Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Control of Indoor Aero flora using Biobased Cups
Authors: Sayana Saleem, Sanjo S Thomas, Nisy S
DOI Link: https://doi.org/10.22214/ijraset.2024.66023
Certificate: View Certificate
Abstract
Indoor air quality is a critical concern, as poor air quality can lead to various health Issues. Microorganisms in indoor air can significantly contribute to air pollution and adversely affect human health. Therefore, effective methods for reducing microbial load in indoor environments are essential. Our study focused on isolating and identifying microbes from indoor air and evaluating a biobased fumigation cup made from natural materials for controlling indoor air microflora. Air samples were collected using the open plate technique, and microbes were isolated and identified based on their characteristics. The predominant bacteria in indoor air identified were Bacillus sp, Pseudomonas sp, Serratia sp, and Micrococcus sp and the fungi identified were Aspergillus sp Penicillium sp, Fusarium sp, and Mucor sp. The Bio-based cup is prepared using Neem, Aparajitha dhooma choornam, and Boswellia serrata resin, effectively reducing microbial load, particularly fungi, in indoor air. However, some bacterial strains showed resistance to the cup. Overall, our study highlights the potential of the biobased fumigation cup as an effective, natural method for improving indoor air quality, showing practical benefits without adverse effects, advocating for broader adoption in various applications, and encouraging environmentally conscious practices.
Introduction
I. INTRODUCTION
Indoor air quality (IAQ) is crucial for health, as poor air can cause various health issues. Microorganisms in indoor air contribute to pollution, impacting health. This project aims to isolate and analyze indoor air microbes, evaluate the effectiveness of biobased fumigation cups in reducing microbial load, and identify antimicrobial components. The study uses the open plate technique to isolate microbes, which are then cultured, analyzed morphologically, and identified using biochemical tests. Extracts from neem leaves, Boswellia serrata gum, and Aparajitha dhooma choornam powder will be tested for antimicrobial properties through antibiotic sensitivity tests. The impact of biobased fumigation cups on microbial load will be assessed in confined spaces, and GC-MS analysis will identify the chemical composition of natural extracts. This research will provide insights into the use of biobased fumigation cups for indoor air purification, offering sustainable, eco-friendly solutions for improving IAQ (Allen JG et al., 2016). IAQ is influenced by factors like humidity, temperature, and organic materials, which affect microbial load, including bacteria (e.g., Staphylococcus, Streptococcus, Pseudomonas), fungi (e.g., Aspergillus, Penicillium, Cladosporium), and viruses (e.g., influenza, rhinovirus, coronavirus) (Hospodsky et al., 2012; Pitkaranta et al., 2008; Prussin et al., 2015). Poor IAQ can cause respiratory symptoms, allergies, and infections (Adams et al., 2015). Biobased fumigation cups offer a sustainable alternative to chemical fumigation, using botanical extracts or microbial agents to control pests with minimal environmental impact (Isman et al., 2006). These cups contain active ingredients which have insecticidal, repellent, or antifungal properties, offering environmental benefits compared to chemical fumigation (Akhtar et al., 2004). Neem leaves (Azadirachta indica) are known for antimicrobial, anti-inflammatory, and antioxidant properties, widely used in organic farming and pest management (Elumalai K et al., 2012; Schmutterer H et al., 2002; Biswas K et al., 2022). Boswellia serrata gum resin, sourced from the Boswellia serrata tree, has anti-inflammatory, analgesic, and immunomodulatory effects, traditionally used in Ayurvedic medicine for respiratory and gastrointestinal conditions (Siddiqui, 2011). Aparajitha Dhooma Choornam powder, used in Ayurvedic "Dhoomapana," includes herbs like Vasaka, Bharangi, Ela, and Twak, known for their respiratory and antimicrobial properties (Sindhu et al., 2007). Biobased cups, used as an eco-friendly alternative to conventional plastic cups, reduce environmental impact by using renewable materials, being biodegradable or compostable, and supporting sustainable agriculture. However, challenges include ensuring performance, sustainable sourcing, and proper disposal to maximize environmental benefits (Narayan, 2020).
