Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Conjugated Hole Transport Molecule based on Triphenylamine and Aminoanthraquinone: A Theoretical Approach
Authors: Priyatha Elody
DOI Link: https://doi.org/10.22214/ijraset.2023.54699
Certificate: View Certificate
Abstract
Quantum chemical calculations were performed by using Density functional theory (DFT) to understand the hole transport properties of the novel Triphenylamine-Aminoanthraquinone azomethine compound, (E)-N-(4-(diphenylamino)benzylidene)-9,10-dihydro-9,10-dimethyleneanthracen-2-amine (C3). The theoretically calculated energy levels of the compound were well matched with the other layers of the perovskite sensitized solar cells to fit as hole transport material in perovskite sensitized solar cells. The results suggest that this compound can be considered as a promising hole transport material for perovskite sensitized solar cells.
Introduction
I. INTRODUCTION
Energy consumption has been increasing at one of the fastest rates in the world, due to the population growth and economic development. The worldwide demand of energy is expected to be doubled by the year 2050 and tripled by the end of century. The energy consumed is produced from conventional resource, fossil fuels, such as coal, petroleum, and natural gas. But the most concerned topic is depletion of fossil fuels, and negative effects on the environment by enhancing the natural greenhouse effect and causing global warming, due to the combustion of fossil fuels [1-4]. For global, economic, and environmental stability, an abundant supply of energy is needed. The renewable energy systems such as sunlight, wind, tides, and biomass are starting to play a significant role in power generation, replacing fossil fuels. In this, solar energy has been considered as the largest single source of clean energy to satisfy our present global energy needs. The amount of solar energy that hits the surface of the earth in one hour is greater than the total amount of energy that the entire human population requires in one year. Scientists have been encouraged by this fact to harvest the solar energy in usable form and ultimately resulted in the form of different types of solar cells or photovoltaic cells [1-5].
Looking at solar cells, Perovskite solar cells (PSC) are the fast-growing solar technology [6-9]. They have recently received an extraordinary attention of the research community due to rapid increase in the photovoltaic conversion efficiency from 3.8% to 26% [6-10]. The challenge in PSC research is the discovery of the hole transport materials (HTMs) that are excellent in performance, low cost and high stability, so as to commercialize the device successfully [11-23]. The ideal HTM for PSC fulfil certain requirements to enhance the efficiency of the cell. The HTM must have highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) level are well aligned with the HOMO level of the perovskite material and conduction band of the TiO2 to participate in efficient charge transfer process. The shift of HOMO level towards perovskite will leads to enhancement in the open circuit voltage. It should have a high hole transfer efficiency to facilitate hole conduction and prevent charge recombination. It should suppress the diffusion between back electrode metals and perovskite layer. The HTM must form amorphous thin film with glass transition temperature above 100°C to avoid crystallization. Furthermore, minimum absorption or transparency in visible region is desirable to hinder sunlight from efficiently reaching the perovskite material. Moreover, the HTM should possess thermal and photo stability and environmentally friendly and have resistance to external degradation factors such as moisture and oxygen for long term device performance. Several small molecule or polymer based HTMs have been synthesized extensively in this aspect. Most of these HTMS are synthesized by time consuming coupling reactions, such as Suzuki [24-26], Buchwald-Hartwig [27-28], Wittig-Horner [29], Ullmann [30]], Stille [31], Sonogashira [32] coupling reactions. These reactions are carried out in the presence of expensive transition metal catalysts and need strict reaction conditions and extensive product purification. Hence, still more efforts must be made to develop efficient and low cost HTMs alternative to spiro-OMeTAD for successful commercialization of the device.
