Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Comparative in Vivo Study on Quality Analysis on Bisacodyl of Different Brands
Authors: Shubham Patel, Aakash Patel, Rahul Lovhare
DOI Link: https://doi.org/10.22214/ijraset.2023.52196
Certificate: View Certificate
Abstract
Bisacodyl is an organic chemical belongs to the diphenylmethane family that is utilised as a laxative stimulant. To induce a bowel movement, it acts directly on the colon. It is commonly used to treat episodic and chronic constipation, as well as neurogenic bowel dysfunction, and as part of bowel preparation before medical procedures such as a colonoscopy. It has a dual prokinetic and secretory activity and must be converted in the stomach into the active metabolite bis-( p-hydroxyphenyl)-pyridyl-2-methane (BHPM) to generate the intended laxative effect. Bisacodyl operates locally in the large bowel by directly improving motility, decreasing transit time, and raising stool water content. The tablets available on the market from various popular brands were chosen for the evaluation based on different quality control tests such as physical appearance, hardness, friability, disintegration time, weight variations, dissolution rate, and potency. The test will be implement of the certain kind of available product such as DULCOFLEX, BISOWEX, BISFLOW and BYLAX-5 which has active contain of 10mg/each respectively. This study is likely to be a point of appreciation in raising awareness among the people and prescriber communities about the importance of having a higher surplus of medicines by selecting the suitable medication from among some sorts of commercial brands which is available in market formulation.
Introduction
I. INTRODUCTION
Tablets made from dry powders are characterised as solid pharmaceutical dosage forms that contain pharmacological ingredients with or without acceptable diluents and are formed using either the wet granulation, dry granulation, or direct compression method . Tablets on the market come in a variety of forms, strengths, and API combinations[1]. Tablets are solid, flat or biconvex dishes and unit dose forms made by compressing a medicine or drug mixture with or without diluents.
The majority of dossiers are accessible as tablets that can be ingested easily and have immediate or sustained release effect[2]. The oral route of administration is the most popular since it is one of the safest and most convenient means of drug administration. As a result, it is critical to assess the optimal quality maintenance of each drug for human health. Tablets are one of the most used dose forms all around the world. Nearly all medication compounds can be formed in a tablet, and the tablet manufacturing technique is very simple and adaptable[3].
It is an efficient and well-tolerated medication for constipation patients. It improves bowel function, symptoms of constipation, and disease-related quality of life (QOL)[4]. It has been used as a first-line laxative for functional constipation treatment and, more recently, to help with bowel preparation before to experimental procedures (e.g., colonoscopy, sigmoidoscopy), surgery, or pharmacologic stimulation during colon manometry[5].
When offered to neuropathy patients, bisacodyl is effective. Bisacodyl appears to outperform the other drugs in terms of reducing the number of bowel movements per week. It has an impact in the large intestine 6 to 12 hours after oral treatment and 15 to 60 minutes after rectal administration[6].
Constipation is characterized by several symptoms, including hard stools, considerable straining, infrequent bowel motions, and/or the sensation of incomplete evacuation[7].It can occur on an irregular/episodic basis, which is often managed by self-medication, or on a chronic basis (usually >4 weeks or, according to consensus criteria, >3 months)[8].which is more likely to receive therapy by a practitioner or through speciality care.
Bisacodyl is a diphenylmethane derivative that is used as a contact laxative. It works directly against the colon wall, strengthening the peristaltic action that softens the stool. Aside from being a typical laxative, it is also frequently used during surgery or x-ray inspection. Bisacodyl resorption in the small intestine can reach 50%, and it is largely eliminated by bile after deacetylation in the liver and passes through enterohepatic cycles. The remaining active metabolites are eliminated by the kidney, and the amount that is not absorbed is helpful to the intestinal wall[9].

Bisacodyl belongs to diphenylmethane class with chemical name 4,4'-(pyridin-2-ylmethylene)bis(4,1-phenylene) diacetate (Figure Bisacodyl contains not less than 98.0 % and not more than 101.0 % of C22H19NO4, calculated on the dried basis[10].
- Molar mass:-361.391g/mol
- Formula:- C22H19NO4
- Pregnancy category:- B
- Classification:- Organic compound
- Elimination half-life:- 16 Hours
A. Objective
- The main purpose is to determine safety and effectiveness of the new drug.
