Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Computer Aided Drug Designing Approach for Prospective Human Metastatic Cancer
Authors: Uma Kumari, Nehal Balhara, Ojas Singh
DOI Link: https://doi.org/10.22214/ijraset.2023.55014
Certificate: View Certificate
Abstract
It is well known that finding new drugs is a difficult, expensive, time-consuming, and expensive project. According to a study, the typical time and cost for developing a new medicine through the conventional drug development pipeline is 12 years and 2.7 billion dollars. The pharmaceutical industry is grappling with the difficult and pressing challenge of how to find new drugs faster and at lower research costs.Insilico,The field of computer-aided drug discovery (CADD) has shown significant promise as an advanced technology for secure, cost-effective, and efficient drug design. In recent times, there has been remarkable progress in computational tools for drug discovery, particularly in the development of anticancer therapies. This progress has had a significant impact on the design of anticancer drugs and has provided valuable insights into the field of cancer treatment. To carry out molecular docking, we utilized AutoDock software and prepared the target protein by loading and converting its PDB file format into a macromolecule. Additionally, the ligand structures underwent energy minimization (EM) and were selected alongside the target proteins in AutoDock. To ensure coverage of the binding site residues, a suitable grid box with appropriate dimensions was chosen.
Introduction
I. INTRODUCTION
Metastatic cancer remains a formidable challenge in oncology, with limited treatment options and poor prognosis. However, the advent of Computer-Aided Drug Design (CADD) has ushered in a new era of precision oncology, offering novel therapeutic opportunities for patients with advanced metastatic cancer. Metastasis, the process by which cancer cells spread from their primary site to distant organs, is a complex and multifactorial phenomenon that eludes conventional treatments. Cancer metastasis remains one of the most pressing challenges in modern medicine. The ability of cancer cells to disseminate from the primary tumor to distant sites makes the management of metastatic cancer exceptionally difficult. Metastasis is a complex and multistep process that involves various molecular and cellular events. This section delves into the key stages of metastasis, including epithelial-mesenchymal transition (EMT), intravasation, extravasation, and colonization. Emphasis is placed on the role of specific genes, signaling pathways, and microenvironmental factors that contribute to cancer cell dissemination and colonization of distant organs. Certain risk factors predispose individuals to the development of metastatic cancer. Genetic mutations, chronic inflammation, immune dysregulation, and lifestyle choices can influence the metastatic potential of cancer cells. Early detection of metastatic cancer is vital for effective treatment. Non-invasive and sensitive diagnostic tools play a crucial role in monitoring disease progression and tailoring personalized treatment plans. Conventional cancer therapies, such as surgery, chemotherapy, and radiation, often demonstrate limited efficacy against metastatic cancer [1,2,3,4]. Brain metastases, which are the most common type of brain tumor, often occur in patients with lung, breast, and colorectal cancers. Approximately 10% to 26% of cancer patients who succumb to their illness develop brain metastases. In a specific patent, ArapetaI. investigated the targeting of aminopeptidase A (APA) for the treatment and diagnosis of cancer. APA is overexpressed and enzymatically active in malignant gliomas and metastatic brain tumors. It is a proteolytic enzyme involved in pathological angiogenesis, predominantly found in tumor vascular endothelium but absent in normal blood vessels. The patent suggests using peptides and antibodies that specifically target APA, coupled with diagnostic and/or therapeutic agents, to diagnose cancer and its metastasis while delivering the therapeutic agent specifically into the tumor [5,6]. Molecular docking is a key CADD technique used to predict the interaction between a small molecule (drug) and its target protein. Through the analysis of binding affinities and interactions, researchers can identify potential lead compounds that show promise in targeting metastasis-related proteins and pathways. Virtual screening involves the in silico evaluation of large chemical databases to identify candidate compounds with the desired pharmacological activity. This approach has revolutionized early-stage drug discovery, expediting the process of lead identification for metastatic cancer targets. With advances in structural biology, researchers can now leverage the 3D structures of target proteins to design novel drugs.
