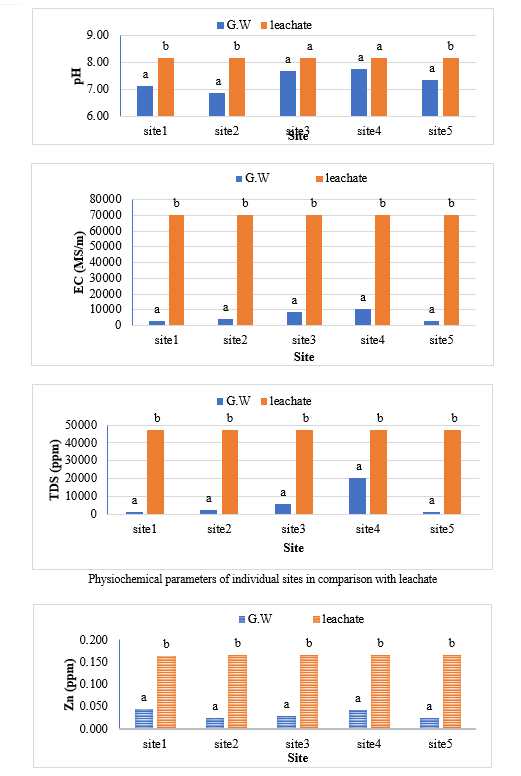
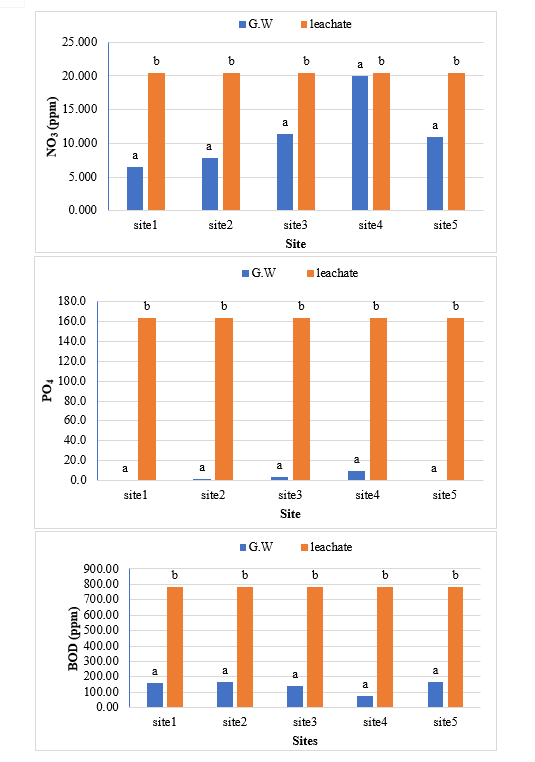
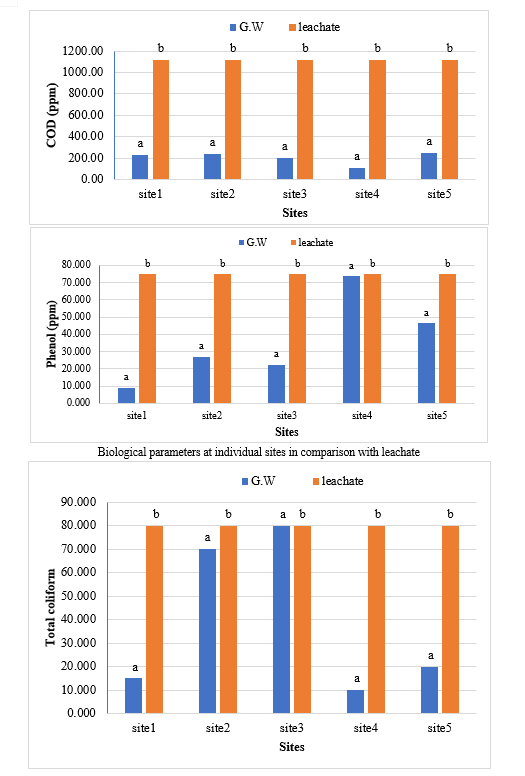
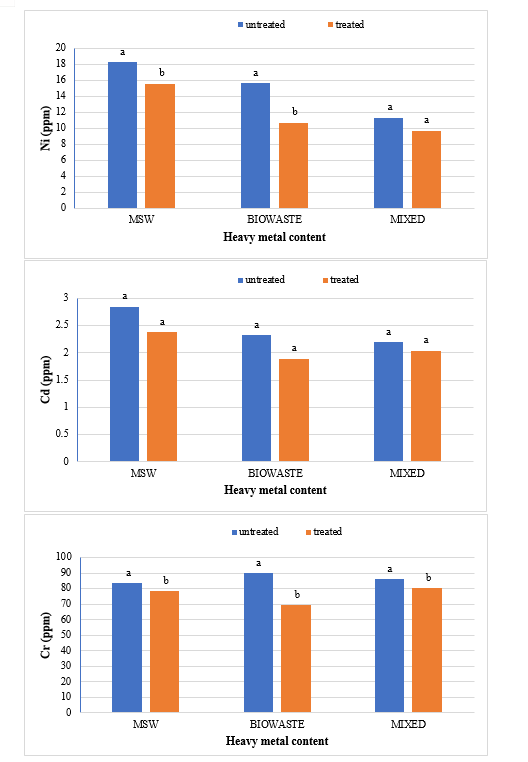
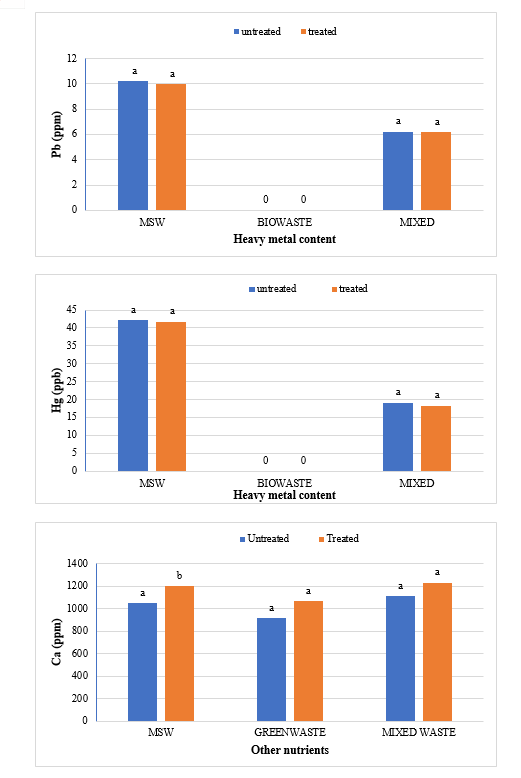
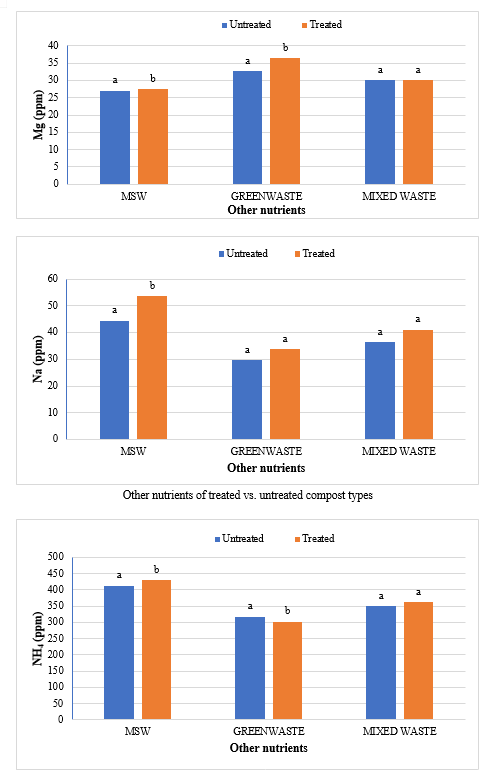
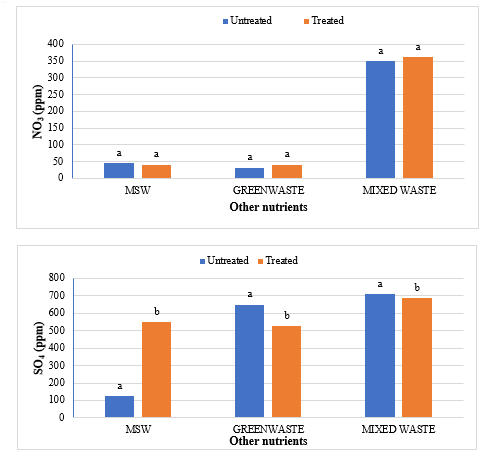
The study comprised ground water, leachate and compost analysis of samples collected from different sampling sites, and compost formed heaps. The major findings of the study are summarized below: 1) The moderately high concentration of some of the parameters like EC (2630-30320 mg/L), TDS (1761-576267 mg/L), NH4+ (9-30 mg/L), Mg (155-487 mg/L), BOD (76-243 mg/L), COD (112-249 mg/L), Phenol (9-73 mg/L), Cd (0.08-0.15 mg/L), Cr (0.34-0.42 mg/L) in groundwater near landfill deteriorates its quality for drinking and other domestic purposes as these concentrations are above the limits of Indian standard for drinking water (BIS-2012) and WHO. In addition to this these also represent heavy pollutant indicators as per the Single Point Pollution Index as well by the Nemerow index as these elements crossed the safe limit for both these pollution indexes. Further, the presence of Cl-, NO3-, NH4+, Phenol and COD can be used as tracer with relation to leachate percolation. The presence of total coliform (TC) and faecal coliform (FC) in most of water samples indicate the contamination possibly due to leachate percolation in groundwater. The presence of faecal contamination is an indicator that a potential health risk exists for individual exposed to this water. As there is no natural or other possible reason for high concentration of these pollutants, it can be concluded that leachate has significant impact on groundwater quality near the area of Srinagar landfill site Achan. The groundwater quality improves with the increase in depth and distance of the well from the pollution source (well 1 and well 2). Although, the concentrations of few contaminants do not exceed drinking water standard even then the ground water quality represent a significant threat to public health. 