- To evaluate a tablet's potential for efficacy.
- The main aim is to study the quality control tests of Tablets which is the most popular conventional dosage forms and to list down the similarities and differences as per various Pharmacopoeias.
- It provide valuable information regarding the effects of a particular substance or disease progression in a whole, living organism.
- To study the effectiness of the formulation available in the outer most market which would define their potency and safety and used for the public wellbeing.
- Comprehend the amount of tolerance encorporate in the remedies which would affect the livelihood.
B. History of Bisacodyl
Bisacodyl is a locally acting laxative that has been used since the 1950s to treat constipation and promote defecation. Bisacodyl belongs to the diphenyl methane derivatives class. One of the oldest members of this compounds group, phenolphthalein, had a modest laxative efficacy and required 30-200 mg daily to produce a reaction in adults. Following thorough structure-activity analyses of substances structurally related to phenolphthalein, the diphenolic laxative group was discovered.
- Structure-Activity Insights
The term diphenolic laxatives refers to the two free hydroxyl groups at para positions on the two benzene rings that are required to elicit a secretagogue and laxative activity. Additionally the structure of the third aryl group influences secretagogue efficacy. If a nitrogen is present in the aromatic ring, the distance from the methyl group's central carbon atom and the nitrogen group's dissociation constant will alter the secretagogue activity.
Bisacodyl and sodium Pico sulfate are both prodrugs that are metabolized in the gastrointestinal tract into the same active metabolite, bis-(p-hydroxyphenyl)-BHPM (pyridyl-2-methane), which has the necessary laxative action.
The action of endogenous deacetylase enzymes on the mucosa of the small intestine and colon mediates the conversion of bisacodyl to BHPM, whereas sodium picosulfate is converted to BHPM by the action of colonic bacteria desulfate enzymes (e.g., arylsulfate sulfotransferase of Eubacterium rectale) (Figure 2). Because sodium picosulfate is dependent on bacterial activity, antibiotic use can impair its activity. Similar considerations apply to senna and cascara, both of which are digested by gut bacteria.

C. Pharmacokinetic
Bisacodyl is supplied as a 5-layer enteric-coated tablet (Figure 3) that does not disintegrate until it reaches the lower intestine, limiting absorption and assuring conversion to BHPM in the colon. Bisacodyl is available as an enteric-coated oral drug/tablet at a dose of 5 mg or as a rectal suppository at a dose of 10 mg for adults and adolescents (over the age of 10 years),and for children aged 4-10 years as an oral tablet or rectal suppository at a dose of 5 mg. The onset of action occurs between 6 and 12 hours after oral administration, while the suppository can take effect between 15 and 60 minutes following rectal administration. Tablets are recommended to be taken at night and the bowel movement typically occurs the following morning, at the time when colonic motor activity is normally highest Only a limited quantity of the drug is absorbed after oral and rectal administration, and it is virtually entirely conjugated in the intestinal wall and liver to generate the inactive BHPM glucuronide. Even after several once-daily dosage with 2 x 5 mg bisacodyl enteric-coated tablets, there is no measurable free BHPM in the plasma, however the inactive glucuronidated BHPM is detected. With bisacodyl pills, the mean plasma elimination half-life of BHPM has been estimated to be 7.7 hours. Following the administration of enteric-coated bisacodyl tablets, an average of 51.8% of the dose was recovered as free BHPM in the faeces and 10.5% as BHPM glucuronide in the urine. The authors of the second study also evaluated the excretion of total and free BHPM into human breast milk in two groups of eight healthy nursing mothers and discovered that it did not accumulate after several bisacodyl administrations and was not excreted into the breast milk. No data from controlled human pregnancy studies are available. Long-term post-marketing surveillance has not shown evidence of undesirable or damaging effects during pregnancy.
Early trials revealed only minor side effects. as well as additional reviews Bisacodyl is considered safe for use during pregnancy. A previous investigation on the suppositories found that no plasma levels of bisacodyl metabolites could be detected in half (n = 6/12) of the healthy volunteers recruited, and only 3.1% of the dose was eliminated in the urine. Bisacodyl is a laxative that acts locally, and absorption is not necessary for the laxative effect. The time it takes for the prodrug to reach the lower intestine and be transformed into the active pharmaceutical component BHPM determines the time to onset. There is a lag time before there is an increase in plasma levels of glucuronidated BHPM. The absence of a temporal relationship between the laxative effects and the plasma levels supports the view that BHPM acts locally[11].

