Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Comprehensive Review on AI in Endodontics
Authors: Abhishek Patel, Divam Shah, Zeel Patel, Hirben Shah Makwana, Aarini Rajput
DOI Link: https://doi.org/10.22214/ijraset.2023.54565
Certificate: View Certificate
Abstract
Artificial intelligence (AI) has substantially grown its presence and importance in many jobs and applications in dentistry, notably endodontics. AI has the capacity to imitate human intelligence to conduct prediction and complicated decision-making in the healthcare industry. Dental technology uses artificial intelligence (AI), a relatively recent technology, extensively. Dental professionals have mostly employed AI technologies to diagnose disorders, plan treatments, make clinical judgements, and determine prognoses. Convolutional neural networks (CNN) and artificial neural networks (ANN) are AI models that have been applied in endodontics to study the anatomy of the root canal system, measure working lengths, find periapical lesions and root fractures, forecast the success of retreatment procedures, and forecast the viability of dental pulp stem cells. These all aspects have been reviewed comprehensively in the present article. Moreover, the works of literature stating the advancements in the field of AI in endodontics are studied for the present work. Furthermore, the future predictions and detailed limitations of AI in endodontics are discussed. Additionally, the future scope of the technology has been discussed in detail in the field of Photorealistic 3D Reconstructions and Robotics and Microbots.
Introduction
I. INTRODUCTION
The aim of endodontic therapy is to provide high-quality care to maintain the tooth's functionality and prevent subsequent issues. The diagnosis of root canal pathology, equipment design elements, components, and treatment options have significantly improved in recent years with the goal of delivering successful endodontic care [1]. In the field of endodontics, AI models such as convolutional neural networks (CNN) and artificial neural networks (ANN) are being used to study the root canal system anatomy, measure working lengths, find periapical lesions and root fractures, forecast the success of retreatment procedures, and forecast the survival of stem cells in dental pulp [2]. Accuracy in treatment judgement and diagnosis is crucial for getting the greatest results. Numerous therapeutic alternatives and diagnosing tools have made it possible to conduct diagnostic tests, make clinical decisions, and plan the optimal course of action for treating illnesses of the root canal system [3]. By identifying answers to a range of clinical issues and simplifying the work of physicians, AI systems have the potential to revolutionise medicine and dentistry. Alongside other dentistry disciplines, endodontic research has expanded [4]. The root canals could be accurately biomechanically prepared when AI and endodontics are integrated [5]. The establishment of clinical treatments like AI-based diagnosis and assisted access cavity preparations to get simple access to root canals even in obliterated roots has also been facilitated by the most recent developments in digital applications [6]. The use of this recently created paradigm for endodontic disease diagnosis and treatment planning was also demonstrated in numerous research [7]. In accordance with the International Caries Detection and Assessment System (ICDAS II), Berdouses et al. [8] devised a computer-aided automated approach (ACDS) for the diagnosis of occlusal caries lesions of posterior permanent teeth using photographic colour dental images. Hiraiwa et al. [9] employed deep learning algorithms (AlexNet and GoogleNet) to recognise the distal root form of the mandibular first molar on panoramic dental radiographs. Both deep learning systems slightly outperformed radiologists with substantial training in performing diagnostic tasks. A deep CNN-based AI diagnosis model was created to determine the specific location of periapical lesions and measure their volume in CBCT images based on a random walk segmentation of a graph, a noninvasive differential diagnosis technique for periapical lesions [10]. After segmentation, the image is classified using a Random Forest (RF) approach, which creates a grey-level cooccurrence matrix of the image by extracting pertinent features from the matrix. Automatic gender determination has a 90% success rate when using an applicable technique that can identify gender from plaster casts of the maxillary teeth [11]. A CAD/ CAM system, which also supplied constraints for the digital positioning of the complete denture's artificial teeth, was used to anticipate the facial deformation of a patient after obtaining a complete denture prosthesis [13]. As part of an automated dental chart filing system, Miki et al. [12] examined a deep convolutional neural network-based automated system for categorising tooth kinds on dental cone-beam CT images. An automated computational method for categorising teeth using artificial neural networks was also introduced by Raith et al. [24]. Moran et al. [15] proposed a superresolution generative adversarial network (SRGAN) as a method to produce high-resolution periapical images with a 4-order-of-magnitude improvement.