2) By performing the statistical analysis using SPSS software-17, it is conclude that there is a significant difference (p-value< 0.05) between most of the parameters of leachate and groundwater at all the sampling site. 3) By performing the statistical analysis using O.P. STAT obtaining Critical Difference (C.D), it is concluded that filtrate and leachate differ significantly from each other. And thus concluded the efficient nature of leachate filtration unit. 4) This study showed that majority of the compost failed to achieve the requisite specification with respect to both fertilizing and heavy metal parameters of the quality control (QC) standard, mainly due to the use of mixed wastes as feedstock material for composting. All of the micronutrient (heavy metals) values are below the standard limit of heavy metals except chromium and copper in all types of compost. The statistical analysis using SPSS-17 software concluded showed that: • In case of MSW Compost there is a significant difference (P < 0.05) between the N, P, OC, EC, Mn, NH4+, SO4+, Ca, Mg, Na, Fe and Zn, in compost treated with effective microorganisms (EM) and the compost without EM. While the parameters like pH, K, NO3, C:N, Cu, Cd, Cr, Ni, Pb, none of them reached to the level of significance. • In case of GW Compost there is a significant difference (p < 0.05) between the N, OC, EC, NH4+, SO4+, Fe, Mn, Cr, in compost treated with (EM) and the compost without EM. Also there is no significant difference between treatments in case of pH, P, K, NO3, C:N, Cd, Cu, Ca, Mg. • In case of MW compost, treated samples vary significantly in terms of N, P, K, EC, pH, C:N, SO4+, Zn, Fe, with untreated samples at (P < 0.05), while the parameters like Ca, Mg, Na, Mn, Cu, Cd, Cr, Ni, Pb, NO3, exhibited non-significant difference between treated and untreated compost types. • The prepared untreated MSW compost and treated MSW compost has Fertilizing Potential (Fi) value of 3.40 and 3.47 respectively. For untreated and treated green-waste compost the Fi value was 2 and 2.73 respectively, also for the untreated and treated mixed waste compost Fi value was 3.27 and 3.47 respectively. From this it is concluded that MSW compost and mixed waste compost are good in terms of fertilizing potential as (FI value >3) while green-waste compost is poor in same as (FI value <3). • Heavy metal polluting potential (CI value) for untreated and treated MSW compost, was 2.8 and 3.0, untreated and treated green-waste, was 3.06 and 3.7 and for untreated and treated