1. Absorption
Bisacodyl oral formulations are only 16% bioavailable. A 10 mg enteric coated oral tablet achieves a Cmax of 26 ng/mL with an 8-hour Tmax, but a 10 mg oral solution achieves a Cmax of 237 ng/mL with a 1.7-hour Tmax. A Cmax of 0-64 ng/mL is achieved using a 10 mg suppository. In lactating women, 10mg of oral bisacodyl achieves a Cmax of 20.5-195 ng/mL, a Tmax of 3-4 hours, and a geometric mean AUC of 471 h*ng/mL following a single dosage. The geometric mean AUC drops to 311 h*ng/mL after numerous doses.
2. Distribution
Bisacodyl's volume of distribution data is not easily available. In lactating women, however, the volume of distribution of the active metabolite, BHPM, is 181 L after a single dosage and 289 L at steady state.
3. Metabolism
An intestine deacetylase converts bisacodyl to the active bis-(p-hydroxyphenyl)-pyridyl-2-methane (BHPM).The gastrointestinal tract absorbs a little quantity of BHPM, which is then glucuronidated before excretion.
4. Excretion
The vast majority of bisacodyl is excreted. The active metabolite BHPM excretes 13.8-17.0% of a bisacodyl dosage in the urine. Bisacodyl half-life data is difficult to come by. In lactating women, the half-life of the active metabolite, BHPM, was 7.3 h after a single 10 mg oral dose and 10.0 h after several doses. regarding the clearance of bisacodyl is not readily available. The apparent plasma clearance of the active metabolite, BHPM, in lactating women after a single 10 mg oral dose is 272 mL/min and after multiple doses is 412 mL/min[12].
D. Mechanism of Bisacodyl
The gastrointestinal tract is the site of action of bisacodyl. Because its composition is a coated tablet, which is resistant to degradation in the stomach and small intestine and hence accomplishes transit to the colon in its intact form, absorption from the GI tract is modest. It then dissolves in the colon, providing a laxative effect following oral administration. Bisacodyl is hydrolyzed by intestinal deacetylase and bacterial enzymes to a deacetylated active metabolite, bis- (p-hydroxyphenyl)-pyridyl-2-methane (BHPM), which activates the intestinal mucosa and causes peristalsis, which is responsible for the laxative activity. In the colon, BPHM has two effects: an anti-absorptive-secretory impact and a direct prokinetic action via stimulating parasympathetic nerve terminals in the colonic mucosa. It operates locally in the large bowel by promoting myoelectrical and motor activity as well as intestinal secretion, improving colon motility, decreasing overall colonic transit time, and raising stool water content.
Ratnaike et al. found that bisacodyl affects fluid absorption by activating adenylate cyclase in small intestine enterocytes, which causes active secretion of Cl- and HCO3-, passive efflux of Na+, K+, and water, and suppression of Na+ and Cl- absorption into the enterocyte.
Bisacodyl may also have a laxative effect by preventing water transfer from the intestinal tract to the vascular side of the cells by lowering the expression of aquaporin 3 (AQP3) in the colon. conducted a study showing that bisacodyl increases sigmoid colon longitudinal muscle tone through direct action on the smooth muscle, but not in the colonic circular muscle, and suggest that bisacodyl could be effectively administered to manage constipation in patients with neuropathy because bisacodyl improves the spatiotemporal-coordinated pattern of HAPCs.
- Administration
Bisacodyl can be administered orally or rectally. Bisacodyl is given to adults in dosages of 5 to 10 mg tablet daily, either at night or before bedtime, because it acts within 6 to 12 hours of administration when taken orally, thus the therapeutic impact occurs in the morning.
While bisacodyl works within 15 to 60 minutes of rectal administration, it can also be given in 10 mg as an enema or suppository in the morning. For full bowel evacuation, 10 to 20 mg are administered orally, followed by 10 mg as a suppository the next morning. Bisacodyl oral or rectal is administered in doses of 5 mg per day, once at night, to children aged 3 to 10 years. Ten-year-olds and older can be given up to ten milligrams per day in one dose at night. Prolonged use of bisacodyl may lessen its efficacy because it increases the creation of PGE2, which decreases the expression of AQP3, and the effectiveness of chemicals that decrease AQP3 expression in the colon, which is reduced by continued usage[13].