The AI-based diagnosis' accuracy was found to be 94.96%, and the degree of difficulty for these patients could be determined with great endodontic precision [16]. In recent years, there has been a significant increase in the prevalence and usefulness of AI in numerous processes and applications in dentistry, particularly endodontics. AI has the capacity to make healthcare decisions and perform diagnostic and prognostic analyses. Regarding the use of AI, the endodontist's knowledge needs to be updated. The objective of this in-depth review is to assess the diagnostic and prognostic precision of artificial intelligence in endodontic dentistry [17].
II. ENDODONTIC DETECTION
AI has primarily been employed virtually in endodontics to detect periapical diseases, and crown and root fractures, determine working length, and identify morphology. These steps outline AI's virtual component in the next section.
A. Periapical Lesions
It might be difficult for clinicians to make a diagnosis and determine a course of therapy for teeth with periapical lesions and/or symptoms. About 75% of radiolucent jaw lesions are caused by the prevalent illness of apical periodontitis. Early identification may improve the effectiveness of treatment, prevent the disease from spreading to nearby tissues, and reduce subsequent problems. Panoramic and intraoral periapical radiographs are the most often utilised 2-dimensional diagnostic tools in daily clinical practice to detect apical periodontitis. On radiographs, periapical lesions are typically identified as radiolucencies. Periapical radiographs do not provide correct information since the 3-dimensional anatomy is reduced to a 2-dimensional picture [18-20].
In order to precisely detect periapical lesions and determine their size and position, cone-beam computed tomographic (CBCT) imaging, a 3-dimensional imaging method, was created. According to a meta-analysis, CBCT imaging, conventional periapical radiography, and digital periapical radiography each had accuracy values for periapical lesions detection of 0.96, 0.73, and 0.72, respectively. However, apical periodontitis in teeth with filled roots could not be diagnosed as accurately with CBCT imaging when it was used. According to Endres et al., a deep learning algorithm model can detect periapical radiolucencies on panoramic radiographs as accurately as 24 oral and maxillofacial surgeons. In one study, the capability of CNN models to identify simulated periapical lesions on intraoral radiographs was compared to that of three oral and maxillofacial radiologists [21, 22].
They came to the conclusion that the CNN group's interpretations had higher mean values of sensitivity, specificity, and area under the receiver operating characteristic curve per fold than those of the oral and maxillofacial radiologists. In comparison to dentists with more than 10 years of clinical experience, Ekert et al. demonstrated that the potential of deep CNNs was accurate in the discriminatory ability to detect apical lesions on panoramic radiographs [23]. However, both trials used panoramic radiography, a technique that endodontists use extremely infrequently for diagnosis and had a small sample size. The ability to discriminate between periapical lesions on a radiograph is typically extremely variable between examiners and heavily dependent on expertise. AI systems can be used to lessen bias and differences between examiners [24, 25].
The use of AI systems in periapical lesion detection from radiographs and CBCT scans may increase accuracy and help physicians achieve detection accuracies comparable to or better than that of seasoned experts. Additionally, reducing assessment time and enabling semi-automated documentation, it may lessen the dentist's diagnostic efforts. Before being used in therapeutic settings, AI systems' sensitivity should be increased and more research should be done [26].
Different gene expressions for a periapical cyst versus a periapical granuloma were reported by Poswar Fde et al. In order to distinguish between a cyst and a granuloma, the authors used a multilayer perceptron neural network for gene categorization. The study was limited by the possibility that some algorithmic data might not be present. The algorithm did not distinguish between inflammatory and physiological cytokines [27]. The scientists did, however, suggest that this methodology might be beneficial for differentiating other biological processes (such as cancer biomarkers). Regarding lesion identification accuracy and dice coefficient indices of a multilabel segmentation, Zheng et al. compared an anatomically constrained Dense U-Net with existing biomedical image analysis techniques. The scientists showed that despite the short sample size, the unique deep learning method enabled CBCT segmentation and the detection of pathosis with improved sensitivity and specificity [28].
B. Working Length
A crucial stage of root canal therapy is identifying the apical limit of the root canal system. The root canal system can be thoroughly mechanically and chemically disinfected with the help of a precise working length (WL) determination. The proper WL also helps to avoid the extrusion of debris, lessens post-operative discomfort, and protects the periodontal tissues from instrumentation beyond the canal terminal.
