Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Cutting Edge Research on Treatment of Type I Diabetes
Authors: Divy Ankurkumar Patel
DOI Link: https://doi.org/10.22214/ijraset.2024.64214
Certificate: View Certificate
Abstract
Type 1 Diabetes Mellitus (T1DM) is a condition where multiple genes play a role. It is characterized by chronic autoimmune destruction of pancreatic beta cells, which produces insulin. Type 1 diabetes is common in children and is more prevalent in India than in many other Asian countries. Treating type 1 diabetes often involves management of sugar levels of the bloodstream. Insulin therapy involving glucose monitors, subcutaneous injections, insulin pumps, etc. plays a pivotal role in the treatment of T1D. Transplantation of pancreatic islets as well as xenotransplantation are emerging mechanisms being employed but have major drawbacks. Ayurvedic therapies help alleviate symptoms and traits in order to boost the body’s immunity to fight the disease. Prevention and following basic lifestyle modifications can help provide resistance from diabetes. This review provides an overview of current and emerging approaches that aim to address these goals, offering new insights into the treatment and management of type 1 diabetes.
Introduction
I. INTRODUCTION
Diabetes, often known as diabetes mellitus, is a long-lasting metabolism mediated disease diagnosed by increased glucose levels in blood (hyperglycaemia) brought on by inadequate insulin synthesis, inadequate insulin utilization, or a combination of the two. The pancreas secretes the insulin hormone, which is needed to control sugar levels in blood as it makes glucose easier for cells to absorb and use as fuel.
Diabetes is divided into several types, each with distinct characteristics and causes namely, Type 1 diabetes, Type 2 diabetes and Gestational diabetes.
1) Type 1 diabetes
Is a lifelong condition where the body's immune system mistakenly attacks the cells that produce insulin, leading to an inability to produce this hormone. Over the years, treatments have advanced, significantly improving the lives of patients Nonetheless, the search for a remedy that can fully reverse type 1 diabetes remains an ongoing challenge (Barnett R, 2018), (Skyler JS, 2018). Type 1 diabetes is common in children (Patterson et al., 2019). Type 1 diabetes is a condition where multiple genes play a role. In this disease, the immune system, specifically cells like CD4+ and CD8+ T cells, along with macrophages, attacks and destroys insulin-producing beta cells within the pancreas's islets (Foulis et al., 1991).
2) Type 2 Diabetes
The most common, affecting around 90% diabetics. In type 2 diabetes, the body either becomes resistant to insulin's effects, or the pancreas doesn't produce enough insulin. This can be due to genetic and lifestyle factors such as obesity, not physically active, and junk diet. While there is no cure for type 2 diabetes, it can often be managed by changing lifestyle, medicines, or a combination of both.
3) Gestational Diabetes
Develops during pregnancy. Some pregnant women experience a temporary reduction in insulin sensitivity which can lead to high blood sugar levels. Gestational diabetes typically resolves after birth of the child, but women who have had GD are at a risk of developing type 2 diabetes after.
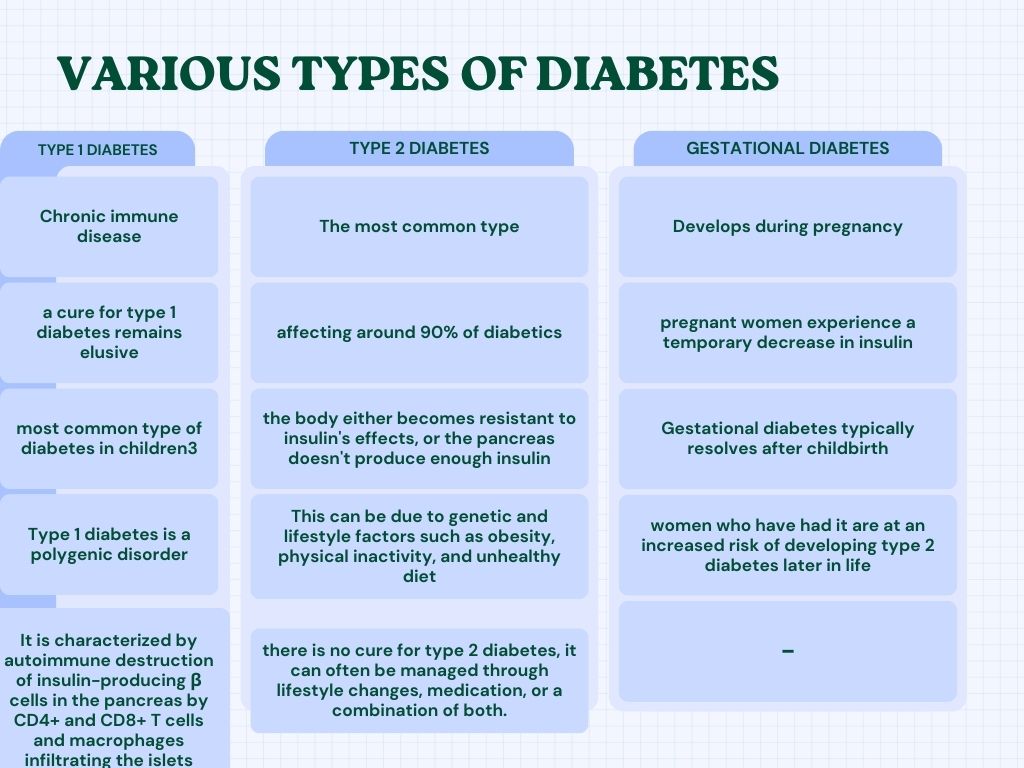 TABLE 1: Types of Diabetes
TABLE 1: Types of Diabetes
T1D and T2D are the two main types of diabetes, but recent research suggests the existence of five distinct types (Ahlqvist et al., 2018).