E. Side Effect
- Mild side effects of bisacodyl such as:
a. Abdominal cramping
b. Electrolyte and fluid imbalance
c. Excessive diarrhea
d. Nausea
e. Rectal burning
f. Spinning sensation (vertigo/unconsciousness)
g. Stomach/abdominal pain
h. Vomiting
i. Weakness
2. Major side effects of bisacodyl such as:
a. Persistent nausea/vomiting/diarrhea
b. Muscle cramps/weakness
c. Irregular heartbeat
d. Dizziness
e. Fainting
f. Decreased urination
g. Mental/mood changes (such as chaos)
This document does not cover all of the potential adverse effects, and more may arise. For further information about side effects, consult your doctor[13].
F. Contraindication
- General Information
In the United States, bisacodyl tannex formulations are no longer available. Bisacodyl tannex was not to be taken in repeated dosages due to the potential of increased tannic acid absorption, which could lead to hepatotoxicity and mortality; specified dosages were not to be exceeded. Bisacodyl tannex was also not recommended for patients under the age of ten since the risk of absorbing tannic acid in this age group had not been studied.
2. Abdominal Pain, Acute Abdomen, Appendicitis, Colitis, Vomiting
Bisacodyl should not be utilised in individuals with GI obstruction or ileus, GI perforation, toxic colitis, or toxic megacolon. Prior to starting laxative medication, patients with symptoms suggestive of bowel blockage (e.g., acute abdomen or appendicitis, abdominal pain, distension, nausea, or vomiting) should be assessed. In the presence of severe ulcerative colitis or other situations where the integrity of the intestinal wall may be damaged, use bisacodyl with caution (e.g., diverticulitis, rectal fissures). Stimulant laxatives can aggravate these symptoms and, in rare cases, result in intestinal perforation. In a patient with faecal impaction, a stimulant laxative is unlikely to cause disimpaction, and other known successful techniques should be used. Bisacodyl tablets should not be used in patients with dysphagia or those who cannot swallow a tablet without chewing it first
3. Diarrhea, Electrolyte Imbalance, Gastrointestinal Hemorrhage, And Hypokalemia
Individuals should be encouraged to quit use and visit their doctor if they observe a rapid change in bowel habits that lasts more than two weeks. Bisacodyl is contraindicated in patients with rectal GI bleeding; if this laxative causes rectal GI bleeding or fails to produce a bowel movement within 12 hours, patients should discontinue treatment and visit their health care practitioner. Patients should not use this product for self-treatment for more than a week without seeking medical advice; prolonged use is not recommended. Individuals who experience undetected rectal bleeding should discontinue use of the laxative and visit their doctor. Laxatives that stimulate the GI tract, such as bisacodyl, are the most likely to produce GI irritation or fluid and electrolyte loss. Patients above the age of 65 may be more prone to hypokalemia or other electrolyte imbalances. If diarrhoea develops, the stimulant laxative should be stopped. Long-term continuous stimulant laxative therapy may result in laxative dependence ('cathartic colon'). Most stimulant laxatives currently available, however, can be used without risk of laxative dependence when used no more than 2 to 3 times per week.
1.6.4 Pregnancy, and workers:-
Before using stimulant laxatives like bisacodyl during pregnancy, women should contact with a trained health care provider. Because of the powerful stimulant activity on the colon, indiscriminate use of stimulant laxatives during pregnancy may cause dehydration and pre-term labour; consequently, stimulant laxatives should be avoided during pregnancy if feasible. Bisacodyl's safety during pregnancy has not been established; however, no indication of adverse effects has been documented, and systemic absorption should be low after either oral or rectal administration. Teratogenic effects are not predicted with occasional use at acceptable levels during pregnancy, when absolutely necessary and when advised by a skilled health care professional. To limit drug exposure to the fetus, the safest first-line therapies to utilize during pregnancy are those that are not absorbed systemically (e.g., fiber, bulk-forming laxatives, stool softeners). Psyllium, docusate sodium, or polyethylene glycol 3350 have low systemic absorption and can be used to treat chronic constipation.
G. Types Of Tablet
- Uncoated tablets: It contains active and excipients which are compressed together without any coat/cover.