It has been demonstrated that while treating infected root canal systems, a millimetre decrease in WL can lower the success rate by 12–14%. Additionally, obturating the canals past the radiographic apex can have a negative impact on the treatment's result [29].
Electronic apex locators and periapical radiography are mostly used in the current methods of determining canal length. The clarity of the image and the clinician's subjective judgement are crucial factors in the accurate interpretation of digital radiography. Additionally, the instrumentation should minate at the cemento-dentin junction, which can be found 0.5 to 2 mm from the radiography apex. Apex locators can be extremely accurate, but they can also give readings that are due to mistakes in damp canals, metallic repair, or damaged wires. All of these factors may contribute to rate measures that have a negative effect on the course of treatment [30].
To help clinicians locate the apical endpoint on radiographs, artificial intelligence systems are currently being developed. Saghiri et al. employed AI to calculate working length measurements on a cadaver model to simulate a clinical setting [31]. They discovered that AI was 100% accurate when calculating the root length when compared to the real measurement after tooth extraction. Additionally, they came to the conclusion that AI could find the small apical constriction 96% of the time. To preserve the apical constriction and avoid over instrumentation, further development of this technology may enable AI to gather information from imaging techniques and translate it to the endodontic handpiece/motor operated by the clinician. This would allow the clinician to drive the endodontic files to the cemento-dentin junction with the least amount of operator interference.
C. Fractures
It is very difficult to identify crown and/or root fractures with radiography. The diagnosis of vertical root fractures (VRF) with CBCT imaging is only 78% reliable, according to a recent systematic review and meta-analysis. Fukuda et al. demonstrated a 75% sensitivity and a 93% positive predictive value in their detection of VRF on panoramic radiographs using AI. Another study that used AI for the same purpose with single-rooted premolar teeth as the test subjects demonstrated 97% accuracy, 93% sensitivity, and 100% specificity in properly detecting VRF. Future studies are being conducted to increase AI's overall diagnostic precision and its ability to identify fractures in multirooted teeth [32].
D. Root and Root Canal Morphology
Understanding the various root and root canal systems is a crucial element in the effectiveness of nonsurgical root canal therapy. Periapical radiography and CBCT imaging have traditionally been employed for this purpose. When compared to radiography, CBCT imaging has been shown to be more accurate at determining the root and root canal geometries. However, it cannot be advised in standard therapeutic practice due to radiation-related concerns. In their study, Hiraiwa et al. found that the deep learning system using panoramic radiographs had a high degree of accuracy in determining if a mandibular first molar's distal roots contained one or many roots. Deep learning systems were used to generate learning models by importing image patches that were extracted from panoramic radiographs. The programme created by information analysis and artificial intelligence (AI) showed the capacity to measure the root canal curvature and its 3-dimensional alteration following instrumentation51. To verify the findings of this study, additional research is needed [33, 34].
An automatic 3-dimensional tooth segmentation using the CNN method was reported by Lahoud et al. In a rapid, accurate, and effective clinical reference, the scientists assessed 433 CBCT radiographic segmentations of teeth and found that AI did just as well as a human operator while working significantly faster. The authors combined two deep CNNs and professional refining in a different investigation that used panoramic radiography. The scientists showed that the AI tool produced high sensitivity and specificity in a very quick performance for the recognition and segmentation of teeth using 153 panoramic radiographs [35].
III. OUTCOME PREDICTION
In terms of endodontic treatment, AI has been utilised to forecast some potential outcomes. In a study by Lee et al., the researchers sought to develop an efficient AI-based module that would enable clinical decision-making on tooth prognosis and consideration of the best possible treatment strategy. This study made use of data from a multidisciplinary study team at Harvard made up of top clinician educators, prosthodontists, periodontists, and endodontists. Their findings demonstrated that an efficient AI-based module enables precise clinical judgements on tooth prognosis with outcomes comparable to clinicians from a variety of specialities. In a study by Campo et al., the outcome of nonsurgical root canal retreatment with benefits and risks was predicted for endodontic retreatment cases. Whether one should retreat or not was reported by the system. Case-based reasoning is the process of developing answers to issues based on prior experiences with issues that are comparable to the current issue. Information is obtained during that process from related instances and other clinical techniques.
The system makes use of information from a variety of sources, including performance, recall, and statistical probabilities. The system's strength is that it might be able to forecast how the therapy will go. The system would only be as accurate as the information in the data, which is the restriction. The sensitivity, specificity, and accuracy of such techniques improve with the amount of data gathered [36, 37].
