Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Computational Analysis and NGS for Human LDHC Complexed with NAD+ and Ethylamino Acetic Acid (Lung Cancer)
Authors: Uma Kumari, Saran Sri Nivas , Mohini Singh, Pinki Raj Sahu
DOI Link: https://doi.org/10.22214/ijraset.2024.60614
Certificate: View Certificate
Abstract
This study presents a multidimensional exploration of the structural and functional characteristics of the human Lactate Dehydrogenase C (LDHC) complexed with NAD+ and ethylamino acetic acid (EAA), focusing on its implications in lung cancer. Leveraging data from the Protein Data Bank (PDB) and Molecular Modeling Database (MMDB), a comprehensive analysis was conducted using various computational tools and resources. Structural insights were gained through PDBsum and RasMol, elucidating molecular interactions and identifying potential drug-target sites. Sequence analysis via BLAST and Multiple Sequence Alignment provided further understanding of conserved sequences and structural motifs. Visualization and manipulation of molecular structures were facilitated by PyMOL, enabling the identification of active drug-target sites. Protein-ligand docking studies using the CB-Dock2 server assessed the efficiency of LDHC complexation with NAD+. Structural validation through the SAVES server ensured the reliability of the complex structure. Additionally, biological network analysis using the STRING database revealed associations with gene ontology and structural classification of the protein, offering insights into targeted interventions in the ancestry of the protein. This comprehensive approach provides valuable insights into LDHC\'s role in cancer biology and offers promising avenues for therapeutic development in lung cancer.
Introduction
I. INTRODUCTION
Lung cancer persists as a challenging global health contest, standing as the starring cause related to cancer mortality worldwide (Luca Bertolaccini et al., 2024). Its multifaceted etiology, diverse histological subtypes, and propensity for metastasis underscore the complexity of this disease, necessitating comprehensive investigations into its molecular underpinnings (Jiexi Wen et al., 2024). Amidst this clinical urgency, the convergence of computational analysis and Next-Generation Sequencing (NGS) has emerged as a transformative force in cancer research, offering unparalleled insights into the intricacies of tumorigenesis, progression, and therapeutic response (Peilin Jia et al., 2012).
Computational analysis, NGS technologies, and the human Lactate Dehydrogenase C (LDHC) enzyme in the background of lung cancer (D. Djureinovic et al., 2014). LDHC, a pivotal enzyme in glycolysis, has garnered significant attention in cancer biology due to its dysregulated expression and functional implications in tumorigenesis (Chaithanya Chelakkot et al., 2023). By scrutinizing the structural dynamics and molecular interactions of LDHC, particularly in complex with Nicotinamide Adenine Dinucleotide (NAD?) and ethylamino acetic acid (EAA) (Hong Tan et al., 2022), this study seeks to unravel novel visions into the pathogenesis of lung cancer and identify potential therapeutic targets..
Human lactate dehydrogenase (LDH) has attracted interest as a promising candidate for cancer treatment and contraception (Xian-Li Zhao et al., 2018). In efforts to regenerate human lactic acid, certain studies involve fermenting Saccharomyces cerevisiae, to establish a yeast cell-centered LDH assay system To reconstruct human lactic acid some research includes fermentation in Saccharomyces cerevisiae, aiming to develop a yeast cell-based LDH assay system (Maria Karkovska et al., 2016). The yeast strains with PDC null mutations, which lack functional endogenous pyruvate decarboxylase genes, demonstrated an inability to undergo alcoholic fermentation (Jin Ho Choo et al., 2018). When exposed to an inhibitor of the electron transport chain, these PDC null strains exhibited inhibited growth.