mixed waste compost, was 3.0 and 3.2 respectively. From the calculated values of CI, it is concluded that MSW treated & untreated as well as mixed waste treated and untreated belongs to marketable Class D (medium fertilizing potential and medium heavy metal content and the green waste treated untreated belong to restricted use Class RU-1 (Should not be allowed to market due to low fertilizing potential. However, these can be used as soil conditioner). It can also be concluded that Pseudomonas spp, Azatobacter played a great role of bioremediation of heavy metals, Shalimar consortium also had a good role in mineralization, solubilization of some elements like iron zinc, and increase in nitrogen content
References
[1] Abbas, A., Jingsong, G., Ping., L., Ya, P. and Al-Rekabi, W. 2009. Review on landfill leachate treatments, Journal of Applied Science Research 5: 534-545. [2] Abiriga, D.2017. Groundwater contamination from an old municipal. landfill at Revdalen, Norway. M. Sc Thesis. University College of Southeast Norway. [3] Adeolu, O. A., Oriaku Ada V., Adewumi, G. A, and Otitoloju, A. A. 2011. Assessment of groundwater contamination by leachate near a municipal solid waste landfill. African Journal of Environmental Science and Technology 5(11): 933-940. [4] Afsar, S. S., Kumar, S., Alam, P. 2015. Characterization of leachate at various landfill site of Delhi, India. International Journal of Advanced Technology in Engineering and Science. ISSN 2348-7550. [5] Agarwal, R., Chaudhary, M. and Singh, J. 2015. Waste management initiatives in India for human well-being. European Scientific Journal ISSN: 1857-7881. [6] Akhtar, M. S., Chali, B. and Azam, T. 2013. Bioremediation of arsenic and lead by plants and microbes from contaminated soil. Research in Plant Sciences 1(3): 68-73. [7] Akinbile, C. O. 2012. Environmental impact of landfill on ground water quality and agricultural soils in Nigeria. Journal of Soil Water Research 7(1): 18-26. [8] Akinbile, C. O. 2012. Environmental impact of landfill on groundwater quality and agricultural soils in Nigeria. Soil Water Research 7(1): 18-26. [9] Alam, P. and Ahmade, K. 2013. Impact of solid waste on health and the environment. Special Issue of International Journal of Sustainable Development and Green Economics 