The asymptomatic phase of the disease, which can last from a moderate duration to a prolonged period, can be easily detected using serological markers that indicate immunity against islet cells. These markers include autoantibodies such as ICA (islet cell cytoplasm), IAA (insulin), GADA (glutamic acid decarboxylase), and ICA512/IA2A. The discovery of biomarkers that predict diseases helped launch clinical trials to develop preventive treatments. Animal studies revealed a predictable disease progression that could be prevented with a wide variety of therapies (Bowman et al., 1994).
After diagnosis, patients with Type 1 Diabetes Mellitus (T1DM) experience ongoing and chronic autoimmune destruction of their beta cells, which produce insulin. T1DM is fatal without treatment because humans cannot survive without insulin. However, insulin replacement therapy is imperfect, as it's challenging to perfectly match insulin injections with the body's needs. moreover, subcutaneous injections of insulin bypass the liver, the primary target organ for insulin secretion from beta cells.
A. Etiology
Our current knowledge of the causes and development of Type 1 Diabetes (T1DM) has allowed us to design trials for preventing and treating it using immune therapy; Primary Prevention: Targeting individuals with a high genetic risk of T1DM, who are likely to develop islet autoimmunity (the destruction of islet cells). The goal is to stop or delay beta-cell destruction before the onset of diabetes. Secondary Prevention: Focusing on individuals who are already developing islet autoimmunity, characterized by the presence of autoantibodies against certain islet proteins. The aim is to intervene and prevent the progression to diabetes by modifying the immune response.
To halt or reverse type 1 diabetes (T1DM), researchers aim to: 1. Prevent beta cell (β cell) destruction by inducing immune tolerance to specific islet autoantigens. 2. Arrest the autoimmune process by suppressing autoantibodies that attack β cells. 3. Monitor residual β cell function using C-peptide levels to identify individuals eligible for intervention therapy. 4. Implement intensive insulin therapy as recommended after the Diabetes Control and Complications Trial (1993) (Pickup JC, 2012).
New technologies have made it easier for people with diabetes to manage their blood sugar levels through intensive treatment, which involves closely monitoring glucose levels and adjusting insulin delivery.
B. Pathogenesis
Type 1 diabetes arises from an immune system malfunction. In genetically susceptible individuals, environmental factors can trigger immune cells to attack insulin-producing beta cells in the pancreas. This destruction steadily reduces insulin production, leading to the characteristic high blood sugar levels of type 1 diabetes. Type 1 diabetes is characterized by the progressive demolition of beta cells in pancreatic islets. T1D doesn't involve insulin resistance; it's a war waged by the body's own immunity against these vital cells. T1D has strong genetic components. The major histocompatibility complex (MHC) genes, particularly in HLA class II region, play a critical role. These genes determine how effectively immune cells present antigens (foreign molecules) to other immune cells. In T1D, specific HLA genotypes increase susceptibility by presenting beta cell proteins as potential threats. Other genes involved in immune regulation also contribute, such as PTPN22 and CTLA-4, influencing the activation and suppression of immune responses. While genetics loads the gun, environmental factors pull the trigger. Immune cells (antigen-presenting cells or APCs) take up beta cell proteins, break them down into fragments (peptides), and present them on their surface along with MHC molecules. T lymphocytes (T cells) with receptors specific for these peptide-MHC complexes become activated. These activated T cells are primed to attack cells presenting the same antigen. Activated T cells can also stimulate B cells to produce antibodies that target beta cell components. Activated T cells move to the pancreatic islets, and directly attack beta cells. Inflammatory molecules released by these T cells further damage surrounding tissues. The combined assault of T cells, antibodies, and inflammatory mediators leads to the progressive destruction of beta cells. As beta cells are destroyed, insulin production declines. This alters the body's capability to manage blood sugar levels, leading to hyperglycaemia (high blood sugar). Chronic hyperglycaemia is the hallmark symptom of T1D and can damage various organs over time.