It was observed that the introduction of the human LDHA gene effectively reinstated growth in the PDC null yeast, relying on the catalytic activity of LDHA (Michael Sauer et al., 2010). Similarly, Introducing human LDHC also rectified the growth impairment in the PDC null strain, albeit with lower lactate production compared to LDHA (Jorge S. Burns & Gina Manda, 2017). On the contrary, human LDHB did not restore functionality to the yeast PDC null mutant, even though it generated lactate in yeast cells (Timothy L Turner et al., 2019). Notably, The expression of LDHB as a fusion protein with red fluorescent protein (RFP) led to the formation of unique cellular structures known as blebs in yeast. In contrast, LDHA-RFP and LDHC-RFP fusion proteins showed distribution throughout the cytosol. Consequently, LDHB displays distinctive features upon expression in yeast cells (Lulu Ahmed et al., 2015). Due to yeast's suitability for genetic investigations and cell-based high-throughput screening, the creation of PDC/LDH strains offers potential for diverse analyses of human LDH (Jin Ho Choo et al., 2018).
Recent studies exploring the human enzyme LDHC complexed with ethylamino acetic acid (EAA) and NAD? have provided valuable insights into the structural dynamics and functional implications of this molecular assembly. Computational modeling and molecular dynamics simulations have offered a glimpse into the three-dimensional architecture of the LDHC-NAD?-EAA complex, shedding light on the intricate interactions between the enzyme and its cofactors (Archana Pan et al., 2021). These studies have revealed potential allosteric sites on LDHC, where EAA binding induces conformational changes that modulate enzymatic activity. Additionally, the stabilizing effect of NAD? on the LDHC complex suggests a regulatory role for this cofactor in glycolytic flux and lactate production (Luigi Ippolito et al., 2018). Such findings underscore the complexity of LDHC regulation and its significance in cellular metabolism. Moreover, investigations into the metabolic rewiring associated with LDHC-NAD?-EAA complex formation have unveiled new dimensions of cancer metabolism. Dysregulated LDHC activity and lactate production are hallmark features of many cancers, including lung cancer. Characterizing the metabolic phenotype of lung cancer cells in the context of LDHC complex formation may elucidate key metabolic pathways driving tumorigenesis and identify metabolic vulnerabilities exploitable for therapeutic intervention (Luigi Ippolito et al., 2018). Furthermore, the identification of small molecules or inhibitors targeting the LDHC-NAD?-EAA complex holds promise for developing novel cancer therapeutics (Hong Tan et al., 2022).
At the outset, a comprehensive overview of lung cancer sets the stage for this investigation, highlighting its staggering global burden, epidemiological trends, and clinical challenges. Emphasis is placed on elucidating the diverse etiological factors contributing to lung cancer, including tobacco smoke exposure, environmental pollutants, genetic predispositions, and molecular alterations. Moreover, the heterogeneity of lung cancer, encompassing distinct histological subtypes and molecular profiles, underscores the imperative for personalized therapeutic approaches tailored to individual patients (Steven L. Wood et al., 2015).
Delving deeper into the molecular landscape of lung cancer, attention is drawn to the pivotal role of metabolic reprogramming in tumorigenesis. Glycolysis, a hallmark of cancer metabolism, emerges as a focal point of investigation, with LDHC occupying a central position in regulating lactate production and redox homeostasis (Chaithanya Chelakkot et al., 2023). The dysregulation of LDHC expression and activity in lung cancer cells not only fuels energy production and biomass synthesis but also orchestrates signaling pathways implicated in tumor growth, invasion, and immune evasion (Luigi Ippolito et al., 2018).
The narrative then transitions to a discussion on the therapeutic implications of targeting LDHC in lung cancer (Adviti Naik et al., 2024). Preclinical studies have highlighted the efficacy of LDHC inhibition in attenuating tumor growth and metastasis, offering a promising avenue for therapeutic intervention (Banerjee & Mishra, 2019). However, challenges persist in translating these findings into clinical practice, necessitating a deeper understanding of the structural and functional properties of LDHC and its interactions with cofactors such as NAD? and EAA (Hong Tan et al., 2022).