2(I) [10] Alslaibi, T. M., Mogheir, Y.K. and Afifi, S. 2009. Assessment of groundwater quality in the municipal solid waste landfill leachate. Journal of environmental science and technology 4 (4): 419-436. [11] Aneja, K. R. 2001. Experiments in microbiology, plant pathology, Tissue Culture and Mushroom Production Technology. pp.68. [12] Antón, M.A., Mu?oz, P., Montero, J.I. and Soliva, M. 2005. Improving waste management in protected horticulture. Agronomy for Sustainable Development 25(4): 447-453. [13] APHA, 1995. Standard methods for the examination of water and waste water, 20th edition. Washington D. C, American Public Health Association. [14] APHA, 2005. Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC. [15] APHA-AWWA-WPCF. 1994, Standard Methods for the Examination of Water and Waste water, 15th edition. American Public Health Association, Washington, DC, USA. [16] Asha Sahu, Renu Shukla, Bhanu P. Singh, Jai P. Rai, Pawan Kumar Sharma and Harshad Lade and Diby Paul. [17] Ashworth, D. C, Elliott, P. and Toledano, M. B. 2014. Waste incineration and adverse birth and neonatal outcomes: A systematic review. Journal of Environment International 69: 120-132. [18] ATSDR, 2002. Toxicological profile for copper (draft for public comment). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (Subcontract No. ATSDR-205-1999-00024). [19] ATSDR. ToxProfile. 2016. Cadmium. ATSDR Tox Profiles. http://www.atsdr.cdc.gov/toxprofiles/tp5-c2.pdf. [20] ATSDR. ToxProfile. 2016. Cadmium. ATSDR Tox Profiles. http://www.atsdr.cdc.gov/toxprofiles/tp5-c2.pdf. [21] Awasthi, G., Chester, A., Chaturvedi, R. and Prakash, J. 2015. Study on Role of Pseudomonas aeruginosa on Heavy Metal Bioremediation. International Journal of Pure & Applied Bioscience 3(4): 92-100. [22] Ayari, F., Hamdi, H., Jedidi, N., Gharbi, N., Kossai, R. 2010. Heavy metal distribution in soil and plant in municipal solid waste compost amended plots. International Journal Environmental Science Technology 7 (3): 465-472. [23] Azim, M. D., Rahman, M. M., Khan, R. H. and Kamal, A. 2011. Characteristics of leachate generated at landfill sites and probable risks of surface and groundwater pollution in the surrounding areas: a case study of Matuail landfill site, Dhaka. Journal of Bangladesh Academy of Sciences 35(2): 