IV. LIMITATIONS
The present section states the limitations of AI in Endodontic treatments. Till now, there are no developments in AI that can schedule patient treatments based on ongoing needs and acquired medical information by programming technology in appointment scheduling, patient management, and recall. As decisions on scheduling, appointment, delegation, and recall are continuous and should be updated to meet the demands of the healthcare system. There is no procedure in place to alert the doctor to drug interactions and/or treatment modifications based on the available electronic health record; as the population rises and more individuals live longer, more medications are being used. AI may be able to predict patient-specific drug-drug problems if healthcare records are made public [38].
Based on medical, dental, and clinical results, there is no procedure to provide a correct endodontic diagnosis; however, using the information and data collected, AI would be able to enhance diagnosis and staging as well as forecast outcomes. This would involve prognostic risk assessment or outcome prediction. The endodontist can do precise manual and robotic microsurgery; with implant technology, robotic-assisted dental surgery can assist the surgeon with accurate navigation of the implant placement [38]. However, there isn't much information on this subject. The same system should aid endodontists in their navigation during endodontic surgery. However, no comparative cohort studies have been carried out yet contrasting robotic technology with conventional endodontic surgery or therapy. The scientists reported that the mean deviations of the implant robotic placement were as accurate as both static and dynamic navigations. Future research should assess the various placement strategies' accuracy and safety using robotic guidance.
V. FUTURE SCOPE
The application of AI-enhanced algorithms to produce photorealistic 3D reconstructions of tooth anatomy, root canal space, and frequent orofacial diseases is an intriguing topic that has been attracting greater attention. Cinematic rendering (CR) is the name given to this innovative reconstruction technique. Based on CBCT data sets, CR can produce these photorealistic 3D photos by employing high dynamic range rendering lightmaps to simulate a natural lighting situation. Better showing the anatomical details, may increase the diagnosis accuracy (Figure 1). Cinematic rendering has been integrated with the usage of augmented reality headsets in the medical industry, enabling users to view and alter images in actual physical space. This technology can be applied to the dental industry as well. A lot of opportunity exists for using such technologies in clinical teaching and training [12].
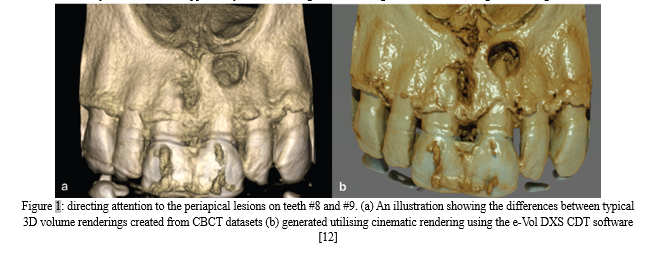
The creation of AI-guided robots to assist in providing actual treatment to patients is another potential future use. Neurosurgery and orthopaedics have both used image-guided robotic surgery regularly. An interactive map of deep anatomy, vascular, and disease is made using tracking technology and preoperative or intraoperative images.
The development of robots to assist in implant placement is currently taking place in the field of implant dentistry. Some studies have revealed that the mean deviations of the implant robotic placement were as precise as both static and dynamic navigations. It is anticipated that a comparable technology can be created to help with normal root canal therapy or possibly endodontic microsurgery. The absence of tactile input is a significant drawback of robotic surgery. Dental professionals rely on sensory feedback, yet robotic systems do not support the pressure or tension that is frequently experienced during endodontic tactile sensations [15]. The newest robotic models under research today are attempting to solve this issue by giving surgeons immediate sensory feedback. Many studies have focused on "sensory substitution," which involves transmitting haptic or sensory information through aural or graphical signals, in order to circumvent the challenges associated with relaying force and tactile information directly to the operators' hands.
In addition to AI-guided robots, another area of endodontics with a lot of potential is AI-guided microrobots. The ultimate therapeutic approach for eliminating bacterial biofilm colonies that attach to the dentin in the most intricate regions of the root canal wall is being constantly improved in endodontics [21]. One of the most clinically difficult areas in the oral cavity is the root canal system because of its known abnormalities and anatomical complexity. As a result, the biofilm that has not been completely removed from the complex canal area continues to be a major factor in treatment failure and recurrent endodontic infections.