- Coated tablets: This tab are those which have additional coating on tablet surface. These coatings include Gums, sugar, plasticizer and waxes.
- Sugar Coated Tablets: These are compressed tablets that have been coated with a concentrated sugar solution in order to improve patient compliance, increase appeal, hide undesirable taste or odor, and promote stability. The issue with this coating is the high expense of the procedure, validation, and delivery.
- Film-Coated Tablets: These are standard tablets coated with a thin layer of polymers such as HPMC, HPC, or a polymer mixture such as Eudragit E100 (forms skin like film).The properties are comparable to sugar coating, but it has the added benefits of being more durable, less bulky, and less time consuming to apply. Water is created in this composition to break and investigate core tablet at desirable areas in GIT, such as Diclofenac potassium USP100 mg.
- Enteric-Coated Tablets: This coating is utilised to provide delayed release features. Cellulose acetate phthalates/Cellulose acetate butyrates, HPMC succinate, and Methacrylate acid copolymers are the coating polymers. These coatings repel gastric fluid while dissolving the tablet and allowing medication breakdown and absorption in the intestine. Enteric coatings are typically used when a medicine is inactivated or destroyed by stomach fluid, such as erythromycin, or when a drug causes irritation to the gastrointestinal mucosa, such as NSAIDs.
- Dispersible tablets: These are film coated/uncoated tablets which undergoes uniform dispersion when suspended in water.
- Effervescent Tablets: When uncoated pills are dissolved with organic acid, CO2 is produced. This carbon dioxide aids in tablet disintegration and provides a powdered material suspension that is easily absorbed.
- Chewable Tablets: Chewable tablets are large and cannot be ingested, thus they are chewed within the buccal cavity. These pills are designed specifically for traditional or anti-acid formulations. Gestid, for example, is a pleasant chewable ant-acid.
H. Tablet Related Issues And Defect
- Capping: When the upper and bottom segments of a tablet separate horizontally, this word is employed.
- Reason:- Air entrapment during compression.
- Dilemmas and remedies:- Capping is caused by a large quantity of fines or overly dried materials, thus remove fines using a 200 mesh screen or moist granules, or add hygroscopic ingredients such as sorbitol, PEG400, and so on.
2. Lamination: It is the horizontal division of a tablet into two or three levels.
- Reason: Air entrapment during compression
- Dilemmas and remedies: Too much oil or waxy components in granules produces lamination; thus, add adsorbents or absorbents and less lubricants.
3. Cracking: On the upper and lower surfaces of the tablets, little, thin cracks can be seen.
- Reason – Rapid expansion of tablets
- Dilemmas and remedies: Big granules that are too dry or too cold cause cracking, thus minimize the granular size most of them and compress at room temperature.
4. Chipping: It is described as tablet edge breaking as the tablet is leaving the press or during handling and coating processes.
- Reason: More binder in formulation
- Dilemmas and remedies: Too much binding produces chipping, as does adhering on punch faces, thus optimize the binding or use dry binders and dry granules correctly.
5. Sticking/Filming: It refers to the material adhering to the die wall, whereas filmmaking is a gradual sort of sticking caused by excess moisture in granulation.
- Reasons: Improper drying and improper lubrication of granules
- Dilemmas and remedies: Sticking is caused by incorrect drying, inadequate lubrication, too much binder, or oily/waxy components, thus dry the granules thoroughly and apply adequate lubricants.
6. Picking: It is defined as when a small amount of material from a tablet is adhered to and removed from the tablet surface using a punch face.
- Reasons: When punch face having engraving or embossing letters.
- Dilemmas and remedies: Low melting point medication in large concentrations, too warm granules during drying/too much binder results in picking, so compress at room temperature, add high melting point ingredients, or lower the amount of binders.
7. Mottling: It is defined as the uneven distribution of colour on the surface of the tablet with light or dark spots standing out in an otherwise uniform surface.
I. Properties Of Tablet
- The tablet must be strong and hard to withstand mechanical shock during dispensing, manufacturing, packaging and shipping.
- Tablet should have uniformity in weight and drug content.
- Tablets should have elegant product identity and free from defects.
- They must be physically and chemically stable during manufacturing, storage and use.
- The tablets must produce sufficient bioavailability i.e., it must be able to release its contents in predictable and reproducible manner[14].
