Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Experimental Study on Compressive Strength of Magnesium Oxychloride Cement Mortar Using Stone Crushed Powder by Varying Molar Ratio
Authors: Pavan Kumar Marupakula, Sai Kiran Rachamalla
DOI Link: https://doi.org/10.22214/ijraset.2023.55006
Certificate: View Certificate
Abstract
One of the major consumers of natural resources and a significant contributor to CO2 emissions is the construction sector. The manufacture of cement and building materials was found to be the source of the highest greenhouse gas emissions. In order to lessen the damaging effects of the building industry on the environment, this paper seeks to determine whether magnesium oxychloride cement (MOC) may be used as a mortar substitute for regular Portland cement. A thorough review of the literature, an experimental investigation, and an internal structure analysis based on the ideal molar ratio are all part of the research technique. In this study, MOC cement with powdered stone is experimentally investigated utilizing various molar ratios with phases 3 and 5. In the first set of results usage of unheated magnesium oxide powder, SEM image shown some amount of MgO left inactive and compressive strength depicted lower values. Eventually, a modification was implemented in order to boost compressive strength. In order to improve the reaction or gel formation that would boost the compressive strength, the MgO powder was heated and added to the cement. Different molar ratios produced various compressive strength values in the mortar cubes. Two ideal molar ratios are chosen from each phase, SEM and EDXA are used to analyse the micro structure. The MgO6 octahedral crystal structures of Phases 3 and 5 are visible in the SEM pictures as double and tripple ribbons of shared edges with water molecules and chloride anions. The percentage of elemental weight in the specimens is displayed by EDXA.
Introduction
I. INTRODUCTION
Portland Ordinary cement is now widely employed in vibrant infrastructure due to its low cost, dependability, and high strength. Portland cement has nonetheless been perceived as a harmful substance due to the huge carbon footprint during its production process and the significant amount of water used during its conformation process, and research interest in creating low-carbon cement has grown.
Magnesium oxychloride cement (MOC), one of the low-carbon cement alternatives, has a good chance of replacing Portland cement due to its superior qualities, such as decreased CO2 emission and the absence of sticky drying. MOC also stands out for having a low alkalinity, a quick setting time, and exceptional mechanical strength. Magnesium chloride hydrate, one of the raw components of magnesium oxychloride cement, is the main derivative and waste of the potash industry. The complete disposal of magnesium chloride hydrate into the lake wastes precious magnesium resources and produces environmental concerns akin to the deposit of solid waste and the destruction of biodiversity. Consequently, the operation of magnesium oxychloride cement may efficiently utilize waste magnesium chloride while reducing environmental dangers. Magnesium oxychloride (MOC) cement, first discovered by Sorel in 1867, is a type of air hardening gel material formed from light burned magnesium oxide (MgO), magnesium chloride (MgCl2), and water (H2O).
A. Sources and Occurrence of Magnesium Chloride (MgCl2)
The hydrated MgCl2 can be extracted from brine or ocean water. The typical active magnesia used for MOC materials is calcined from magnesite at 700-900 degrees Celsius. The extraction of potassium fertilizer in the salt lake area will result in a large amount of magnesium chloride waste liquid. Many magnesium leftovers will be produced during the extraction of lithium carbonate products from salt lake brine. For every tonne of potash fertilizer produced, 8-10 tonnes of magnesium chloride are produced.
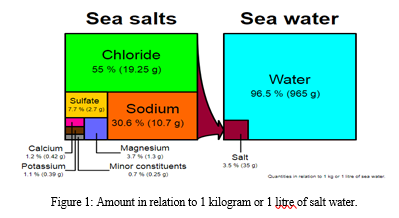
B. Sources and Occurrence of Magnesium Oxide (MgO)
The majority of magnesium oxide produced today is made from minerals that are found in nature through a process called calcination. The most typical kind of magnesite is MgCO3. Seawater, brine deposits underground, and deep salt beds where the production of magnesium hydroxide, Mg(OH)2, are additional significant sources of magnesium oxide.
Magnesium oxide through moist route:
- Caustic-calcined at 600 –1300 °C
- Deadly burnt, 1600 –2200 °C
- Fused, greater than 2800 °C.
Magnesium oxide through dry pathway:
a. Lightly burnt, 700 to 1000 °C
b. Hardly burnt, 1000 –1500 °C
c. Dead-burned, 1500 to 2000 °C
C. Objective of Work
The main aim of the present study is to determine the compressive strength of magnesium oxychloride cement mortar using stone crushed powder by varying molar ratio to arrive at ideal molar ratio with highest strength and analyse the internal structure of the cube using SEM and EDXA.