Reports suggests that in monozygotic twins, the onset of type 1 diabetes varies (Guarnotta et al., 2018). It is evident that non-genetic factors also play a vital role in triggering overt type 1 diabetes. Studies on animals have suggested that many viral infections may prevent the development of type 1 diabetes (Cohen et al., 2016), (Yeh et al., 2012). People with type 1 diabetes remain dependent on external insulins as a therapeutic approach (Priya et al., 2018).
???????C. Prevalence
Deficiency of insulin leads to type 1 diabetes mellitus (T1DM) and is autoimmune or idiopathic in nature and present in 9% cases of insulin deficiency. It is also on the increase like T2DM, though not same, but still with a 3–5% of increase per year. T1DM is rising in India, Finland, Sweden, Colorado and Germany. There has been an increase of incidence by almost 10 per 1,00,000 children to around 60 per 1,00,000 children in Finland in the last half decade. Around 78,000 children of 15 years of age are said to develop T1DM worldwide. The existing 4,90,000 children with the disease, 24% is of European region and 23% of South-East Asian region. Children develop T1DM before they are 35 years of age is only about 6% (Aguirre et al., 2013). Type 1 diabetes mellitus (T1DM) is one of the most common paediatric endocrine illnesses. Of these, over half are living in developing nations, with India being home to an estimated 97,700 children with T1DM (Kumar et al., 2012). People of 15 years of age are constitute about 1%–4% of the total diabetic population, a 1990 report stated (Menon et al., 1990).
India experiences a high prevalence of type 1 diabetes (T1DM) in children, with onset often before age 15. The incidence continues to rise by 3% annually. In Europe alone, over 90,000 children are affected, with 75,000 new cases diagnosed annually. This trend, along with improved access to insulin and higher survival rates, is projected to increase the prevalence of T1DM. Despite this, there is a data gap on diabetes prevalence in India, and a comprehensive registry is lacking. In 1992, research on 30 children with insulin-dependent diabetes in South India showed a prevalence rate of 0.26 per 1,000 children below 15 years. The highest number of cases were diagnosed when children were around 12 years old. This study highlights that insulin-dependent diabetes is not as rare as previously thought and is more prevalent in South India than in several other Asian countries (Ramachandran et al., 1992).
In Karnataka, over 13 years, the rate of type 1 diabetes (T1DM) was higher in girls (4.0 per 100,000) than in boys (3.7 per 100,000). In Karnal, Haryana, the average T1DM prevalence was 10.2 per 100,000, with higher rates in urban areas (26.6 per 100,000) than rural areas (4.27 per 100,000). Notably, Karnal city had a particularly high prevalence of 31.9 per 100,000 (Kalra et al., 2010).
A government study conducted in three cities has revealed that over 1% of Indian children have diabetes. The study, conducted as part of the National Program for Prevention and Control of Chronic Diseases, surveyed 92,047 schoolchildren in Nainital, Ratlam, and Bhilwara. It found that 1,351 (1.467%) children exhibited symptoms of diabetes. Approximately 18,000 children under 15 years old were newly diagnosed with Type 1 Diabetes Mellitus (T1DM) in these regions in 2011. According to a World Health Organization report, the prevalence of T1DM among children in South East Asia is estimated to be around 1,11,500.
According to a nationwide survey conducted by the Indian Council of Medical Research (ICMR), approximately 8.1% of individuals surveyed in Gujarat were diagnosed with diabetes, while another 10.5% were identified as having pre-diabetes.