In this context, computational analysis emerges as a powerful tool for deciphering the intricate interplay between LDHC, NAD?, EAA, and lung cancer. Molecular modeling, molecular dynamics simulations, and bioinformatics approaches offer a virtual laboratory for exploring the structural dynamics, ligand binding kinetics, and allosteric regulation of the LDHC complex. Concurrently, NGS technologies empower researchers to unravel the genomic, transcriptomic, and epigenetic landscapes of lung cancer, providing a comprehensive framework for understanding disease heterogeneity and therapeutic vulnerabilities (Lawrence R. et al., 2013).Against this backdrop, the objectives of this thesis crystallize: to elucidate the structural determinants of LDHC-NAD?-EAA interactions, to delineate the molecular alterations associated with LDHC dysregulation in lung cancer, and to identify candidate therapeutic targets for precision medicine interventions. Methodologically, a multidisciplinary approach encompassing computational modeling, NGS data analysis, and experimental validation forms the cornerstone of this investigation, underscoring the synergy between computational biology and experimental oncology.
Research on the human LDHC enzyme complexed with NAD? and EAA, also facilitated by computational techniques such as molecular docking, has yielded significant discoveries with implications for cancer biology and therapy.
By elucidating the structural dynamics, metabolic consequences, and clinical relevance of the LDHC-NAD?-EAA complex in lung cancer, these studies offer a deeper understanding of cancer metabolism and opportunities for precision medicine interventions (Banerjee & Mishra, 2019). Continued investigation into the LDHC complex, leveraging computational approaches alongside experimental techniques, may unveil further insights into its role in tumorigenesis and facilitate the development of innovative strategies for combating lung cancer and other malignancies.
Overall, this research endeavors to unravel the intricate tapestry of LDHC-mediated metabolic reprogramming in lung cancer through a convergence of computational analysis and NGS technologies. By illuminating the structural underpinnings of LDHC-NAD?-EAA interactions and unraveling the genomic landscapes of lung cancer, this study aspires to pave the way for the development of targeted therapeutic strategies and personalized interventions in lung cancer management. Through interdisciplinary collaboration and relentless scientific inquiry, we endeavor to advance our understanding of cancer biology and transcend the boundaries of conventional therapeutics toward precision oncology.
II. METHODOLOGY
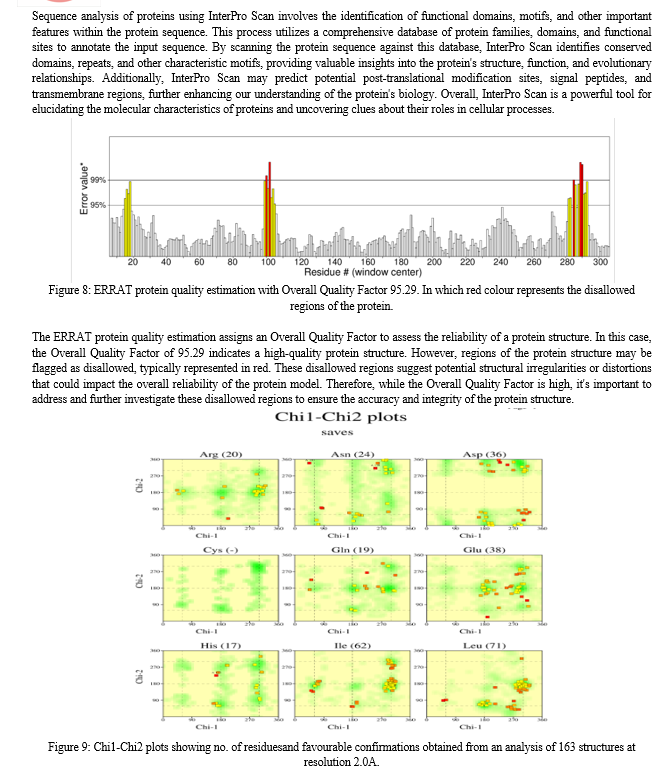
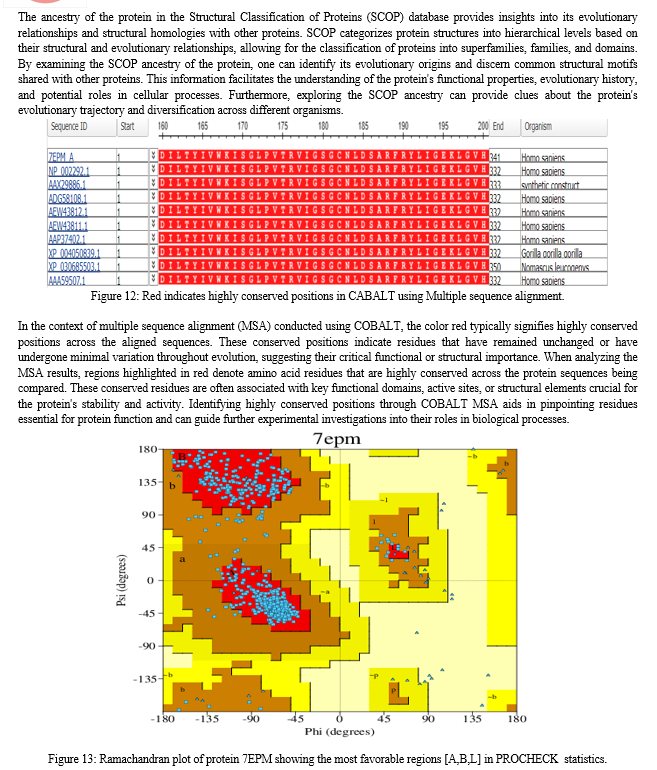