Structure-based drug design allows for the rational development of compounds that precisely fit into the active sites of metastasis-promoting proteins, offering specificity and potency. Inhibiting angiogenesis, the formation of new blood vessels that support tumor growth, is a promising approach in metastatic cancer therapy [7,8]. CADD techniques have enabled the discovery of anti-angiogenic agents that disrupt VEGF signaling and impede tumor vascularization. Dysregulated cell signaling pathways play a pivotal role in cancer metastasis. CADD has facilitated the identification of small molecules that inhibit key kinases and downstream effectors, thereby interfering with cancer cell proliferation, migration, and invasion. Immunotherapy has revolutionized cancer treatment, including metastatic cancer. CADD has been instrumental in designing immune checkpoint inhibitors that unleash the patient's immune response against metastatic tumors. CADD empowers clinicians to identify patient-specific drug candidates based on the molecular profile of metastatic cancer. Personalized medicine approaches have shown great promise in clinical trials, offering tailored treatments with improved efficacy and reduced side effects. Metastatic cancers often acquire resistance to conventional therapies. CADD enables the development of novel drugs that circumvent existing resistance mechanisms, providing new therapeutic options for resistant tumors [9,10].
Computer-Aided Drug Design represents a transformative approach in the fight against human metastatic cancer. Through a systematic and data-driven approach, CADD has expanded our understanding of the molecular intricacies of metastasis and facilitated the discovery of innovative drug candidates. As research progresses and CADD techniques evolve, the future holds great promise for personalized and effective therapies for patients facing the daunting challenge of metastatic cancer [11].
The treatment of brain metastases, a devastating complication of cancer, has witnessed remarkable progress through the integration of Computer-Aided Drug Design (CADD) methodoIogies. CADD involves the utiIization of computationaIaIgorithms and tools to expedite the process of drug discovery and design.One of the primary contributions of CADD lies in its ability to facilitate the screening of large chemical libraries to identify potential drug candidates. By leveraging virtual screening techniques, such as molecular docking and ligand-based similarity searches, CADD can evaluate the interactions between small molecules and target proteins implicated in brain metastases. This approach enables the rapid assessment of a vast number of compounds, significantly reducing the time and cost associated with experimental screening. CADD also facilitates the exploration of complex biological systems by integrating data from various sources, including genomics, proteomics, and structural biology. By mining and analyzing large datasets, CADD can identify key molecular targets and signalling pathways that drive brain metastases. This knowledge, combined with computational modelling techniques, enables the rational design of drugs that interfere with specific molecularinteractions, disrupt critical cellular processes, and inhibit tumor growth in the brain.By employing computational simulations, researchers can optimize the pharmacokinetic properties of drug candidates, predict their blood-brain barrier permeability, and assess their distribution within the brain tissue. These insights guide the formulation of efficient drug delivery systems, such as nanoparticle-based carriers or targeted drug conjugates, capable of traversing the blood-brain barrier and selectively accumulating within metastatic tumors [12,13,14].
II. MATERIALS AND METHODS
The Protein Data Bank (PDB) is a globally recognized and widely utilized resource that serves as a repository forthree-dimensional structural data of biological macromolecules, primarily proteins and nucIeic acids. The PDB stores atomic coordinates, experimental data, and related metadata, allowing scientists to study the folding, function, and interactions of biomolecules. The data in the PDB is obtained through techniques such as X-ray crystaIIography, NMR spectroscopy, and cryo-EM [15,16]. PubChem is a comprehensive database maintained by the National Center for BiotechnoIogy Information (NCBl), a division of the United States National Library of Medicine. Ligands were foundfrom the literature and downloaded in SDF format from PubChem. It is the world’s Iargest archive of openly accessible chemical data.Additionally, PubChem offers a range of tools and resources for data analysis, visualization, and integration with other databases, making it an invaluable platform for researchers worldwide [17,18]. PyMOL is a Python-basedopen-source molecular visualization toolkit. It provides an interactive and user-friendly environment for the visualization and analysis of three-dimensional biomolecular structures. PyMOL allows users to render high-quality images and animations, explore protein structures, and analyze various molecular properties.PyMOL was used to visualise the neuroglobin 1OJ6and prepare partialligands while also analyzing the binding of ligands to the target [19,20]. RasMol is a pioneering molecular visualization program that has played a significant role in the field of structural biology. Developed in the early 1990s, RasMol provides a user-friendly interface for the visualization and analysis of biomolecular structures, particularly protein and nucleic acid structures. It enables researchers to interactively explore and manipulate 3D models, visualize secondary structure elements, and analyze molecular interactions.