Based on the extensive literature survey it was observed that azomethine based HTMs have great attention in Perovskite solar cells in recent years due to its simplicity and easiness of reaction to develop hole transport materials [33]. Azomethines are isoelectronic with their vinylene counterparts [34]. Since azomethines are prepared by straightforward condensation of complimentary amines and aldehydes and can purify by straightforward precipitation, drastically reduce the cost of PSCs [35]. The studies clearly shows that highly conjugated aromatic azomethines are stable and resist hydrolysis and are thermally stable above 300ºC [36-38]. Particularly, Triphenylamine (TPA) azomethines have received appreciable recognition due to the excellent hole transport properties of TPA [33]. Triphenylamine (TPA) has excellent electron donating capability. The three N-C bonds of TPA, conjugation through lone pair of electrons on the N atom makes TPA and its derivatives as appropriate HTMs in PSC. The study demonstrates the design and theoretical study of novel Triphenylamine-Aminoanthraquinone azomethine compound, (E)-N-(4-(diphenylamino)benzylidene)-9,10-dihydro-9,10-dimethyleneanthracen-2-amine (C3).
The specific objective of this work is to demonstrate the design of novel Triphenylamine-Aminoanthraquinone azomethine based conjugated organic hole transport material, (E)-N-(4-(diphenylamino)benzylidene)-9,10-dihydro-9,10-dimethyleneanthracen-2-amine (C3) for perovskite solar cells. The investigation was initially focused on theoretical study of designed material and evaluate the adaption of these material in perovskite solar cells as hole transport material and to confirm the structure and energetic properties to fit in perovskite solar cells. This conjugated system can act as push pull system due to the nature of constituents. Triphenylamine can act as a good electron donor molecule due to its lower ionization potential than many organic and inorganic materials. The aminoanthraquinone molecule accepts electron through azomethine bond to complete the conjugated system. The proposed structure of azomethine has given in Fig 1.

II. METHOD
Quantum chemical calculations were performed by using Density functional theory (DFT) to understand the structure and electronic properties of the compound. Density functional theory (DFT) computations were performed to understand the structure and electronic properties of the compound using Gaussian 09 with the basis set of B3LYP/6-311G (d, p) and the results were visualized using Gauss view. DFT method was used to calculate the most ground state electronic structure calculations of atoms, molecules and solids. DFT calculation focuses its attention on electron density instead of wave function to compute the energy. B3LYP is the most popular DFT model. The molecules were first optimized by the method B3LYP with 6-311G (d, p) basis set in Gaussian 09 program. The frontier molecular orbitals were visualized by molecular visualization program Gauss view 5.
III. RESULT AND DISCUSSION

In this work, we report the theoretical study of conjugated hole transport material based on Triphenylamine and Aminoanthraquinone unit linked with azomethine bond. This conjugated system can act as push pull system in which Triphenylamine can act as a good electron donor molecule and aminoanthraquinone molecule accepts electron through azomethine bond to complete the conjugated system (Fig 1). Quantum chemical calculation by using Density functional Theory (DFT) can be able to predict the structural and electronic properties of the new compound (Fig 1). In this work, DFT/B3LYP with 6-311 G (d, p) basis set in Gaussian 09 was used to optimize the structure of the new HTM. The molecular visualization program, Gauss view 5.0.8 was used to obtain frontier molecular orbitals of the new HTM.