Hence, to achieve the objective, the work has been carried out in the following stages:
- In the first stage, the molar ratio of 3:1 MgO:MgCl2 and 5:1 MgO:MgCl2 were selected by varying molarity using unheated MgO and crushed stone powder.
- In the second step, cubes of size 70x70x70 mm3 were cast in the requisite numbers and were left to cure in the open air for 7, 14, and 28 days.
- In the third stage, the cubes were subjected to testing under compressive testing machine and if any flaws or improvements required were examined and experimental work is carried out accordingly.
- Finally, the tested specimens internal structure is analyzed using SEM and EDAX.
II. LITERATURE REVIEW AND METHODOLOGY
Conclusion of literature on comprehensive study:
In this group the detailed study of magnesium oxychloride cement mortar is described. This includes the history of magnesium oxychloride cement, the numerous materials used to make magnesium oxychloride cement mortar, and the variables that affect the various qualities of magnesium oxychloride cement. Moreover, various casting and curing techniques are described. The high levels of CO2 released during portland cement production set new records.
The large use of cement in the construction industry can be decreased by using cement containing magnesium oxychloride. The components that can be utilized in the manufacturing of magnesium oxychloride cement are magnesium oxide powder derived from the calcination of naturally existing minerals, most typically magnesite, and magnesium chloride from brine or fertilizer industries. For the preparation of magnesium oxychloride cement mortar, a variety of cementitious materials including fly ash, GGBS, Alko