RasMol supports a variety of rendering options, including wireframe, space-filling, and ribbon representations, allowing users to customize the display of molecular structures. While it may lack some of the advanced features of modern molecular visualization software, RasMol remains widely used and respected for its simplicity, versatility, and contributions to the understanding of macromolecular structures [21,22]. AutoDockVina is widely utilized and swift open-source molecular docking software. Along with AutoDock4 (AD4), AutoDock-GPU, AutoDock-FR, and AutoDock- CrankPep, it is one of the docking tools included in the AutoDock Suite. Vina is regarded as one of the most widely used applications due to its simplicity and efficiency in comparison to other docking programs, both within and beyond the AutoDock Suite. In addition, the program is open-source. It is a set of tools used for automating docking, which attempts to anticipate how smaIImolecuIes, such as substrates or potential drugs, will bind to a 3D-structured receptor. Numerous modifications and enhancements have been made to the program over time, resulting in the development of multiple engines and the addition of new features. AutoDock 4 consists of two primary programs: autodock and auto grid. Autodock is responsible for docking the Iigand to a set of grids representing the target protein, while auto grid generates these grids beforehand [23,24].
III. RESULT AND DISCUSSION
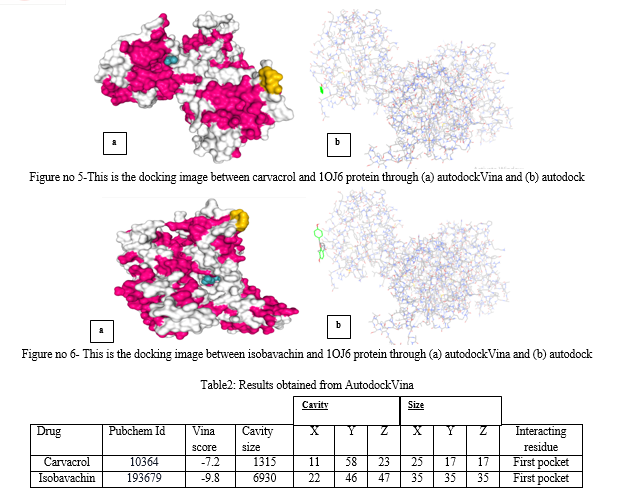
For conducting computational analysis and molecular docking, the required target information was obtained from the National Center for Biotechnology Information (NCBI) database, including the PDB ID (1OJ6). Subsequently, the 3D structure of the target was downloaded in PDB format from the Protein Data Bank (PDB) database. In this project, the target is identified as 1OJ6, which is a three-dimensional arrangement of human neuroglobin.In the subsequent step, 2 ligands are chosen for molecular docking against the target protein: Cavacrol&Isobavachin.
Carvacrol is a natural compound that belongs to the class of chemicals known asmonoterpenoids. It is a monoterpene derivative and is found in the essential oil of variousplants, particularly in the oil of oregano (Origanumvulgare) and thyme (Thymus vulgaris). Carvacrol is known for its strong aromatic and medicinal properties. It possessesantimicrobial, antifungal, antiviral, and antioxidant activities. It has beenextensively studied for its potential health benefits and is commonly used in traditional medicine and natural remedies.
Isobavachin, also known as psoralidin, is a natural compound belonging to the class ofchemicals called flavonoids. It is found in various plants, including Psoraleacorylifolia, which is commonly used in traditional medicinesystemslike Ayurveda and Traditional Chinese Medicine. Isobavachin has been the subject ofscientific research due to its potential biological activities and health benefits.
Next, Carvacrol and Isobavachin regimens were required as ligands. To obtain these structures, the PubChem database was accessed and downloaded the structures of these metals. These structures will be utilized in the later stages of the project. In order to study the structure of the target molecule, we utilized the RasMol software. This software allows us to read molecular coordinates from a file and interactively display the molecule in various representations. Specifically, we used RasMol to analyze the structure of target 1OJ6 and gain a deeper understanding of its molecular composition. After launching RasMol, the target structure was loaded into the software. We accomplished this by transferring the appropriate file from.
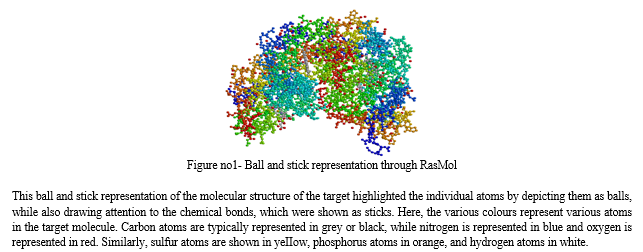

Approximately 10% to 15% of cancer patients experience the development of metastatic brain tumours. Among these cases, the lung is the most common primary site of metastasis, also including the breast, gastrointestinal tract, kidneys, and skin. The occurrence of brain metastasis is influenced by the genetic characteristics of the cancer. Patients with advanced non-small cellIung cancer (NSCLC) harbouring an epidermal growth factor receptor (EGFR) mutation exhibit a higher incidence of brain metastasis, even when accounting for differences in survival, compared to patients without the mutation.