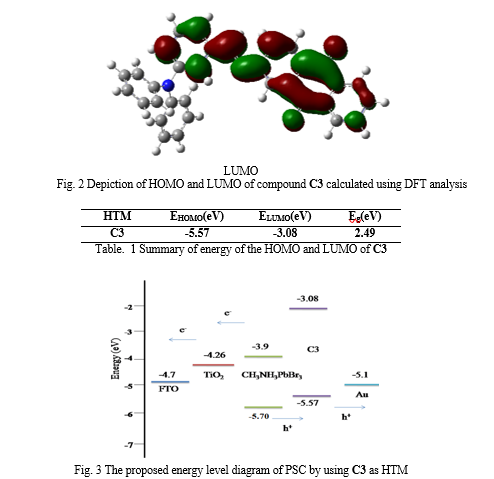
The frontier molecular orbitals of the HTM are presented in Fig 2. The analysis of frontier molecular orbitals will give a first insight on the charge transport properties of the HTM. The delocalized HOMO orbitals are favourable for hole transport materials. The analysis shows that electron density of HOMO of the molecule was concentrated on the electron donating Triphenylamine moiety and LUMO found on Aminoanthraquinone moiety through azomethine linkage. The distribution of frontier molecular orbital favours the intramolecular charge transfer through the system. The energy of the HOMO and LUMO of these HTM are summarized in Table 1. The HOMO-LUMO gap of C3 was calculated to be 2.49 eV. The basic requirement for hole transport material is that its HOMO and LUMO levels are compatible with the HOMO level of the dye and the conduction band of TiO2 to achieve the maximum charge transfer process. The energy levels and energy gap calculated theoretically are well matched with CH3NH3PbBr3 based perovskite solar cells. The all results indicates that this new hole transport material can fit in CH3NH3PbBr3 based perovskite solar cells as HTM and will show high performance.
IV. ACKNOWLEDGMENT
The author acknowledges Dr. V. Sathyanarayanamoorthy and Dr. R. Venkateswaran for the support during the study and the PSG Institutions for the infrastructure to carry out the work.
Conclusion
The quantum chemical calculations by using Density functional theory (DFT) study found that Triphenylamine-Aminoanthraquinone azomethine based conjugated organic hole transport material, (E)-N-(4-(diphenylamino)benzylidene)-9,10-dihydro-9,10-dimethyleneanthracen-2-amine (C3) can perform as efficient HTM for perovskite solar cell applications from the comparison of the HOMO-LUMO values and the predicted energy band alignment of this compound confirms that the exciton dissociation and charge transfer at the interface are energetically favourable for perovskite solar cell.
References
[1] In Basic Research Needs for solar energy utilization, U.S. Department of Energy, 2005 [2] A. Fahrenbruch and R. Bube, Fundamentals of Solar cells: Photovoltaic Solar Energy Conversion, Academic press, INC, 1983 [3] H. P. Garg and J. Prakash, Solar Energy: Fundamentals and Applications, Tata McGraw-Hill Education, 2000 [4] N. Kannan and D. Vakeesan, Renewable and Sustainable Energy Reviews, 2016, 62, 1092-1105 [5] A. M. Bagher, M. M. Abadi Vahid and M. Mohsen, American Journal of Optics and Photonics, 2015, 3, 94 -113 [6] J. Yan, T. J. Savenije, L. Mazzarellaa and O. Isabella, Sustainable Energy Fuels, 2022, 6, 243–266 [7] A. S. R. Bati, Y. L. Zhong, P. L. Burn, M. K. Nazeeruddin, P. E. Shaw and M. Batmunkh, https://doi.org/10.1038/s43246-022-00325-4 [8] J. Y. Kim, J-W. Lee, H. S. Jung, H. Shin and N-G Park, Chem. Rev. 2020, 120, 15, 7867–7918 [9] A. Kojima, K. Teshima, Y. Shirai, and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 [10] https://www.nrel.gov/pv/cell-efficiency.html [11] S. Pitchaiya, M. Natarajan, A. Santhanam, V. Asokan, A. Yuvapragasam, V. M. Ramakrishnan, S. E. Palanisamy, S. Sundaram