Conclusion
The discipline of endodontics can benefit from the current AI models\' promising applications in the identification of periapical pathosis, root fractures, working length estimation, and treatment result prediction. To ensure accuracy and consistency, it is essential to create these AI models using data gathered from knowledgeable clinicians. The use of AI in endodontics is still somewhat underutilised when compared to other disciplines of dentistry, although there are promising areas where utilisation could increase. When considering how dentistry as a profession may change in the future, endodontics is not an anomaly. Opportunities within our discipline are constantly growing. Endodontics\' potential as a dental treatment method in the future offers a wide range of opportunities for permanently preserving teeth. Clinical uses of AI in endodontics may help identify periapical pathosis, identify root fractures, calculate working length, and anticipate disease. Before widespread acceptance into standard clinical practice, there is a need for high-quality research to assess the performance of AI in terms of its dependability, applicability, legal and ethical considerations, and cost-effectiveness.
References
[1] Shtar G, Rokach L, Shapira B. Detecting drug-drug interactions using arti?cial neural networks and classic graph similarity measures. PLoS One 2019;14:e0219796. [2] Bhandari M, Zef?ro T, Reddiboina M. Arti?cial intelligence and robotic surgery: current perspective and future directions. Curr Opin Urol 2020;30:48–54. [3] Moyle W, Arnautovska U, Ownsworth T, Jones C. Potential of telepresence robots to enhance social connectedness in older adults with dementia: an integrative review of feasibility. Int Psychogeriatr 2017;29:1951–64. [4] Barry DT. Adaptation, arti?cial intelligence, and physical medicine and rehabilitation. PM R 2018;10:S131–43. [5] Abdi J, Al-Hindawi A, Ng T, Vizcaychipi MP. Scoping review on the use of socially assistive robot technology in elderly care. BMJ Open 2018;8:e018815. [6] Langley P, Laird JE. Arti?cial intelligence and intelligent systems. Ann Arbor 2006;1001. 48109–2121. [7] Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Arti?cial intelligence in medicine. Ann R Coll Surg Engl 2004;86:334–8. [8] LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521:436–44. [9] Hiraiwa T, Ariji Y, Fukuda M, et al. A deep-learning arti?cial intelligence system for assessment of root morphology of the mandibular ?rst molar on panoramic radiography. Dentomaxillofac Radiol 2019;48:20180218. [10] Schwendicke F, Golla T, Dreher M, Krois J. Convolutional neural networks for dental image diagnostics: a scoping review. J Dent 2019;91:103226. [11] Nebeker C, Torous J, Bartlett Ellis RJ. Building the case for actionable ethics in digital health research supported by arti?cial intelligence. BMC Med 2019;17:137. [12] Schwendicke F, Samek W, Krois J. Arti?cial intelligence in dentistry: chances and challenges. J Dent Res 2020;99:769–74. [13] Ilhan B, Lin K, Guneri P, Wilder-Smith P. Improving oral cancer outcomes with imaging and arti?cial intelligence. J Dent Res 2020;99:241–8. [14] Heo MS, Kim JE, Hwang JJ, et al. Arti?cial intelligence in oral and maxillofacial radiology: what is currently possible? Dentomaxillofac Radiol 2021;50:20200375. [15] Prados-Privado M, García Villalo´n J, Martínez-Martínez CH, et al. Dental caries diagnosis and detection using neural networks: a systematic review. J Clin Med 2020;9:3579. [16] Mahmood H, Shaban M, Indave BI, et al. Use of arti?cial intelligence in diagnosis of head and neck precancerous and cancerous lesions: a systematic review. Oral Oncol 2020;110:104885. [17] Grischke J, Johannsmeier L, Eich L, et al. Dentronics: towards robotics and arti?cial intelligence in dentistry. Dent Mater 2020;36:765–78. [18] Becconsall-Ryan K, Tong D, Love RM. Radiolucent in?ammatory jaw lesions: a twenty-year analysis. Int Endod J 2010;43:859–65. [19] Setzer FC, Shi KJ, Zhang Z, et al. Arti?cial intelligence for the computer-aided detection of periapical lesions in cone-beam computed tomographic images. J Endod 2020;46:987–93. [20] Chapman MN, Nadgir RN, Akman AS, et