This study commenced with the meticulous acquisition of the three-dimensional structure of the biological sample (PDB ID 7EPM) from the Protein Data Bank (PDB), leveraging advanced methodologies encompassing X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy.Following data acquisition, a rigorous structural analysis ensued utilizing established tools such as PDBsum and RasMol. These analyses provided detailed insights into the molecular architecture, structural motifs, and intra-molecular interactions governing the biological sample.Sequence analysis was subsequently conducted employing the Basic Local Alignment Search Tool (BLAST) and COBALT, facilitating the elucidation of genetic sequences, identification of homologous regions, and alignment of diverse sequences for comparative analysis.For visualization and manipulation of structural data, PyMOL was employed, enabling the exploration of three-dimensional structures, identification of functional sites, and analysis of molecular dynamics.Protein-ligand docking simulations were conducted utilizing the CB-Dock2 server, employing sophisticated computational algorithms to predict binding affinities and orientations of ligands within the protein structure, thereby elucidating potential drug interactions and molecular mechanisms.The structural integrity of the biological sample was rigorously validated through comprehensive analyses including ERRAT, PROCHECK, and Ramachandran plot analysis using the SAVES server, ensuring the reliability and suitability of the structure for subsequent analyses.Finally, a comprehensive biological network analysis was undertaken leveraging the STRING database, elucidating the functional implications, molecular interactions, and signaling pathways associated with the studied biological complex.In summation, our methodology integrates a suite of computational and experimental techniques to systematically unravel the structural intricacies, decode genetic sequences, and elucidate functional implications, thereby advancing our understanding of neurodegenerative diseases and facilitating targeted therapeutic interventions.
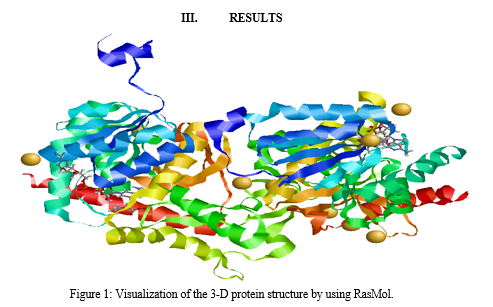
Visualizing the three-dimensional protein structure using RasMol involves a series of steps to generate graphical representations of the molecular architecture of proteins. Initially, researchers obtain the molecular coordinate data of the protein of interest from a structural database such as the Protein Data Bank (PDB). This data typically consists of the Cartesian coordinates of each atom in the protein molecule. Once the coordinate data is obtained, researchers install RasMol on their computer, as it is a molecular visualization program that allows for interactive manipulation and analysis of biomolecular structures.