Computational analysis and molecular docking techniques are valuable tools in studying liver cancer. In this study, research was conducted via molecular docking analysis using the 3D structure of human neuroglobin (PDB ID: 1OJ6). This approach allows for the investigation of potential interactions between the target protein and ligands. Two ligands, cavacrol and isobavachin, were selected for molecular docking against the target protein. Stanford V, is derived from a combination of chemotherapy drugs including mechlorethamine, doxorubicin, vinblastine, vincristine, bleomycin, etoposide, and prednisone. The docking analysis revealed that both ligands were able to partially bind to the target protein, and specific amino acid residues within the HEM were identified as involved in the binding of Stanford V. The docking results were visualized and analyzed using PyMol software. The analysis provided insights into the interactions between the target protein and ligands, shedding light on potential binding sites and affinity. The grid scoring was used in Autodock Vina's docking procedure to determine how well the protein receptor might bind to the ligands. The calculated grid scores from the analysis showed that carvacrol (with x-center = 11, y-center = 58 and z-center = 23) and isobavachin (with x-center = 22, y-center = 46, z- center = 47). It was seen that the ligand with the lowest grid score is carvacrol. A ligand's binding energy is directly proportional to its grid score, meaning that a lower score indicates a higher binding energy. If the ligand has a lower grid score, it is predicted to have a stronger affinity for the protein.
Conclusion
Approximately 20% of individuals diagnosed with cancer are estimated to develop brain metastases, primarily seen in patients with lung, breast, colorectal cancers, melanoma, or renal cell carcinoma. Brain metastases occur when tumor cells spread through the bloodstream and settle in the brain\\\'s small blood vessels. This unique environment promotes tumor growth while limiting the effectiveness of systemic treatments. Despite advancements in various treatment approaches such as surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapies, the prognosis for patients with advanced-stage cancer and brain metastases remains unfavorable. Therefore, there is a strong interest in comprehending the underlying mechanisms of brain metastasis formation to develop preventive therapeutic strategies. Furthermore, understanding the molecular characteristics of brain metastases compared to the primary tumor can guide the selection of targeted therapies. Enhanced knowledge at the molecular level will also facilitate the development of innovative immunotherapies and targeted therapies capable of crossing the blood-tumor barrier, along with advancements in radiotherapy and minimally invasive surgical techniques. As these discoveries and innovations progress from basic research to preclinical and clinical applications, it is highly likely that future outcomes for patients with brain metastases will significantly improve.
References
[1] Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A., & Fares, Y. (2020). Molecular principles of metastasis: a hallmark of cancer revisited. Signal transduction and targeted therapy, 5(1), 28. https://doi.org/10.1038/s41392-020-0134-x [2] Seyfried, T. N., & Huysentruyt, L. C. (2013). On the origin of cancer metastasis. Critical reviews in oncogenesis, 18(1-2), 43–73. https://doi.org/10.1615/critrevoncog.v18.i1-2.40 [3] Jin, X., Demere, Z., Nair, K. et al. A metastasis map of human cancer cell lines. Nature 588, 331–336 (2020). https://doi.org/10.1038/s41586-020-2969-2 [4] Hapach, L.A., Mosier, J.A., Wang, W. et al. Engineered models to parse apart the metastatic cascade. npj Precis. Onc. 3, 20 (2019). https://doi.org/10.1038/s41698-019-0092-3 [5] Guantao Zheng and others, HCMDB: the human cancer metastasis database, Nucleic Acids Research, Volume 46, Issue D1, 4 January 2018, Pages D950–D955, https://doi.org/10.1093/nar/gkx1008 [6] Xiangming Guan,Cancer metastases: challenges and opportunities,Acta Pharmaceutica Sinica