al. Periapical lucency around the tooth: radiologic evaluation and differential diagnosis. Radiographics 2013;33:E15–32. [21] Patel S, Dawood A, Whaites E, Pitt Ford T. New dimensions in endodontic imaging: part 1. Conventional and alternative radiographic systems. Int Endod J 2009;42:447–62. [22] Leonardi Dutra K, Haas L, Porporatti AL, et al. Diagnostic accuracy of cone-beam computed tomography and conventional radiography on apical periodontitis: a systematic review and meta-analysis. J Endod 2016;42:356–64. [23] Kruse C, Spin-Neto R, Evar Kraft DC, et al. Diagnostic accuracy of cone beam computed tomography used for assessment of apical periodontitis: an ex vivo histopathological study on human cadavers. Int Endod J 2019;52:439–50. [24] Endres MG, Hillen F, Salloumis M, et al. Development of a deep learning algorithm for periapical disease detection in dental radiographs. Diagnostics (Basel) 2020;10:430. [25] Pauwels R, Brasil DM, Yamasaki MC, et al. Arti?cial intelligence for detection of periapical lesions on intraoral radiographs: comparison between convolutional neural networks and human observers. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131:610–6. [26] Y. Miki, C. Muramatsu, T. Hayashi et al., “Classi?cation of teeth in cone-beam CT using deep convolutional neural net- work,” Computers in biology and medicine, vol. 80, pp. 24– 29, 2017. [27] S. Raith, E. P. Vogel, N. Anees et al., “Arti?cial neural networks as a powerful numerical tool to classify speci?c features of a tooth based on 3D scan data,” Computers in biology and med- icine, vol. 80, pp. 65–76, 2017. [28] M. B. Moran, M. D. Faria, G. A. Giraldi, L. F. Bastos, and A. Conci, “Using super-resolution generative adversarial net- work models and transfer learning to obtain high resolution digital periapical radiographs,” Computers in biology and med- icine, vol. 129, article 104139, 2021. [29] C. D. Naylor, “On the prospects for a (deep) learning health care system,” Journal of the American Medical Association, vol. 320, no. 11, pp. 1099-1100, 2018. [30] K. Becconsall-Ryan, D. Tong, and R. Love, “Radiolucent in?ammatory jaw lesions: a twenty-year analysis,” Interna- tional Endodontic Journal, vol. 43, no. 10, pp. 859–865, 2010. [31] H. Eriksen, “Epidemiology of apical periodontitis,” in Essential endodontology: prevention and treatment of apical periodonti- tis, D. Ãrstavik and T. R. Pitt Ford, Eds., pp. 179–191, Black- well Science Ltd., Oxford, 1998. [32] S. Patel, A. Dawood, E. Whaites, and F. T. Pitt, “New dimen- sions in endodontic imaging: part 1. Conventional and alter- native radiographic systems,” International endodontic journal, vol. 42, no. 6, pp. 447–462, 2009. [33] F. C. Setzer, N. Hinckley, M. R. Kohli, and B. Karabucak, “A survey of cone-beam computed tomographic use among end- odontic practitioners in the United States,” Journal of End- odontics, vol. 43, no. 5, pp. 699–704, 2017. [34] M. G. Endres, F. Hillen, M. Salloumis et al., “Development of a deep learning algorithm for periapical disease detection in dental radiographs,” Diagnostics, vol. 10, no. 6, p. 430, 2020. [35] T. Ekert, J. Krois, L. Meinhold et al., “Deep learning for the radiographic detection of apical lesions,” Journal of Endodon- tics, vol. 45, no. 7, pp. 917–922.e5, 2019, e5. [36] I. Tsesis, E. Rosen, A. Tamse, S. Taschieri, and A. K?r, “Diag- nosis of vertical root fractures in endodontically treated teeth based on clinical and radiographic indices: a systematic review,” Journal of Endodontics, vol. 36, no. 9, pp. 1455– 1458, 2010. [37] M. Fukuda, K. Inamoto, N. Shibata et al., “Evaluation of an arti?cial intelligence system for detecting vertical root fracture on panoramic radiography,” Oral Radiology, vol. 36, no. 4, pp. 337–343, 2020. [38] D. Prithviraj, H. Balla, R. Vashisht, K. Regish, and P. Suresh, “An overview of management of root fractures,” Kathmandu University Medical Journal, vol. 12, no. 3, pp. 222–230, 2015.
Copyright
Copyright © 2023 Abhishek Patel, Divam Shah, Zeel Patel, Hirben Shah Makwana, Aarini Rajput. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54565
Publish Date : 2023-07-01
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