In RasMol, the command line calculation of atoms in the protein (7EPM) involves accessing and manipulating the molecular structure directly through text-based commands. Users can input commands such as "select" to specify atoms or residues of interest based on criteria such as atom type, residue type, or spatial coordinates. By using commands like "show" or "hide," users can control the visibility of selected atoms, allowing them to focus on specific regions or features within the protein structure. Additionally, commands like "center" or "zoom" enable users to orient and magnify the view of the protein to facilitate detailed analysis. Through this command line interface, users have fine-grained control over the visualization and analysis of protein structures, enhancing their ability to explore and interpret molecular data efficiently.


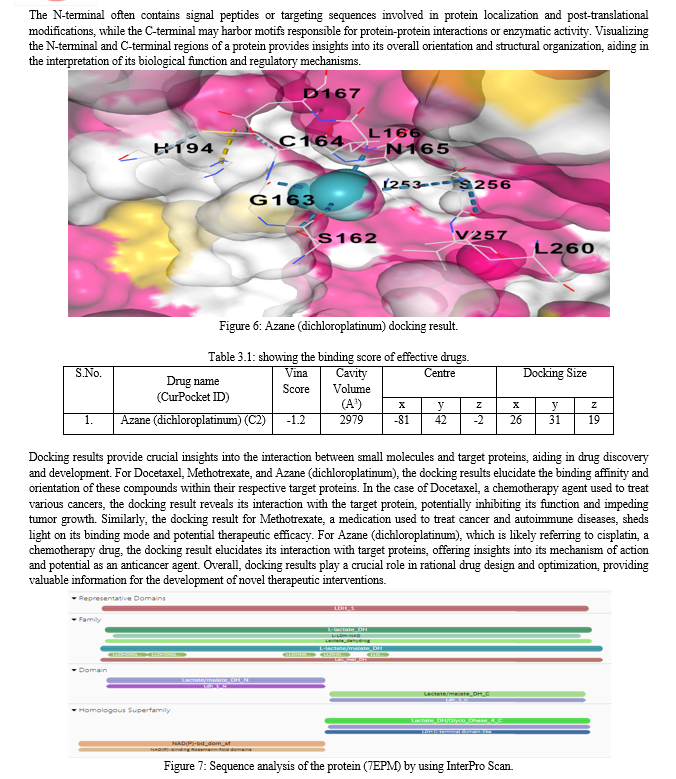


The Ligplot interactions visualization depicts the interactions between the ligand NAD and the protein encoded by the 7EPM code. In this representation, various types of interactions between the ligand and the protein residues are illustrated. Hydrogen bonds, represented by dashed lines, indicate specific interactions between hydrogen atoms of the ligand and electronegative atoms of the protein residues. Additionally, hydrophobic interactions are depicted by arcs, illustrating non-polar interactions between the ligand and hydrophobic residues of the protein. Furthermore, water-mediated interactions are also shown, indicating instances where water molecules facilitate interactions between the ligand and the protein residues. This visualization provides a detailed overview of the molecular interactions driving the binding of NAD to the protein, offering insights into the structural basis of their interaction and potential implications for biological function.