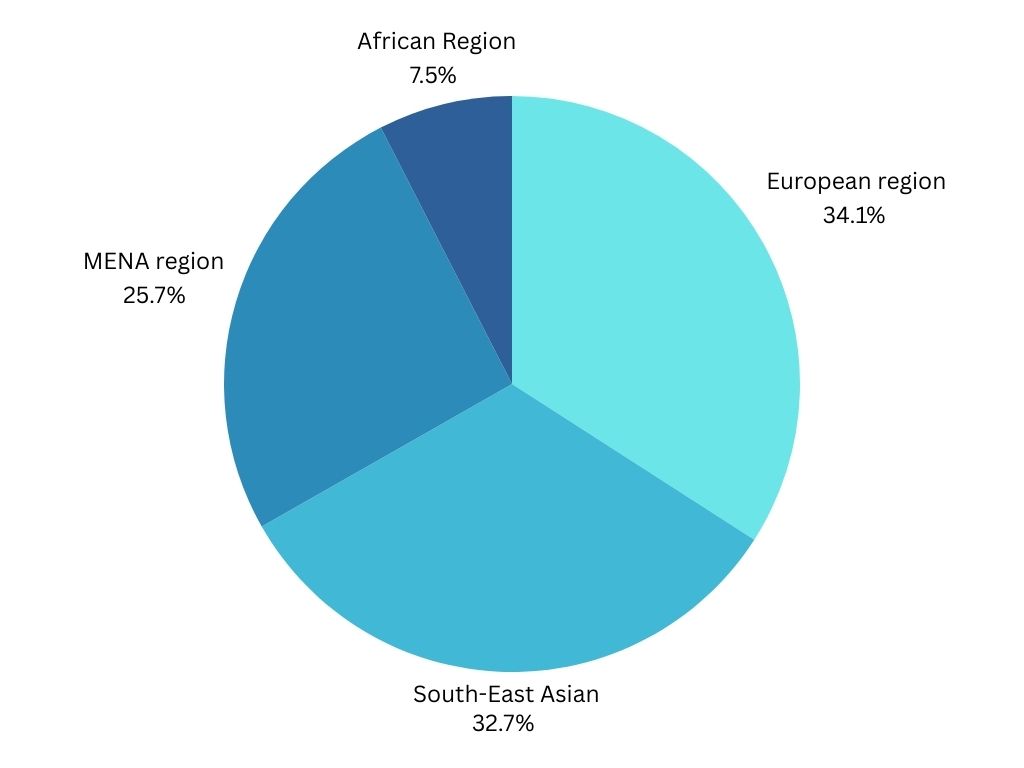 Figure 1: Prevalence of T1D
Figure 1: Prevalence of T1D
II. TREATMENT
Over the past decade, significant progress has been made in treating Type 1 Diabetes (T1D). In the initial stage of T1D, known as Stage 1, it often does not cause noticeable symptoms. Despite this, signs of the immune system attacking the body (autoimmunity) can often be detected early on through the identification of specific antibodies circulating in the blood. These antibodies target proteins associated with T1D, including insulin, GAD65, IA2, and ZNT8 (Nguyen et al., 2013).
As our understanding of T1D evolves, it is becoming clear that effectively managing the condition involves not only providing insulin but also implementing additional strategies.
- Preserve Beta Cell Mass: Prevent or slow the destruction of healthy beta cells, the insulin-producing cells in the pancreas, through immunomodulation or other treatments.
- Enhance Insulin Secretion: Stimulate or reprogram the remaining beta cell mass to release insulin effectively, minimizing blood glucose fluctuations and reducing the need for exogenous insulin.
- Reduce Long-Term Complications: Address the risk of developing cardiovascular and kidney problems associated with type 1 diabetes.
This review provides an overview of current and emerging approaches that aim to address these goals, offering new insights into the treatment and management of type 1 diabetes.
??????????????A. Allopathic approach
Over the past quarter century, there has been a significant shift in the approach to managing type 1 diabetes. The Diabetes Control and Complications Trial, published in 1993, paved the way for intensive insulin therapy to become the recommended treatment, leading to notable changes in disease management (Nathan et al., 1993).
1) Insulin Therapy
The risk of low blood sugar levels can prevent patients from reaching normal blood sugar levels with insulin treatment (DCCT Research group, 1991).
a) Types of Insulin
Short-acting: Humulin R, Novolin R
Rapid-acting: Insulin glulisine (Aphidra), Insulin aspart (Novolog)
Intermediate-acting: Novolin N, Humulin N
Long-acting: Insulin glargine (Lantus, Toujeo Solostar), Insulin detemir (Levemir), Insulin degludec (Tresiba) (Tripathi KD, 2013), (Newre et al., 223)
b) Mechanism
The body's immunity targets the beta cells in the pancreas, resulting in diminished natural insulin production. Consequently, glucose moves in the bloodstream than being utilized for energy. Insulin therapy intervenes to rectify this imbalance. In type 1 diabetes, insulin functions by binding to specific receptors on cell surfaces, predominantly in muscle, fat, and liver tissues. This binding mechanism acts as a key unlocking cellular door. Upon activation of the insulin receptor, a signal is transmitted in the cell, prompting movement of GLUT4 transporter proteins to cell membrane. These transporters function as gates, facilitating the entry of glucose into the cell. The glucose that permeates the cell can be promptly utilized for energy requirements via glycolysis or stored as glycogen for future use, primarily in the liver and muscles. Additionally, insulin indirectly regulates the liver's glucose production, thereby averting spikes in blood sugar levels.