II. METHODOLOGY
A. Test To Perform
- General Appearance
A tablet's overall aesthetic determines its individuality and attractiveness. It is crucial for ensuring consumer approval, lot-to-lot consistency management, and tablet-to-tablet uniformity. General appearance involves measurement of size, shape, colour, taste, presence absence of odor.
2. Size & Shape
Compressed tablets shape and dimensions are determined by the tooling during the compression process. Crown thickness of tablet measured by micrometer Total crown thickness is measured by vernier calliper. Tablet thickness should be controlled with ±5% of standard value.
3. Organoleptic Characters
The tablet's surface should be completely consistent in colour and free of any mottling. Compare the sample's colour to the standard to provide a visual comparison.
4. Hardness of Tablet
The force required to crush a tablet that has been put on its edge is how tablet hardness or tablet crushing strength is typically defined [7]. The ability of a tablet to withstand mechanical shocks during handling during manufacture and guard against tablet deterioration from packaging and transportation is referred to as hardness. The strength required to crush the tablets was used to determine the hardness by sandwiching them between the two jaws of hardness tester . The standard deviation and average crushing strength were then determined. This was triplicated . 4 to 7 kg-f is the acceptable range for tablet hardness or crushing strength (kilogram of force).
5. Weight Variation
In this test, the average weight of 20 tablets of bisacodyl has taken and which is determined by individually weighing each one. If no more than two tablets fall outside of the permitted ranges in terms of percentage and if no tablet deviates by more than twice the acceptable range, the tablet passes the USP test
Percentage weight variation= {(average weight – individual weight)/ individual weight} x 100 %
6. Friability
Tablets chip, cap, or crack in the majority of cases due to friction and stress pressures. The friability test is important to assess the tablet's ability to survive abrasion during packaging, handling, and shipping and has a tight relationship to tablet hardness. The measure of the tablet friability is easily done from the loss due to abrasion. Friability was measured in percentage (%) terms. Each brand's 20 tablets are weighed before being put into the friabilator, where they are repeatedly shocked and rolled as they fall from 6 inches with each spin. The tablets are weighed and the weight is compared with the original weight after this treatment has taken place for 4 minutes or 100 rotations. During the friability test, a weight loss of no more than 1% of the weight of the tablets being evaluated is generally regarded as acceptable. then the following formula was used to get the % reduction in weight of the tablets:
Percentage friability= {(Initial weight- Final weight)/ Initial weight} x100
7. Disintegration of Tablet
The compressed tablet's solid structure needs to be dissolved after oral administration for the active components to work in the body. The disintegration test is a crucial quality control method mechanical process of breaking down tablets into smaller granular particles, and the amount of time needed to cause disintegration is known as the disintegration time. The standard disintegration time for enteric coated tablet is 2hrs in gastric fluid region and 1hrs in intestinal fluid(alkaline medium). The tablet disintegration tester was used to measure tablet disintegration. Throughout the experiment, distilled water was kept at a constant temperature of 37°C for every tablet from every brand. The disc was utilised after 6 tablet of each brand had been chosen and placed in each of the cylindrical tubes of the basket. Each tablet's time to disintegrate into tiny pieces and pass through the 10-mesh screen was timed. The disintegration time was defined as the period of time during which no particle was present on the system's basket[15].
a. Tablet-6 selected randomly
b. Glass tubes - 6
c. Glass tube length - 3inches
d. Mesh Screen -10 mesh i.e. 1.7 mm (USP); 8 mesh i.e. 2mm (IP)
e. Upper and lower end closed with #10 mesh screen.
f. Beaker contains - 900 ml of water simulated gastric fluid or simulated intestinal fluid.
g. Temperature-37± 2°C (Remember with reference to difference with dissolution)
h. Speed-28-32 rpm- Tablet raised and lowered
i. Tablets up and down through a distance 5 to 6 cm.
8. Dissolution Rate Test of Tablet
This test is used to measure the extent and rate of solution formation from dosage forms such as tablets, capsules etc. The dissolution of drug is important for its bioavailability and therapeutic effectiveness. The apparatus for dissolution of tablet are regarded as per IP/USP are as follow:
Type 1-Basket type
Type 2-Paddle type
Type 3-Reciprocating cylinder
Type 4-Flow through cell
Type 5-Paddle over disc
Type 6-Rotating cylinder
Type 7-Reciprocating disc
According to IP Type 1 is the paddle and Type 2 is the basket type apparatus.