fine, Meta kaolin, Fumed silica, rice-husk ash, granite powder, river sludge, industrial residue, etc. are employed. Depending on what is readily available on the market, fine aggregate such as fine sand, coarse sand, stone crushed powder or robo sand is employed. Due to its greater availability, stone-crushed powder is the main ingredient used to prepare magnesium oxychloride cement mortar and cost effectiveness.
Conclusion of literature on experimental work:
This group is categorized based on the detailed methodology of experimental work. This involves mix design procedure, method of chemical handling, casting, and curing of magnesium oxychloride cement. It is described how the researchers used various substitutions and molarities. Using MgO, MgCl2, F.A, and pozzolonic ingredients in various molar ratios, MOC cement mortar is mixed. Internal bonding and compressive strength metrics are impacted by varying molar ratios. Magnesium oxychloride cement mortar's growth of strength is mostly due to the concentration of molarity solution and replacement of F.A. Unlike traditional methods, ambient air curing is used.

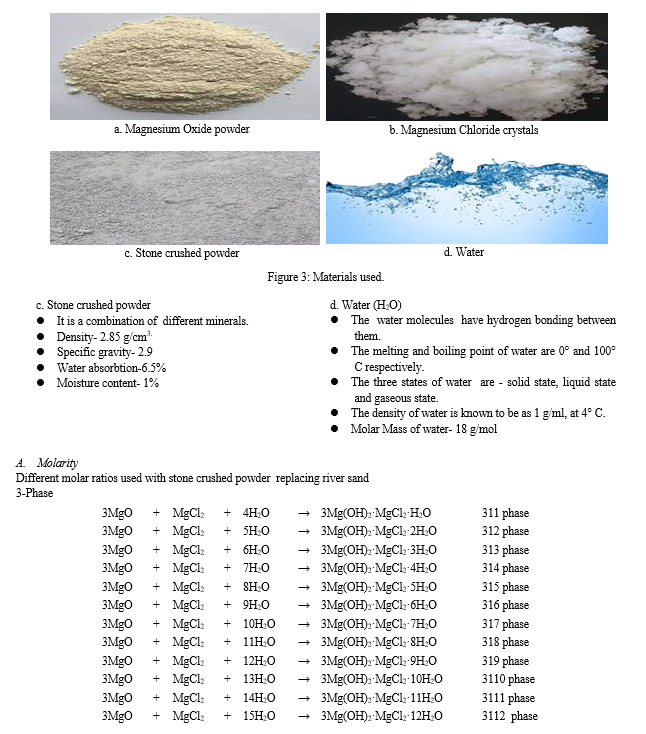




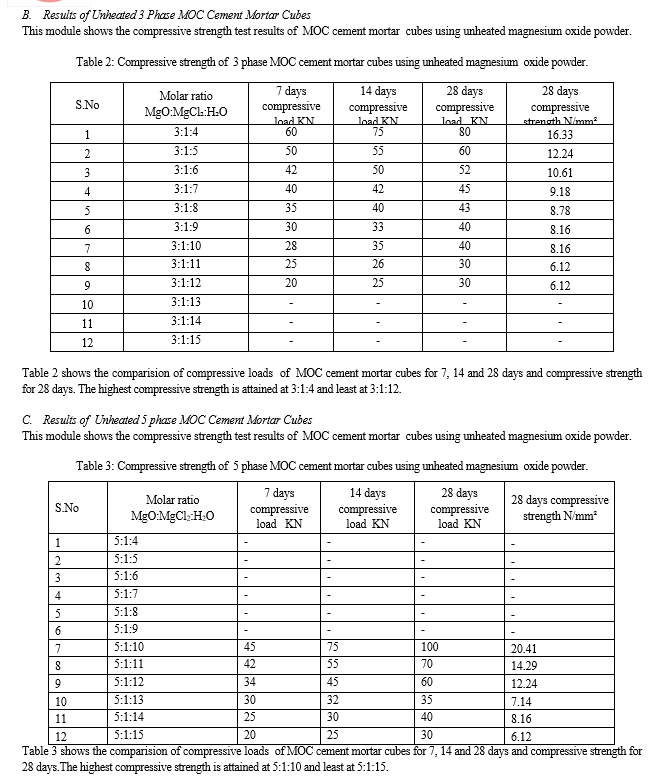



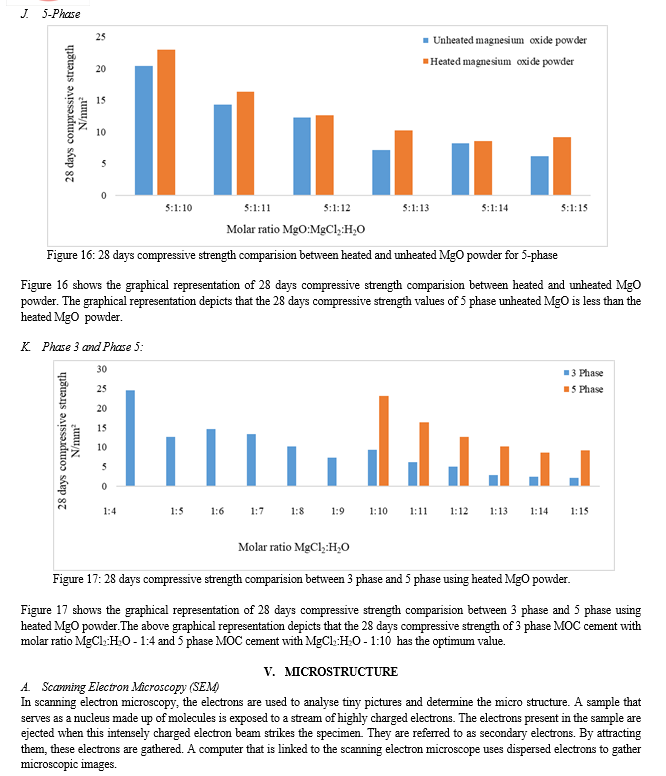


Figure 23 show the elemental composition present in the cubic specimen. It comprises the percentage of each element present in the specimen out of total 100% of elemental composition. In the figure the highest percentage can be seen for oxygen element which helps in forming the oxides for other elements. Even the magnesium element showing highest percentage as indicated in the stoichiometric formula which helps in forming bonds with other elements.