II. MATERIALS AND METHODS
1) Isolation of Microbes From Indoor Air:
Microbes were isolated from a 150 sq. ft. room in the microbiology lab at Sree Narayana Guru College, Coimbatore, using the open plate technique. Sterile Petri dishes with nutrient agar and Sabouraud Dextrose Agar (SDA) were exposed for 15-30 minutes, then incubated at 30-37°C for 24-48 hours (nutrient agar) and 48-72 hours (SDA) (Stryjakowska et al., 2007).
2) Identification of bacteria and fungi from indoor air:
Predominant bacteria present in indoor air were identified based on colony morphology, staining, motility and biochemical tests. Predominant fungi present in indoor air were identified based on colony Morphology and fungal Staining (Cappuccino et al., 2020).
3) Preparation of Biobased cup:
Biobased cups were prepared using Neem leaves, Boswelia serrata gum and Aparajitha dhooma choornam, an ayurvedic composition (Commercial Product) with total 8 ingredients - Acorus calamus , Actiniopteris dichotoma, Aquilaria agallocha, Azadirachta indica , Calotropis gigantea, Cedrus deodara, Commiphora mukul and Shorea robusta- used for fumigation. Fresh neem leaves were collected and thoroughly cleaned to remove any dirt or impurities. The cleaned leaves were then placed in a blender, and a small quantity of distilled water was added to facilitate proper blending and binding. Aparajitha dhooma choornam was powdered, and the powder was added to the blended neem mixture in a 1:1 ratio. This mixture was blended again to ensure uniform consistency. The homogeneous mixture was transferred to a mold and pressed to obtain the desired shape of the fumigation cup. The cups were carefully removed from the mold and placed in a shaded area to dry. They were left to dry for several days until they reached the desired texture and hardness. Finally, the cups were filled completely with 10g of Boswellia serrata gum. Size of the cup was 4 cm long and 2 cm wide. This biobased cup which emits fumes after burning is used for fumigation purposes.
4) Fumigation using a Biobased cup incorporated with Boswellia serrata gum resin:
Fumigation of Biobased cups is carried out by igniting till fumes start to arise. After fumes start evolving from a biobased cup for fumigation it is kept in the desired area on a ceramic stand and allowed to stand till emission of fumes is complete. Boswellia serrata gum resin, renowned for its antimicrobial properties, was a key component used as a fumigant in the biobased cup. This resin was incorporated into the cup, which was primarily composed of neem leaves and Aparajitha dhooma choornam powder. This combination creates a potent fumigant harnessing the natural antimicrobial properties of its ingredients. The inclusion of Boswellia serrata gum resin enhanced the cup's efficacy in purifying indoor air, making it a safe and natural alternative for indoor air purification.
5) Preparation of extracts from neem leaves , Boswellia serrata gum resin and Aparajitha dhooma choornam.
a) Neem leaves extract:
Fresh neem leaves were collected and thoroughly washed with sterilized water. The leaves were then shade-dried, and the dried leaves were powdered. To prepare the extract, 33.3 g of the dried powder was mixed with 100 ml of 90% ethanol and kept at room temperature for 36 hours. The slurry was filtered using Whatman No. 1 filter paper. The filtrate was evaporated and dried at 40°C, then stored in an airtight bottle at 4°C. (Subapriya et al., 2003)
b) Boswellia serrata gum resin extract:
Oleo-gum resin of Boswellia serrata was purchased from an Ayurvedic pharmacy in Alathur, Palakkad. To prepare the extract, the gum resin was powdered, and 10 g of the powder was mixed with 100 ml of 95% ethanol and kept at room temperature for 36 hours. The slurry was then filtered using Whatman No. 1 filter paper. The filtered extracts were evaporated and dried at 40°C and stored at 4°C in an airtight bottle. (Mohammadi et al., 2017)
c) Aparajitha dhooma choornam extract:
Aparajitha Dhooma Choornam (50g commercial packet) was purchased from an Ayurveda pharmacy in Alathur, Palakkad. For the preparation of the extract, Aparajitha Dhooma Choornam was powdered, and 16.6 g of the powder was mixed with 100 ml of 90% ethanol and kept at room temperature for 36 hours. The slurry was filtered using Whatman No. 1 filter paper. The extracts were then evaporated and dried at 40°C and stored at 4°C in an airtight bottle. (Pratap et al., 2019)
6) Chemical Analysis of Biobased Cup:
Chemical analysis of the components of Biobased cup were carried out using GC-MS .Separation and identification of chemical constituents of extracts from neem leaves, Aparajitha dhooma choornam, and Boswellia serrata gum resin were done using GC -MS analysis . (Microtech lab, Coimbatore, Tamil Nadu).