and D. Velauthapillai, Arabian Journal of Chemistry, 2020, 13, 2526-2557 [12] S. Li, Y-Li. Cao, W-H. Li and Z-S. Bo, Rare Met., 2021, 40, 2712-2729 [13] I-Y. Shao and Y-W. Zhong, Chem. Phys. Rev., 2021, 2, 021302 [14] E. Sheibani, L. Yang and J. Zhang, rrl solar, 2020, 4, 2000461 [15] R. Singh, P. K. Singh, B. Bhattacharya and H-W. Rhee, Applied Materials Today, 2019, 14, 175-200 [16] J. Urieta-Mora, l. Garcia-Benito, A. Molina-Ontoria and N. Martin, Chem. Soc. Rev., 2018, 47, 8541-8571 [17] X. Zhao and M. Wang, Materials Today Energy, 2018, 7, 1-13 [18] C. H. Teh, R. Daik, E. L. Lim, C. C. Yap, M. A. Ibrahim, N. A. Ludin, K. Soipana and M. A. Teridi, J. Mater. Chem. A, 2016, 4, 15788-15822 [19] B. Salhia, Y. S. Wudila, M. K. Hossaina, A. Al-Ahmeda and F. A. Al-Sulaimana, Renewable and Sustainable Energy reviews, 2018, 90, 201-222 [20] X. Yang, H. Wang Q, B. Cai, Z. Yu and L. Sun, Journal of Energy Chemistry, 2018, 27, 650-672 [21] Z. H. Bakra, Q. Walia, A. Fakharuddin, L. Schmidt-Mendec, T. M. Browne and R. Josea, Nano Energy, 2017, 34, 271-305 [22] Y-K. Wang, Z-Q. Jiang and L-S. Liao, Chinees Chemical Letters, 2016, 27, 1293-1303 [23] M. A. Mutaliab, F. Aziz, A. F. Ismail, W. N. W. Salleh, N. Yusof, J. Jaafar, T. Soga, M. Z. Sahdane and N. A. Ludina, Applied Materials Today, 2018, 13, 69-82 [24] H. Li, K. Fu, A. Hagfeldt, M. Gra?tzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem. Int. Ed., 2014, 53, 1-5 [25] N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457-2483 [26] A. Yokoyama, H. Suzuki, Y. Kubota, K. Ohuchi, H. Higashimura and T. Yokozawa, J. Am. Chem. Soc., 2007, 129, 7236-7237 [27] K. Do, H. Choi, K. Lim, H. Jo, J. W. Cho, M. K. Nazeeruddin and J. Ko, Chem. Commun., 2014, 50, 10971-10974 [28] D. D. Astridge, J. B. Hoffman, F. Zhang, S. Y. Park, K. Zhu and A. Sellinger, ACS Appl. Polym. Mater., 2021, 3, 5578-5587 [29] S. Lv, L. Han, J. Xiao, L. Zhu, J. Shi, H. Wei, Y. Xu, J. Dong, X. Xu, D. Li, S. Wang, Y. Luo, Q-Meng and X. Li, Chem. Commun., 2014, 50, 6931-6934 [30] E. Xu, P. Sheibani, J. Liu, H. Zhang, N. Tian, G. Vlachopoulos, L. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Energy. Mater., 2014, 26, 6629-6634 [31] L. Calio, S. Kazim and M. Graetzel, Angew. Chem., 2014, 50, 10971-10974 [32] A. Abate, M. Planells, D. J. Hollman, V. Barathi, S. Chand, H. J. Snaith and N. Robertson, Phys. Chem. Chem. Phys., 2015, 17, 2335-2338 [33] E. Priyatha, C. Sathishkumar, N. Palanisamy, S. Venkatachalam and R. Venkateswaran, J. Mol. Struct, 2019, 1179, 145-153 [34] A. Bolduc, A. A. Ouahabi, C. Mallet and W. G. Skene, J. org. Chem., 2013, 78, 9258-9269 [35] H. Schiff and J. Liebigs, Ann. Chem.1864, 131, 118 [36] M. Grigoras and C. O. Catanescu, J. Macromol. Sci. Part C Polym. Rev., 2004, 44, 131 [37] M. Bourgeaux and W. G. Skene, Macromolecules, 2007, 40, 1792-1795 [38] S. Dufresne, S. A P. Guarin, A. Bolduc, A. N. Bourque and W. G. Skene, Photochem. Photobiol. Sci., 2009, 8, 796-804
Copyright
Copyright © 2023 Priyatha Elody . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54699
Publish Date : 2023-07-08
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