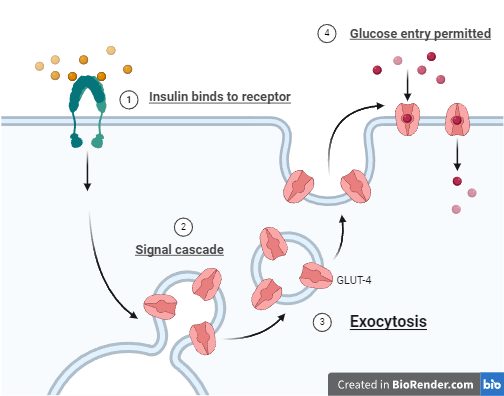
Figure 2: Insulin-Mode of action
For people with type 1 diabetes, insulin is typically administered via injections or an insulin pump. Injection therapy involves long-acting basal insulin injected daily or twice daily, along with rapid-acting insulin injected before meals. This approach is known as multiple daily injections.
Insulin therapy has been explored for treating subclinical Type 1 Diabetes Mellitus (T1DM) with two potential benefits:
Reduced β-cell load: Insulin treatment may help lower the workload on the pancreatic β-cells, slowing down the progression of T1DM.
Immunological tolerance: Insulin may potentially reprogram the immune system to tolerate the β-cells, preventing further damage. Insulin therapy has been administered in various forms, including parenteral (injections under the skin), oral, and intranasal (in the nose).
i) Parenteral
Researchers examined patients with Type 1 Diabetes (T1DM) for islet cell antibodies. They administered low-dose insulin as an intervention, injecting it under the skin twice a day at a total dose of 0.25 units per kilogram of body weight daily. However, at this dosage, the insulin did not delay or prevent the progression of T1DM (Trial DP, 2002).
The intravenous part of this study could not repeat the findings of the earlier, smaller studies. Since only one dose of insulin was tested and the participants already had weak insulin-producing cells when the study started, it was not clear if the insulin given had any effect on protecting or boosting the cells' health.
ii) Oral
A later analysis of an earlier study found that people with high levels of insulin autoantibodies who took oral insulin experienced a significant delay in the onset of type 1 diabetes, even though the original study did not show an overall benefit (Skyler et al., 2005).
Long-term follow-up (13 years) of individuals with high levels of insulin autoantibodies who received oral insulin revealed that their pancreas's ability to produce insulin (β-cell function) remained stable as long as they continued taking the treatment (Vehik et al., 2011).
iii) Nasal
In addition to treating diabetes, insulin has been tested through the nose in studies aimed at training the immune system to tolerate insulin. One such study, the Diabetes Prediction and Prevention (DIPP) trial in Finland, gave nasal insulin to children who were genetically at risk of developing Type 1 Diabetes (T1DM) and who had signs of islet cell and insulin autoantibodies present in their blood (Näntö-Salonen et al., 2008).
The Intranasal Insulin Trial (INIT) and The Diabetes Prediction and Prevention (DIPP) trials were significant in showing that nasal insulin was safe to use. Additionally, studies linked to these trials found that nasal insulin administration might induce immune tolerance to insulin.
c) cInsulin Pumps
Traditional insulin pumps use tubing to connect to the infusion site, but patch pumps offer a tubeless option, with insulin delivered through a pod attached to the skin. Modern insulin pumps allow for more flexibility, enabling users to program multiple basal insulin rates throughout the day and night. They also support various bolus delivery patterns, such as dual or square waves, to accommodate mealtime insulin needs (Pickup JC, 2012).
Insulin pumps often include calculators that help determine the appropriate insulin dose for meals, sometimes with access to a database of food nutritional values. Additionally, modern pumps allow data transfer to computers, enabling the integration of insulin pump information with glucose data, which helps healthcare providers make informed decisions about diabetes management. Study shows that using an insulin pump improved blood sugar control, especially in individuals who significantly lowered their HbA1c (a measure of long-term blood sugar levels). Additionally, it reduced the number of severe low blood sugar (hypoglycaemia) episodes, particularly in patients with a history of frequent and severe hypoglycaemia (increased HbA1c concentrations leads to hypoglycaemia). Research studies that have followed people with type 1 diabetes over time have shown that using an insulin pump can provide ongoing benefits. After starting to use a pump, most people continue to experience improved blood sugar control (HbA1c) and a reduced risk of low blood sugar (hypoglycaemia) for at least five years (Nixon et al., 2014), (Quirós et al., 2016).