7) Determination of Antimicrobial activity of Neem, Aparajitha dhooma choornam, and Boswellia serrata gum resin extracts:
Antimicrobial properties were analyzed using the well diffusion method. (Khan et al., 2012). Using a sterile swab, broth cultures of the inoculum was swabbed on to Mueller-Hinton agar (MHA) media and 0.1ml Extracts of neem, Boswellia serrata gum, and Aparajitha dhooma choornam were added to bored wells on the agar. The plates were then incubated at 37°C for 24 hours for bacteria and at room temperature for 24 -72 hours for fungi. The zones of growth inhibition were recorded against the test microorganisms.
8) Indoor air fumigation using Biobased Cup:
One biobased cup for fumigation containing neem leaves, Aparajitha dhooma choornam, and Boswellia serrata gum resin was used to fumigate a room of 150 square feet at Sree Narayana Guru College. Effectiveness was assessed by comparing microbial load before and after fumigation by open plate technique using sterile nutrient agar plates and Sabouraud Dextrose Agar plates (Prasannakumar and Balachandran, 2021).
9) Effectiveness of fumigation using a Biobased cup for controlling indoor air microflora:
Effectiveness was evaluated by comparing the microbial load on agar plates exposed before and after fumigation of indoor air. Open plate technique was carried out before 15 minutes of fumigation and after 15 minutes of fumigation. After incubating nutrient agar plates at 30-37°C for 24 hours and Sabouraud's Dextrose Agar plates at room temperature for 24-72 hours the colonies grown in all the plates were tabulated , identified, compared and assessed.
III. RESULT
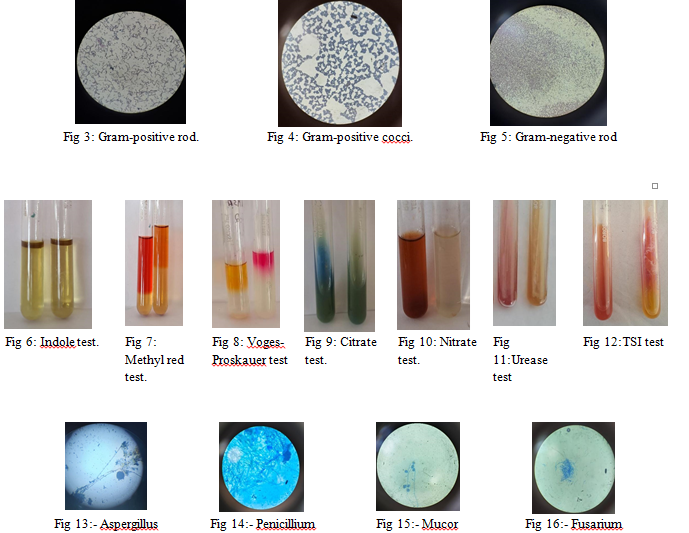
1) Isolation of Microbes from Indoor Air: Using the open plate technique, bacterial and fungal colonies were isolated (Fig. 1 and Fig. 2). Four bacterial and four fungal colonies with distinct morphologies were selected for identification.

2) Identification of Microorganisms from Indoor air: Bacterial and fungal isolates were identified based on colony morphology, staining, motility, and biochemical tests. The predominant bacteria in indoor air were identified as Bacillus, Pseudomonas, Serratia, and Micrococcus, and fungi were identified as Aspergillus, Penicillium, Fusarium, and Mucor. (Fig 3-16)
3) Preparation of Biobased Cup: Fresh neem leaves were collected and thoroughly cleaned to remove any dirt or impurities. These cleaned leaves were then placed in a blender, and a small quantity of water was added to facilitate proper blending and binding. Aparajitha Dhooma Choornam was powdered, and the powder was added to the blended neem mixture. The mixture was blended again thoroughly to ensure a homogeneous consistency. The mixture was then transferred into a mold and pressed to obtain the desired shape of the fumigation cup. The cups were carefully separated from the mold and placed in a shaded area to dry. They were left to dry for several days until they achieved the desired texture and hardness. The cups were then incorporated with Boswellia serrata gum and prepared for fumigation.