Generally, the process of a solid medicine being dissolved into a liquid influences the rate of drug absorption under standardized parameters of temperature, solvent composition, and the liquid or solid interface. For a medicine to work perfectly in an internal organ of a human body at a specific time, a quality assurance type drug release pattern over a set length of time is crucial. Dissolution test for each brand of bisacodyl tablet was carried out by according to IP dissolution type apparatus. In this apparatus, 900 ml phosphate buffer (pH: 7.40) was used as dissolution medium. A constant temperature bath with a chosen speed of 50 rpm from a variable speed motor kept the process at 37 ± 0.5°C. Typically, one tablet of each brand is placed in a basket fastened to the base of a shaft that is attached to a motor. Samples were taken out of the medium at a rate of 25 ml, and they had to be promptly replaced with an equal volume of fresh dissolving medium (phosphate buffer).
B. Solvent Preparation
- Preparation of 0.1 N Hydrochloric Acid
Mix up to 1000 mL of pure water without carbon dioxide with 85 mL of strong hydrochloric acid. Pipette 100 mL of this solution into a 1000 mL volume flask, top it off with distilled water free of carbon dioxide, and stir until homogeneous.
2. Preparation of 0.1 N Sodium Hydroxide
Dissolve 162 g of sodium hydroxide crystals in 150 mL carbon dioxide free water. Chill the mixture to room temperature before filtering it through rough filter paper. Pour 54.5 mL of the clear filtrate into a container that is sealed, then dilute it with up to 1000 mL of carbon dioxide. Pipette 100 mL of this solution into a 1000 mL volume flask, top it off with carbon dioxide-free water, and stir until it is completely homogeneous.
3. Preparation of Phosphate-buffered pH 7.2
Mix 13.0 mL of citric acid solution R 2.1% with 87.0 mL of sodium dihydrogen phosphate solution R 7.15%.
4. Preparation of Bisacodyl Standard Solution 1000 ppm
a. In 0.1 N Hydrochloric Acid:-
By carefully weighing 50 mg of bisacodyl on an analytical scale, placing it in a 50 mL measuring flask, adding a portion of 0.1 N HCl, shaking until it dissolves, and then adding enough 0.1 N HCl to reach the boundary mark, you can make a standard solution of bisacodyl with a concentration of 1000 ppm.
b. In 0.1 N Sodium Hydroxide:-
By carefully weighing 50 mg of bisacodyl on an analytical scale, placing it in a 50 mL measuring flask, adding a portion of 0.1 N NaOH, shaking until it dissolves, and then adding enough 0.1 N NaOH to reach the mark limit, you can create a standard bisacodyl solution with a concentration of 1000 ppm.
c. In phosphate buffer pH 7.2:-
By carefully weighing 50 mg of bisacodyl on an analytical scale, placing it in a 50 mL measuring flask, adding a partial solution of phosphate citrate pH 7.2, shaking until dissolved, and then adding phosphate citrate pH 7.2 to the limit mark, you can make a bisacodyl standard solution with a concentration of 1000 ppm[16].




Conclusion
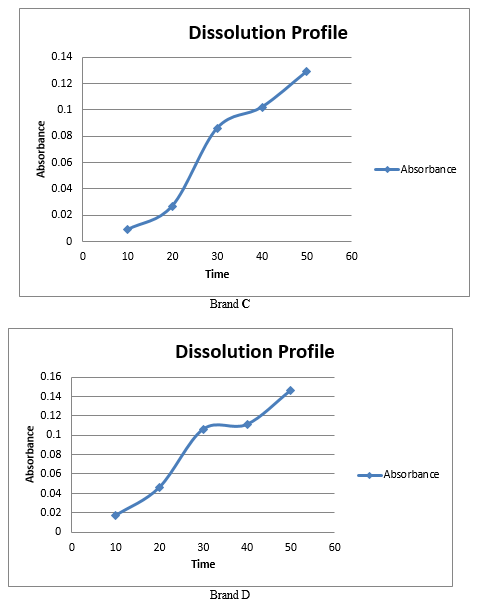
Bisacodyl is a prodrug with a unique dual mechanism of action on gut secretion and motility. The present analysis has reconfirmed that it is well tolerated and effective in the treatment of constipation, and the present review of the literature suggests that the treatment should be started with the lowest dose of 5 mg. The quality parameter, considerable cost, time consumption and scientific expertise of any formulation are important because therapeutic response and safety depends on its quality maintenance . The quality maintenance in a pharmaceutical industry depends on the number of atmospheres including personnel qualifications, active pharmaceutical ingredients quality, validation of the manufacturing process and the area etc. Organoleptic properties, Weight variation, hardness, friability, disintegration time, dissolution test, of all branded tablets used in the study were within BP/USP specified limits. Most of the brands showed acceptable disintegration time, potency, hardness, friability and dissolution profile.