While concerns exist that insulin pump therapy may heighten the risk of diabetic ketoacidosis due to potential disruptions in insulin delivery, research suggests otherwise. Studies have found that the occurrence of diabetic ketoacidosis is comparable, or possibly reduced, with insulin pumps compared to traditional multiple daily injections (Cengiz et al., 2013), (Karges et al., 2017), (Weinstock et al., 2013).
d) Glucose monitoring
From urine tests prior to the 1980s, self-monitoring blood glucose levels with a handy blood glucose meter (launched in 1978), to continuous glucose monitors (CGMs), developed in 1999, glucose monitoring techniques have evolved. The most significant technological advancement in treating type 1 diabetes has probably been the accessibility of blood glucose meters, which make it easy to monitor blood glucose levels throughout 24 hours.
i) Blood Glucose Meters
The advent of at-home blood glucose meters significantly altered the way type 1 diabetes was treated by enabling patients to take charge of their own care and increase their insulin dosages. Since they were first introduced, blood glucose meters have shrunk in size and improved in accuracy. The measurement of glucose concentration now takes seconds, and the volume of the blood sample has shrunk to fractions of a microlitre. Many blood glucose meters transfer the glucose readings to a phone and storage, as well as directly communicate with other instruments like insulin pumps. The majority of meters satisfy the International Organization for Standardization's precise requirements (Freckmann et al., 2015)
Figure 3: Blood Glucose Meter
(Sodhi M, 2023)
But when compared to reference blood glucose levels, the average absolute percentage difference varies from 6% to over 10%, indicating significant variation in blood glucose meter accuracy (Ekhlaspour et al., 2017), (Klonoff et al., 2018).
ii) Continuous Glucose Monitors
Continuous Glucose Monitoring (CGM) systems use a sensor that checks glucose levels in the fluid surrounding cells every few minutes. The sensor sends this information to a transmitter that either stores it or shares it every few minutes with a receiver or device like a smartphone or watch, which can also connect to online storage. The glucose levels in fluid usually match those in blood, especially when blood sugar is steady. However, monitoring glucose in fluid can be delayed by around four minutes compared to blood glucose readings (Shah et al., 2018).
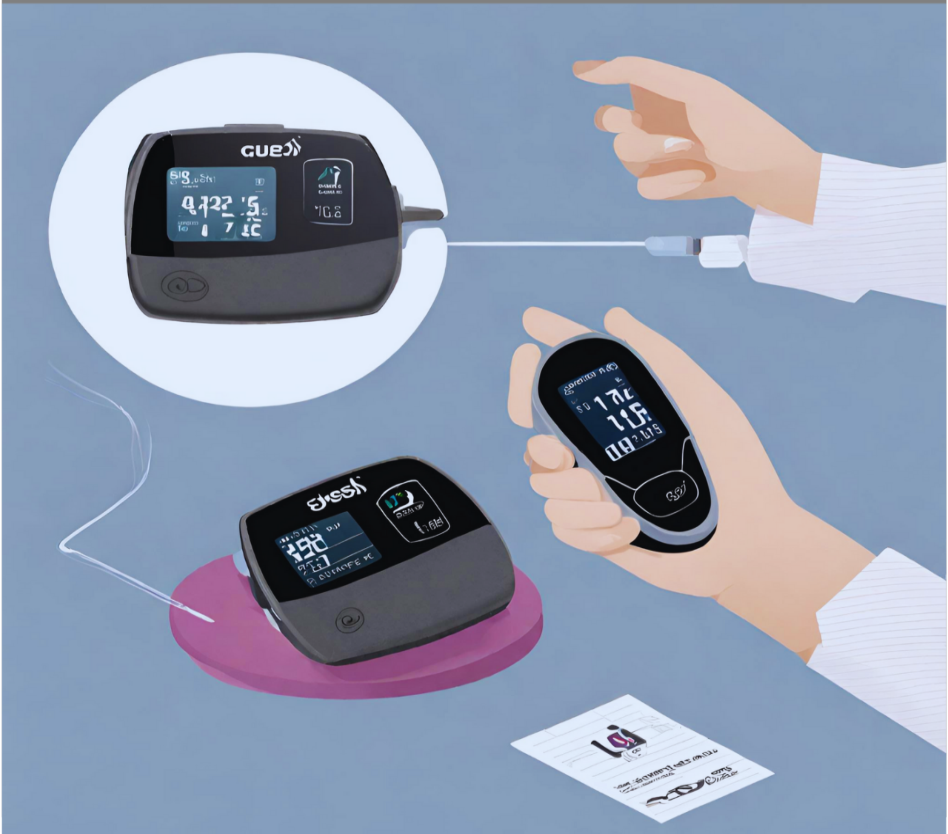
Figure 4: Continue glucose monitoring
When glucose levels fluctuate quickly, the body may experience a delay in its response. The glucose results may be shared with other individuals via devices that upload glucose data to the cloud. This feature has shown to be quite helpful, especially for mom, dad and other lovers of individuals diagnosed with TID. Certain CGM models include the additional capability of gathering blindfolded glucose data for a duration of 7–14 days, which is not visible to the patient in real time. The data is then kept for a health care professional to review at a later time. This is commonly known as professional continuous glucose monitoring.