4) Preparation of Extract: Ethanolic extracts of Neem, Aparajitha dhooma choornam, and Boswellia serrata gum resin were prepared. The Neem extract was dark green and syrupy, the Aparajitha dhooma choornam extract was dark brown and watery, and the Boswellia serrata extract was yellowish-brown and sticky.
5) GC-MS Analysis: By GC-MS analysis most abundant 10 major compounds present in the mixture of biobased cup with neem, Boswelia serrata gum and Aparajitha dhooma choornam containing 8 different herbs is 2R-Acetoxymethyl-1,3,3-trimethyl-4T-(3-methyl-2-buten-1-yl)-1T-cyclohexanol as the most abundant, known for its strong antimicrobial properties.
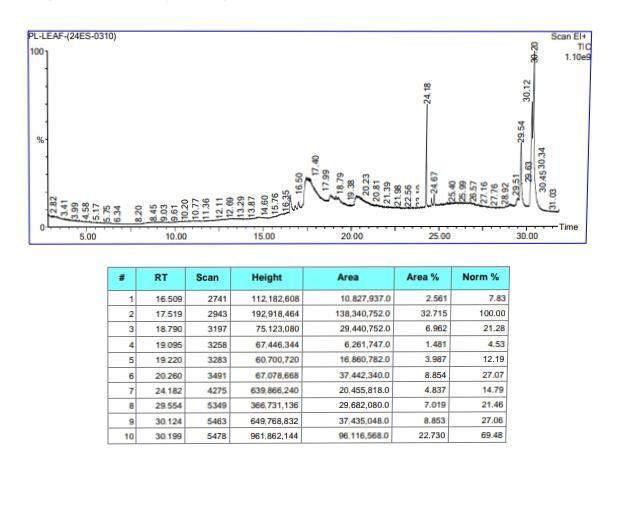
Table 4: Compounds present in mixture of Biobased cup
|
Sl.No |
RT |
Name of compound |
Properties |
|
1 |
16.509 |
3,7,11,15-TETRAMETHYL-2-HEXADECEN-1-OL (phytol) |
• Solubility: It is insoluble in water but soluble in organic solvents. • It is stable under normal conditions but may undergo oxidation over time. • Activity: It has antioxidant, anti-inflammatory, and anticancer properties.
|
|
2 |
17.519 |
2-O-METHYL-D-MANNOPYRANOSA( D-mannose) |
•Solubility: It is soluble in water. •Stability: It is stable under normal conditions. •Prevent urinary tract infections by inhibiting microbe's adhesion to the urinary tract lining.
|
|
3 |
18.790 |
UNDECANOIC ACID (undecylic acid) |
•Solubility: It is slightly soluble in water but more soluble in organic solvents. • Stability: Stable under normal conditions. •Biological properties: Antifungal and Antibacterial properties.
|
|
4 |
19.095 |
BETA.-D-MANNOFURANOSIDE, METHYL ( Methyl) |
•Solubility: It is soluble in water. •Stability: It is stable under normal conditions. • Lacks antimicrobial properties but can enhance antimicrobial activity when part of a compound. |
|
5 |
19.220 |
ETHER, HEXYL ISOPROPYL |
•Solubility: It is soluble in organic solvents but not very soluble in water. •Odor: It has a mild, ether-like odor. •Flammability: It is flammable |
|
6 |
20.260 |
3-DECYN-2-OL |
•Odor: It has a characteristic odor. •Solubility: It is slightly soluble in water but soluble in organic solvents. •Biological properties: Antifungal And Antibacterial properties |
|
7 |
24.182 |
SQUALENE |
•Solubility: Insoluble in water, soluble in organic solvents. •Medical Use: Investigated for its potential health benefits, including antioxidant and anti-inflammatory properties. •Generally considered safe and non-toxic for topical and oral use. |
|
8 |
29.554 |
OCTAMETHYL |
•Solubility: Insoluble in water, soluble in organic solvents like benzene, ether, and acetone •Reactivity: Generally stable under normal conditions, • Odor: Odorless |
|
9 |
30.124 |
2R-ACETOXYMETHYL-1,3,3-TRIMETHYL-4T-(3-METHYL-2-BUTEN-1-YL)-1T-CYCLOHEXANOL |
•It has antimicrobial properties •It has antioxidant activities •It has larvicidal activities |
|
10 |
30.199 |
5H-3,5A-EPOXYNAPHTH[2,1-C]OXEPIN, DODECAHYDRO-3,8,8,11A-TETRAMETHYL |
•It exists as a colorless to pale yellow liquid. • It has Antibacterial activities •It has antifungal activities |
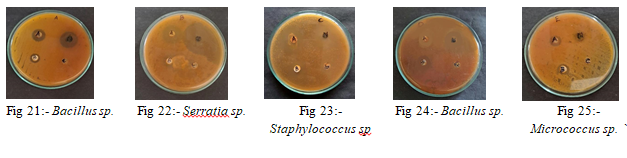
6) Determination of Antimicrobial Properties: The antimicrobial effect of Neem, Aparajitha dhooma choornam, and Boswellia serrata gum extracts were tested on bacteria (Serratia, Staphylococcus, Bacillus, Micrococcus) and fungi (Aspergillus, Penicillium, Mucor, Fusarium) using the well diffusion technique. All microorganisms showed inhibition zones, indicating they could not grow in the presence of these extracts (Fig.18a - Fig.18e).