153-160. [24] Aziz, S. Q., Aziz, H. A., Yusoff, M. S., Bashir, M. J. K. and Umar, M. 2010. Leachate characterization in semi-aerobic and anaerobic sanitary landfills: A comparative study. Journal of Environmental Management 91: 2608-2614. [25] Aziz, S.Q., Aziz, H.A., Bashir, M. J. K. and Mojiri, A. 2014. Waste water Engineering: Advanced waste water treatment systems. International of Scientific Research Books pp.208-224. [26] Banu, S. and Berrin, T. 2015. Parametric fate and transport profiling for selective groundwater monitoring at closed landfills: a case study. Journal of Waste Management 38: 263-270. [27] Barrena, R. 2006. Effect of inoculum dosing on the composting of source-selected organic fraction of municipal solid wastes. Journal of Chemical Technology and Biotechnology 81: 420 -425. [28] Bashir, M. J. K., Aziz, H. A., Aziz, S. Q. and Abu Amr, S. S. 2012. An over view of electro oxidation processes performance in stabilized landfill leachate treatment. Journal of Desalinisation and Water Treatment 51: 2170-2184. [29] Beck-Friis, B., Smars, S., Jonsson, H., Kirchmann, H. 2001. Gaseous € emissions of carbon dioxide, ammonia and nitrous oxide from organic household waste in a compost reactor under different temperature regimes. Journal of Agricultural Engineering Research 78(4): 423-430. [30] Benito, M., Masaguer, A. and Moliner, A. 2003. Chemical and microbial parameters for the characterization of the stability and maturity of pruning waste compost. Biological Fertilizing Soils 37: 184-189. [31] Bertran, E., Sort, X., Soliva, M. and Trillas, I. 2004. Composting winery waste: sludges and grape stalks. Bioresource Technology 95(2): 203-208. [32] Bhalla, B., Saini, M. and Jha, M. 2013. Effect of age and seasonal variations on leachate characteristics of municipal solid waste landfill. International Journal of Research in Engineering and Technology 2: 223-232. [33] Bhalla, G., Swamee, P. K., Kumar, A. and Bansal, A. 2012. Assessment of ground water quality near municipal solid waste landfill by an Aggregate Index Method. International Journal of Environment. M. 2003. The Groundwater Geochemistry
Copyright
Copyright © 2024 Dr. Bashir Ahmad Pandit. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET58183
Publish Date : 2024-01-26
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