e) Future Approach of Insulin Therapy
i) Ultra-fast-acting insulins
A subset of rapid-acting insulin analogues known as ultra-fast-acting insulins has been developed to function even more quickly and for a shorter amount of time than regular rapid-acting insulins. These insulins function more quickly to assist effectively manage postprandial blood glucose levels because they are made to more precisely resemble the physiological response of the pancreas to meals. Type 1 diabetes or type 2 diabetes patients who have noticeable blood sugar rises after meals may benefit most from ultra-fast-acting insulins. They might also be beneficial for people who struggle to plan their meals or whose blood sugar management is difficult to control when using conventional rapid-acting insulins.
A 1996 study (Torlonr et al. 1996), contrasted human standard insulin therapy with the practical benefits of utilizing ultra-fast-acting insulins in people with type 1 diabetes. Patients were given a typical meal twice. The first time, they got human ordinary insulin one hour beforehand, and the second time, they got insulin lispro right before the meal. Following the insulin lispro injection, postprandial glucose levels hardly increased at all. We came to the conclusion that all type 1 diabetic patients ought to take an ultra-fast-acting insulin analogue during meals based on the results of this easy experiment.
ii) Smart Insulin Pens
Many insulin users utilize reusable insulin pens with already filled insulin cartridge; however, some still pull insulin from a vial for each injection. The technology of insulin pens is developing such as customized or retrofitted smart pens can now wirelessly connect to smartphone apps or the cloud for data sharing, bolus calculation, and recording insulin dosage and time. There is currently a plethora of smartphone applications that assist with diabetes self-management. These apps provide patient education, tracking of physical activity, recommendations for dosage, food consumption (including photo recognition), data transmission, and blood glucose data logging. Similar to smart pens, there isn't much proof of these applications' efficacy from controlled trials.
iii) Closed-loop systems (Artificial Pancreas)
The advancements in continuous glucose monitoring and insulin pumps have paved the way for ongoing developments in artificial pancreas systems, which aim to improve the management of diabetes (Slover et al., 2018). An artificial pancreas is a technological marvel designed to mimic the function of a healthy pancreas for people with T-1-D. It's not a single device, but rather a system comprised of three key components working together. It integrates a pump with CGM and a manageable process that automatically changes insulin dosage in response to current blood glucose levels. By precisely administering insulin to keep blood C6H12O6 levels within a specified range, this system seeks to replicate the actions of the natural pancreas, lessening the strain of managing diabetes and enhancing glycaemic control in general. It also provides a Fine-tuned insulin administration, minimized fluctuations in insulin levels, and decreased occurrences of dangerously low blood sugar levels (Boughton et al., 2019). Multiple artificial pancreas systems have been created and evaluated for safety and effectiveness in various medical trials. These systems have demonstrated favourable outcomes (Haidar et al., 2015).