Table 5: Antimicrobial properties of herbal extracts against isolated bacteria.
|
Extracts |
Zone of inhibition |
|
||||
|
|
Bacillus sp |
Serratia sp |
Staphylococcus sp |
Bacillus sp |
Micrococcus sp |
|
|
Neem |
21mm |
35mm |
31mm |
24mm |
13mm |
|
|
Aparajitha dhooma choornam |
16mm |
21mm |
25mm |
17mm |
12mm |
|
|
Bosweilla seratta gum |
13mm |
14mm |
16mm |
17mm |
12mm |
|
|
|
|
Extracts |
Zone of inhibition |
|||
|
|
Aspergillus |
Penicillium |
Mucor |
Fusarium |
|
Neem |
20mm |
23mm |
21mm |
12mm |
|
Aparajitha Dhooma Choornam |
17mm |
20mm |
20mm |
13mm |
|
Bosweilla seratta gum |
14mm |
15mm |
19mm |
14mm |
Table 6: Antimicrobial properties of herbal extracts against isolated fungi.
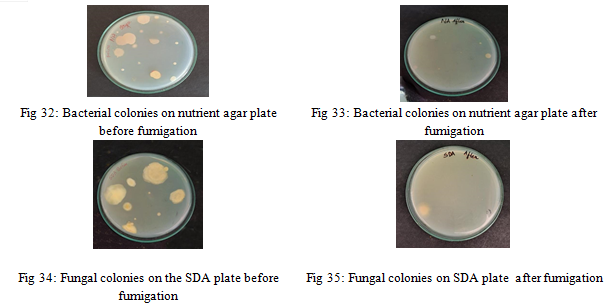
7) Fumigation using Biobased Cup: The cup was dried and then incorporated with Boswellia serrata gum. It was fumigated in a single room from the UG lab of Sree Narayana Guru College, Chavadi, Coimbatore, for 20 minutes, where the microbes had been previously isolated, to assess the reduction in microbial load using the open plate technique. After fumigation, Sabouraud Dextrose Agar (SDA) and Nutrient Agar plates were exposed using the open plate technique to observe the reduction in microbial load.

8) Effectiveness of Fumigation: After fumigation there was a noticeable reduction in microbial load in indoor air.The biobased cup was more effective against fungi than bacteria. Although Bacillus sp. and Micrococcus sp. showed resistance, their numbers were reduced. Aspergillus sp. also exhibited resistance, while other fungi were sensitive. Fumigation was particularly more effective in controlling mold than bacteria.
IV. DISCUSSION
The Indoor air microflora was isolated using the open plate technique and identified through Gram staining and biochemical tests, revealing five predominant bacterial cultures: Bacillus, Serratia, Staphylococcus, and Micrococcus. Fungal colonies were identified using lactophenol cotton blue staining, resulting in Aspergillus, Penicillium, Mucor, and Fusarium. The biobased cup (Neem, Aparajitha dhooma choornam, Boswellia serrata gum resin) exhibited a greenish-brown color and slightly rough texture, indicating organic materials. The extracts showed significant antimicrobial activity against the isolated bacteria and fungi. Fumigation with the cup led to a noticeable reduction in microbial presence, especially fungi. After fumigation, Bacillus and Micrococcus were identified, suggesting resistance to the fumigant, while Penicillium was the predominant fungus, indicating resilience to the fumigant. These findings highlight the potential of biobased fumigation cups for indoor air purification (public health, environmental sustainability). Future research could optimize the fumigation process and explore additional natural extracts.