B,Volume 5, Issue 5,2015,Pages 402-418,ISSN 2211-3835, https://doi.org/10.1016/j.apsb.2015.07.005. [7] Meng, X. Y., Zhang, H. X., Mezei, M., & Cui, M. (2011). Molecular docking: a powerful approach for structure-based drug discovery. Current computer-aided drug design, 7(2), 146–157. https://doi.org/10.2174/157340911795677602 [8] Torres, P. H. M., Sodero, A. C. R., Jofily, P., & Silva-Jr, F. P. (2019). Key Topics in Molecular Docking for Drug Design. International journal of molecular sciences, 20(18), 4574. https://doi.org/10.3390/ijms20184574 [9] Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384-13421. https://doi.org/10.3390/molecules200713384 [10] Ravichandran R, Sundararajan R (2017) In silico-Based Virtual Drug Screening and Molecular Docking Analysis of Phyto Chemical Derived Compounds and FDA Approved Drugs against BRCA1 Receptor. J Cancer Prev Curr Res 8(2): 00268. DOI: 10.15406/jcpcr.2017.08.00268 [11] Folkman J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine, 1(1), 27–31. https://doi.org/10.1038/nm0195-27 [12] Yip, H. Y. K., & Papa, A. (2021). Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells, 10(3), 659. https://doi.org/10.3390/cells10030659 [13] Hidalgo, M., Amant, F., Biankin, A. V., Budinská, E., Byrne, A. T., Caldas, C., Clarke, R. B., de Jong, S., Jonkers, J., Mælandsmo, G. M., Roman-Roman, S., Seoane, J., Trusolino, L., & Villanueva, A. (2014). Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer discovery, 4(9), 998–1013. https://doi.org/10.1158/2159-8290.CD-14-0001 [14] Longo D. L. (2013). Personalized cancer care is not new. The oncologist, 18(6), 644–645. https://doi.org/10.1634/theoncologist.2013-0203 [15] Burley, S. K., Berman, H. M., Kleywegt, G. J., Markley, J. L., Nakamura, H., & Velankar, S. (2017). Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods in molecular biology (Clifton, N.J.), 1607, 627–641. https://doi.org/10.1007/978-1-4939-7000-1_26 [16] Uma kumari, NavjotKaurVirk, Identification of new potential drug for lung adenocarcinoma causing protein RMB10 using computer aided drug design approach, Publication date- 2022/6/11, Journal-IJBTR, Volume-12, Issue-2, Pages 1-8 [17] Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G., Gindulyte, A., Han, L., He, J., He, S., Shoemaker, B. A., Wang, J., Yu, B., Zhang, J., & Bryant, S. H. (2016). PubChem Substance and Compound databases. Nucleic acids research, 44(D1), D1202–D1213. https://doi.org/10.1093/nar/gkv951 [18] Sunghwan Kim and others, PubChem in 2021: new data content and improved web interfaces, Nucleic Acids Research, Volume 49, Issue D1, 8 January 2021, Pages D1388–D1395, https://doi.org/10.1093/nar/gkaa971 [19] Seeliger, D., & de Groot, B. L. (2010). Ligand docking and binding site analysis with PyMOL and Autodock/Vina. Journal of computer-aided molecular design, 24(5), 417–422. https://doi.org/10.1007/s10822-010-9352-6 [20] Sefika Feyza Maden, Selin Sezer, Saliha Ece Acuner, Fundamentals of Molecular Docking and Comparative Analysis of Protein–Small-Molecule Docking Approaches, Molecular Docking - Recent Advances, 10.5772/intechopen.105815, (2023) [21] Dr Uma kumari, Devanshi Gupta, In silico RNA aptamer drug design and modelling,2022/4, Journal-JETIR,Volume-9, Issue-4, Pages 718-725 [22] Roger A. Sayle, E.James Milner-White,RASMOL: biomolecular graphics for all,Trends in Biochemical Sciences,Volume 20, Issue 9,1995,Pages 374-376, ISSN 0968-0004, https://doi.org/10.1016/S0968-0004(00)89080-5. [23] Cole, J.C., Murray, C.W., Nissink, J.W., Taylor, R.D. and Taylor, R. 2005. Comparing protein-ligand docking programs is difficult. Proteins 60: 325- 332. [24] Cozzini, P., Kellogg, G.E., Spyrakis, F., Abraham, D.J., Costantino, G., Emerson, A., Fanelli, F., Gohlke, H., Kuhn, L.A., Morris, G.M., Orozco, M., Pertinhez, T.A., Rizzi, M., and Sotriffer, C.A. 2008. Target flexibility: An emerging consideration in drug discovery and design. J. Med. Chem. 51: 6237- 6255.
Copyright
Copyright © 2023 Uma Kumari, Nehal Balhara, Ojas Singh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55014
Publish Date : 2023-07-25
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