References
[1] Rana AS, Kumar SH. Manufacturing defects of tablets-a review. J Drug Delivery Thery. 2013;3(6):200-206. [2] Ansel H, Allen L. Popovich N. Ansel‘s Pharmaceutical Dosage Forms and Drug Delivery Systems. (8th edn): 227-259. [3] Kamble ND, Chaudhari PS, Oswal RJ, Kshirsagar SS, Antre RV. Innovations in tablet coating technology. International Journal of Applied Biology and Pharmaceutical Technology. 2011; 2(1):214-218. [4] Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clinical. Gastroenterol. Hepatol. 2011 Jul;9(7):577-83. [PubMed: 21440672] [5] Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebocontrolledstudy. Aliment. Pharmacol. Thery. 2006 May 15;23(10):1479-88. [PubMed:16669963] [6] Min YW, Ko EJ, Kim JH, Lee JY, Kim HC, Lee WY, Rhee PL. Increased Tone of the Human Colon Muscle by Bisacodyl In Vitro. J Neurogastroenterol Motil. 2018 Apr 30;24(2):317- 323. [7] Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers. 2017;3:17095. [8] Friedrich C, Richter E, Trommes hauser D, et al. Absence of excretion of the active moiety of bisacodyl and sodium picosulfate into human breast milk: an open-label, parallel-group, multiple-dose study in healthy lactating women. Drug Metab Pharmacokinet. 2011;26:458-464. [9] Tjay TH, Rahardja K. Obat-obat penting, khasiat, penggunaan, dan efek sampingnya. (Edisi4). Jakarta: PT Elex Media Komputindo Kelompok Kompas – Gramedia, 2007 [10] The United States Pharmacopeial Convention. First Supplement to USP 35–NF 30 Official Monographs / Bisacodyl 5437, 2012. [11] Corsetti M, Landes S, Lange R. Bisacodyl: A review of pharmacology and clinical evidence to guide use in clinical practice in patients with constipation.Neurogastroenterology & Motility. 2021;00:e14123. [12] Friedrich C, Richter E, Trommeshauser D, de Kruif S, van Iersel T, Mandel K, Gessner U: Absence of excretion of the active moiety of bisacodyl and sodium picosulfate into human breast milk: an open-label, parallel-group, multiple-dose study in healthy lactating women. Drug Metab Pharmacokinet. 2011;26(5):458-64. doi: 10.2133/dmpk.dmpk-11-rg-007. Epub 2011 Jun 21 [13] Sherly Lawrensia; Avais RNCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.June 5, 2020. [14] Sunitha Reddy Baddam, Anusha Manthani Department of Pharmaceutics, Jawaharlal Nehru Technological University, Hyderabad, India : Baddam SR, et al. Drug Des, Vol. 10 Iss. 5 No: 1000192. [15] Abdullah Al Ragib, Tariqul Islam, Sazaul M. Sazib, Faruk Hosain DOI: 10.26479/2018.0404.32 /July 2018 1.Department of Applied Chemistry and Chemical Engineering, Noakhali Science and Technology University, Noakhali-3814, Bangladesh. 2.Department of Pharmacy, Noakhali Science and Technology University, Noakhali-3814, Bangladesh. [16] Harrizul Rivai, Nia Pratama, Ridho Asra ISSN: 2519-9889 Impact Factor: 3.426 et al, Int. Journal of Pharmaceutical Sciences and Medicine (IJPSM), Vol.2 Issue. 12, December- 2017, pg18.
Copyright
Copyright © 2023 Shubham Patel, Aakash Patel, Rahul Lovhare. This is an open access article distributed under the Creative Commons Attribution Lense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET52196
Publish Date : 2023-05-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