References
[1] Adeleh Mohammadi, SaeedehArabshahi-Delouee, Kyriaki G. Zinoviadou3, Charis M. Galanakis4(2017), Antioxidant Properties of Various Solvent Extracts of Indian Frankincense (Boswellia serrata) Oleogum Resin, Iranian Food Science and Technology Research Journal, Vol. 13(3). [2] Akhtar, Y., & Isman, M. B. (2004). Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. Journal of Applied Entomology, 128(1), 32-38. [3] Hany M. Yehia, (2016), Methanolic Extract of Neem Leaf (Azadirachta indica) and its Antibacterial Activity Against Foodborne and Contaminated Bacteria on Sodium Dodecyl [4] Hospodsky, D., Qian, J., Nazaroff, W. W., Yamamoto, N., Bibby, K., Rismani-Yazdi, H., & Peccia, J. (2012). Human occupancy as a source of indoor airborne bacteria. PLoS ONE, 7(4), e34867. [5] Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology, 51, 45-66. [6] Khan Venkatesh Kamath (2019), Total phenolic and flavonoid content, antioxidant effects and anti-diarrheal activity of balacturbhadrika churna- An ayurvedic preparation, Indian Journal of traditional knowledge vol 18(3),485-492 [7] Pitkäranta, M., Meklin, T., Hyvärinen, A., Paulin, L., Auvinen, P., Nevalainen, A., & Rintala, H. (2008). Analysis of fungal flora in indoor dust by ribosomal DNA sequence analysis, quantitative PCR, and culture. Applied and Environmental Microbiology, 74(1), 233-244. [8] Preeti Chaturvedi, Rajeswary Majhi, Harsha Gupta. Review of method of preparation of Dhoomvarti. J Ayurveda Integr Med Sci 2022;7: 101-104. [9] Preeti Chaturvedi, Rajeswary Majhi, Harsha Gupta. Review of method of preparation of Dhoomvarti. J Ayurveda Integr Med Sci 2022;7:101-104. [10] Priya Prasannakumar, Divya S Balachandran, New interventions in fumigating with Aparajitha dhooma choorna, An International Journal of Research in AYUSH and Allied System(2021),8(2):3130-3138. [11] Prussin II, A. J., Marr, L. C., & Bibby, K. J. (2015). Challenges of studying viral aerosol metagenomics and communities in comparison with bacterial and fungal aerosols. FEMS Microbiology Letters, 362(11), fnv119. [12] R.Subapriya, Kumaraguruparan,V.P.Chandramohan,S.Nagini,(2003), Chemoprotective effects of ethanolic extract of neem leaf against MNNG-induced oxidative stress, Pharmazie 58: 512–517. [13] Rahul Pratap, HN Aswatha Ram, KS Chandrashekar, Vasudev Pai1,^, Saleemulla Khan, Venkatesh Kamath,(2019), Total phenolic and flavonoid content, antioxidant effects and antidiarrheal activity of balacaturbhadrika churna – an Ayurvedic preparation, Indian Journal of Traditional Knowledge Vol 18(3), 485-492. [14] Shaik Mannur Ismail, Sudheer Aluru, KRS Sambasivarao, Bhaskar Matcha(2014), Antimicrobial activity of frankincense of Boswellia serrata, Int. J. Curr.Microbiol.App.Sci,3(10),1095-1101. [15] Siddiqui, M. Z., Boswellia serrata, A potential antiinflammatory agent: an overview. Indian Journal of Pharmaceutical Sciences, 73(3), 255-261. Gupta, S., & Patel, R. Plant-based air purifiers: A review of their effectiveness in reducing indoor air pollutants. Indoor Air Quality, 2021, 14(3), 112-125.
Copyright
Copyright © 2024 Sayana Saleem, Sanjo S Thomas, Nisy S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66023
Publish Date : 2024-12-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