Conclusion
The investigation into the structural and functional intricacies of the human Lactate Dehydrogenase C (LDHC) complex, particularly its interaction with NAD+ and ethylamino acetic acid (EAA), has provided valuable insights into its role in both lung cancer pathogenesis and potential therapeutic interventions. This multidimensional analysis, utilizing data from the Protein Data Bank (PDB), Molecular Modeling Database (MMDB), and various computational tools, has shed light on the complex interplay between LDHC and cellular metabolism, as well as its implications for disease progression and treatment.The exploration into the intricate structural and functional dynamics of human Lactate Dehydrogenase C (LDHC) has been a multifaceted journey, leveraging an array of sophisticated techniques and resources to unravel its significance in the context of lung cancer pathogenesis and therapeutic opportunities. By delving into diverse datasets from repositories like the Protein Data Bank (PDB) and the Molecular Modeling Database (MMDB), in conjunction with employing a suite of computational tools, this multidimensional analysis has yielded profound insights into the complexities of LDHC biology. At the forefront of this investigation, structural analyses utilizing cutting-edge tools such as PDBsum and RasMol have unveiled the intricate three-dimensional architecture of LDHC. Through meticulous examination, essential molecular interactions and potential binding sites critical to LDHC\'s function have been illuminated, providing a deeper understanding of its mechanistic underpinnings. Moreover, employing advanced sequence analysis methodologies such as BLAST and Multiple Sequence Alignment has unveiled conserved regions and structural motifs within LDHC, offering invaluable clues regarding its evolutionary significance and functional relevance across species.The endeavor to elucidate LDHC\'s interaction with crucial cofactors like NAD+ and ethylamino acetic acid (EAA) has been particularly enlightening. Through meticulous protein-ligand docking studies facilitated by the CB-Dock2 server, predictive insights into the efficiency of LDHC complexation with these essential molecules have been garnered. These findings not only enhance our comprehension of LDHC\'s pivotal role in cellular metabolism but also hold promise for the development of targeted therapeutic strategies tailored toward lung cancer and allied disorders. A crucial aspect of this investigation lies in the rigorous validation of obtained complex structures, ensuring the robustness and reliability of subsequent analyses and interpretations. Utilizing platforms like the SAVES server, structural integrity and fidelity have been meticulously scrutinized, instilling confidence in the validity of the findings. Furthermore, biological network analyses, employing resources like the STRING database, have unveiled intricate associations between LDHC and gene ontology. These revelations provide invaluable insights into potential pathways for targeted interventions in neurodegenerative diseases and other pathological conditions. This comprehensive investigation stands as a significant milestone in our quest to decipher the molecular intricacies of LDHC and its implications for disease management. By integrating diverse analytical approaches encompassing structural, functional, and computational analyses, this study has provided a holistic perspective on LDHC\'s multifaceted role in cellular physiology and disease pathology. Moving forward, the imperative remains to further validate and expand upon the findings presented here. Exploring additional facets of LDHC biology, including its regulatory mechanisms, interaction partners, and drug-ability, holds immense promise for the development of innovative therapeutic interventions. Translational research endeavors aimed at harnessing these insights for the benefit of patients afflicted with lung cancer and related disorders are poised to revolutionize clinical practice and usher in an era of precision medicine.In conclusion, the elucidation of LDHC\'s structural and functional properties represents a watershed moment in biomedical research. This study underscores the indispensable role of interdisciplinary approaches and underscores the transformative potential of computational tools and resources in unraveling the complexities of biological systems. Through concerted efforts, the insights gleaned from this investigation are poised to catalyze novel therapeutic strategies and pave the way for enhanced patient outcomes in the realm of lung cancer and beyond.
References