Conclusion
After testing, some MgO oxide powder was left unreacted, and modifications were made to heat the MgO powder at 210°C for one hour before using it in the MOC cement to cast the specimens. Following testing, the specimens compressive strength increased. The specimens with the highest compressive strength and ideal molar ratio were chosen, and internal microstructure was analyzed using SEM and EDXA. The further work comes to the following findings. 1) It is clear from the experimental study that pozzolonic-based replacements have a beneficial effect on the compressive strength of MOC cement mortar. 2) When unheated magnesium oxide powder is used in the MOC cement mortar using crushed stone powder with varying molar ratios of 3-phase and 5-phase showed some amount of MgO left unreacted. 3) The draw back causing to loose the strength is identified and change was adopted i.e by heating the MgO powder at 210oC for 1-2 hours in order to activate the reaction. 4) Higher compressive strength was obtained when both unheated and heated magnesium oxide powder with 3-phase with mole to mole ratio MgO:MgCl2:H2O - 3:1:4 and 5-phase molar ratio MgO:MgCl2:H2O - 5:1:10.This demonstrates that we can notice a significant impact on compressive strength when using light burnt magnesia 5) This demonstrates that we can notice a significant impact on compressive strength when using light burnt magnesia 6) The ideal molar ratio is obtained for 3-phase with molar ratio MgO:MgCl2:H2O - 3:1:4 and 5-phase with molar ratio MgO:MgCl2:H2O - 5:1:10 when MgO is heated at 210o C and highest compressive strength values obtained than previous test results. 7) The SEM images exhibited the formation of crystal structures of Phase 3 and phase 5 by double and tripple ribbons of edges shared by MgO6 octahedral with H2O and Cl- anions in the interstitial region. 8) The EDXA approach indicates the percentage of weight of the chemical constituents in the 3-phase and 5-phase MOC cement samples Magnesium oxychloride cement as an alternative to cement has shown better results in the present study. In the previous studies used as references of this work, Magnesium oxychloride cement mortar prepared for high strength shown less strength than OPC cement But we can predict that the strength for heating of MgO at higher temperature can increase the compressive strength. At lower molarities of 3-phase and 5-phase we have obtained higher strength in both the cases using stone crushed powder along with unheated and heated MgO powder which may be cost effective and may have an ease to work. A. Future Scope We can predict from test results that the heating of MgO powder even at higher temperatures can exhibit good results in increasing the strength.The main draw back of MOC cement is that it cannot resists the extreme exposure to moist environmental conditions. Hence there is a need to overcome the limitation obstacle to completely use in construction industry.Even addition of super plasticizers and reducers can be studied as future research. MOC cement can even be employed in dry wall construction due to its resistance to extreme heat conditions
References
[1] Li, G., Yu, Y., Li, J., Wang, Y., & Liu, H. (2003). Experimental study on urban refuse/magnesium oxychloride cement compound floor tile. Cement and concrete research, 33(10), 1663-1668. [2] Misra, A. K., & Mathur, R. (2007). Magnesium oxychloride cement concrete. Bulletin of materials science, 30(3), 239-246. [3] Li, Z., & Chau, C. K. (2007). Influence of molar ratios on properties of magnesium oxychloride cement. Cement and Concrete Research, 37(6), 866-870. [4] Sglavo, V. M., De Genua, F., Conci, A., Ceccato, R., & Cavallini, R. (2011). Influence of curing temperature on the evolution of magnesium oxychloride cement. Journal of materials science, 46(20), 6726-6733. [5] Li, Y., Yu, H., Zheng, L., Wen, J., Wu, C., & Tan, Y. (2013). Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Construction and Building Materials, 38, 1-7. [6] Walling, S. A., & Provis, J. L. (2016). Magnesia-based cements: a journey of 150 years, and cements for the future?. Chemical reviews, 116(7), 4170-4204. [7] Abdel-Gawwad, H. A., & Khalil, K. A. (2018). Preparation and characterization of one-part magnesium oxychloride cement. Construction and Building Materials, 189, 745-750. [8] Wang, D., Di, S., Gao, X., Wang, R., & Chen, Z. (2020). Strength properties and associated mechanisms of magnesium oxychloride cement-solidified urban river sludge. Construction and Building Materials, 250, 118933. [9] Gomes, C. M., Garry, A. L., Freitas, E., Bertoldo, C., & Siqueira, G. (2020). Effects of Rice Husk Silica on microstructure and mechanical properties of Magnesium-oxychloride Fiber Cement (MOFC). Construction and Building Materials, 241, 118022. [10] Qu, Z. Y., Wang, F., Liu, P., Yu, Q. L., & Brouwers, H. J. H. (2020). Super-hydrophobic magnesium oxychloride cement (MOC): From structural control to self-cleaning property evaluation. Materials and Structures, 53(2), 1-10. [11] Wang, D., Benzerzour, M., Hu, X., Huang, B., Chen, Z., & Xu, X. (2020). Strength, permeability, and micromechanisms of industrial residue magnesium oxychloride cement solidified slurry. International Journal of Geomechanics, 20(7), 04020088. [12] Hao, Y., & Li, Y. (2021). Study on preparation and properties of modified magnesium oxychloride cement foam concrete. Construction and Building Materials, 282, 122708.’ CODE BOOKS [1] Code of practice for preparation and use of masonry mortar IS 2250 - 1981. [2] IS: 2250 – compressive strength test for cement mortar cubes. [3] IS: 269-2015 – specifications for 33, 43 and 53 grade OPC. [4] Specification for coarse and fine aggregate IS 383-2016.
Copyright
Copyright © 2023 Pavan Kumar Marupakula, Sai Kiran Rachamalla. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55006
Publish Date : 2023-07-25
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