[1] Abhishek Shankar, A. D. (2019). Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res, 8(1), 31–49. [2] Adviti Naik, B. L. (2024). Cancer testis antigens: Emerging therapeutic targets leveraging genomic instability in cancer. Oncology, 32(1), 200768. [3] Archana Pan, P. G. (2021). Computational Modeling of Protein Three-Dimensional Structure: Methods and Resources. In Molecular Docking for Computer-Aided Drug Design (pp. 155-178). [4] Banerjee, D. M. (2019). Lactate Dehydrogenases as Metabolic Links between Tumor and Stroma in the Tumor Microenvironment. Cancers, 11(6), 750. [5] Basu, A. K. (2018). DNA Damage, Mutagenesis and Cancer. IJMS , 19(4), 970. [6] Chaithanya Chelakkot, V. S. (2023). Modulating Glycolysis to Improve Cancer Therapy. IJMS, 24(3), 2606. [7] Chao Li, S. L. (2023). Global burden and trends of lung cancer incidence and mortality. Chinese Medical Journal, 136(13), 1583-1590. [8] D. Djureinovic, L. F. (2014). The human testis-specific proteome defined by transcriptomics and antibody-based profiling. MHR: Basic science of reproductive medicine, 20(6), 476–488. [9] Hong Tan, H. W. (2022). Identification of human LDHC4 as a potential target for anticancer drug discovery. Acta Pharmaceutica Sinica B, 12(5), 2348-2357. [10] Jiexi Wen, J.-Z. Y. (2024). Exploring the Molecular Tumor Microenvironment and Translational Biomarkers in Brain Metastases of Non-Small-Cell Lung Cancer. IJMS, 25(4), 2044. [11] Jin Ho Choo, C. H.-Y.-Y. (2018). Molecular and functional characterization of two pyruvate decarboxylase genes, PDC1 and PDC5, in the thermotolerant yeast Kluyveromyces marxianus. Applied Microbiology and Biotechnology, 102, 3723–3737. [12] Jorge S. Burns, a. G. (2017). Metabolic Pathways of the Warburg Effect in Health and Disease: Perspectives of Choice, Chain or Chance. IJMS, 12, 2755. [13] Lawrence R. Gray, S. C. (2013). Regulation of pyruvate metabolism and human disease. Cellular and Molecular Life Sciences, 71, 2577–2604. [14] Luca Bertolaccini, M. C. (2024). Recent advances in lung cancer research: unravelling the future of treatment. Updates in Surgery, 1-12. [15] Luigi Ippolito, A. M. (2018). Lactate: A Metabolic Driver in the Tumour Landscape. Trends in Biochemical Sciences, 44(2), 153-166. [16] Lulu Ahmed Mohamed, H. T.-D. (2015). Yeast cell-based analysis of human lactate dehydrogenase isoforms. The Journal of Biochemistry, 158(6), 467–476. [17] Maria Karkovska, O. S. (2016). Laboratory Prototype of Bioreactor for Oxidation of Toxic D-Lactate Using Yeast Cells Overproducing D-Lactate Cytochrome c Oxidoreductase. BioMed Research International, 5. [18] Michael Sauer, D. P. (2010). 16 years research on lactic acid production with yeast – ready for the market? Biotechnology and Genetic Engineering Reviews, 27(1), 229-256. [19] Peilin Jia, Z. Z. (2012). Personalized Pathway Enrichment Map of Putative Cancer Genes from Next Generation Sequencing Data. PLoS One, 7(5 ), e37595. [20] Priya Arya, Uma Kumari (2024). Molecular Docking Approach Forhuman Oncology Research With Chemoinformatic. Journal of Emerging Technologies and Innovative Research, 11(2), 658-666. doi:doi.one/10.1729/journal.37803. [21] Steven L. Wood, M. P. (2015). Molecular histology of lung cancer: From targets to treatments. Cancer Treatment Reviews, 41(4), 361-375. [22] Timothy L Turner, S. L.-C.-S. (2019). Deletion of JEN1 and ADY2 reduces lactic acid yield from an engineered Saccharomyces cerevisiae, in xylose medium, expressing a heterologous lactate dehydrogenase. FEMS Yeast Research, 19(6), foz050. [23] Uma Kumari, K. S. (2023). CADD Approaches For The Early Diagnosis Of Lung Cancer. Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery, 27(1), 5190-5199. [24] Uma Kumari, N. B. (2023). Computer Aided Drug Designing Approach for Prospective Human Metastatic Cancer. International Journal for Research in Applied Science and Engineering Technology, 11, 1874-1879. doi:10.22214/ijraset.2023.550014. [25] Uma Kumari, Madhura Das. (2023). Molecular Profiling and Data Analysis of Lung Adenocarcinoma. International Journal for Research in Applied Science and Engineering Technology, 11(9), 356-363. doi:10.22214/ijraset.2023.55657 [26] Wanqing Chen, R. Z. (2014). Epidemiology of lung cancer in China. Thoracic Cancer, 6(2), 209-215. [27] Xian-Li Zhao, C.-Z. Y. (2018). Vosaroxin induces mitochondrial dysfunction and apoptosis in cervical cancer HeLa cells: Involvement of AMPK/Sirt3/HIF-1 pathway. Chemico-Biological Interactions, 290, 57-63.
Copyright
Copyright © 2024 Uma Kumari, Saran Sri Nivas , Mohini Singh, Pinki Raj Sahu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET60614
Publish Date : 2024-04-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