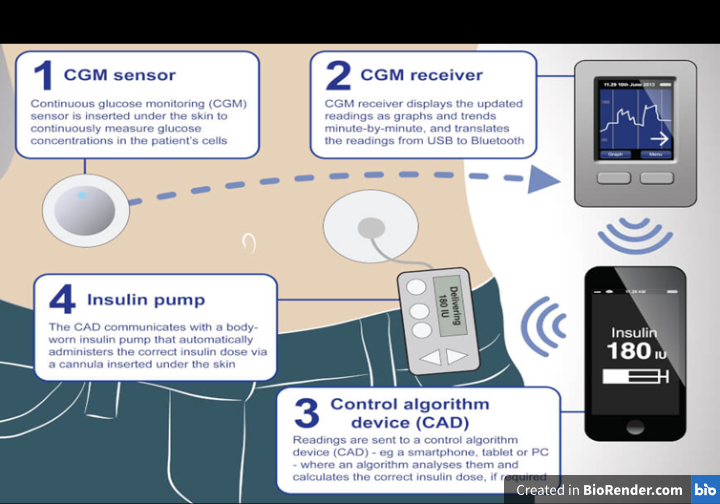
Figure 5: Artificial Pancreas
Due to increasing scientific evidence showing metabolic advantages, the FDA recently approved the first artificial pancreas system for individuals with Type 1 Diabetes (T1D) (Beikari et al., 2018). It's important to remember that although the artificial pancreas is a major development in diabetes technology, it cannot treat diabetes. To get the most out of their diabetes treatment, users still need to frequently check their blood glucose levels, modify their carbohydrate intake, and adopt other lifestyle changes. Furthermore, research and development into artificial pancreas systems is ongoing with the goal of enhancing its use, safety, and effectiveness.
2) Transplantation
While doctors who treat diabetic patients are exuberant regarding the prospect of better glucose checking and insulin-therapy, individuals with disease and their loved ones would undoubtedly acknowledge that the condition be treated without using insulin injections. Both pancreatic transplantation (Robertson RP, 1999) and, more recently, islet transplantation (Shapiro et al., 2000) has culminated in triumphant cures.
a) Xenotransplantation of islets
Pancreatic transplants have proven to be an effective treatment option over an extended period (Burke et al., 2004), (Gruessner et al., 2005). Using whole pancreases and islet cells from deceased donors as transplants could provide patients with lifelong freedom from insulin dependence. However, a major obstacle is the lack of available donor organs. The promising field of transplantation presents options to address the donor shortage. In 1994, the initial documented effort to transplant islets from pigs into T1D patients was completed (Groth CG, 1995).
Due to their similar structures and functioning to human organs and the potential for an unlimited supply, pigs have become a highly valuable model for translational research (Hering et al., 2006). For many years, doctors have been treating diabetes with insulin that comes from the pancreas of pigs (Home et al., 1982), (Sonnenberg et al., 1983), (Cheng M, 2015), (Koike et al., 2002). Due to the similarity of pigs' islet-produced insulin patterns to those of humans, and their ample availability (Cheng M, 2015), extensive research indicates that pig-derived islets hold great potential as a viable alternative to human islets for T1D treatment.
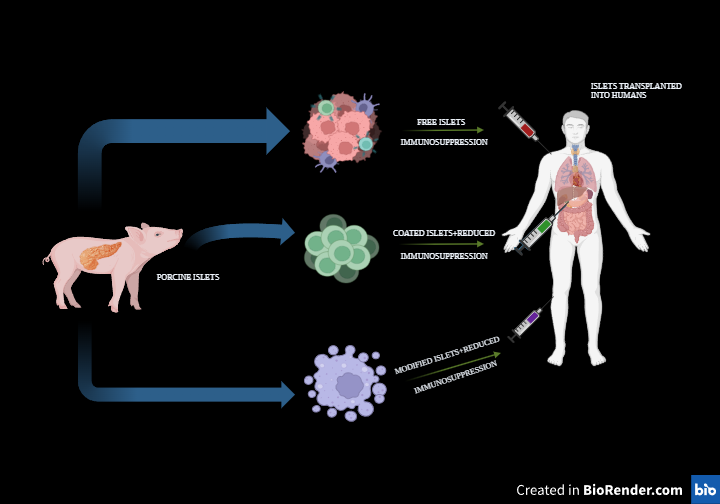
Figure 6: Porcine islet transplantation
Reports have also shown that genetically engineered Xeno-islet grafts can be transplanted to provide stable glycaemic control. For example, after being genetically modified to express the gene Bcl-2, islets have been transplanted and have shown a consistent functional profile with low islet loss (Contreras et al., 2004). Moreover, pigs that express human anti-thrombotic or anti-coagulant genes, including CD39 or thrombomodulin, can reduce the incidence of IBMIR and improve transplant outcomes (Ekser et al., 2010). To improve the success of organ transplants, scientists may create pigs with genetically altered immune system genes. These changes would enhance the compatibility of pig organs with the human body, promoting their survival and functionality after transplant. Xenotransplantation requires continuous immunosuppression. Immunosuppression is required to stop autoimmune islet destruction from recurring as well as to avoid rejection (Sutherland et al., 1984).
Over the last ten years, there have been significant improvements in the immunosuppressive regimens for pancreatic transplants. Medication like mycophenolate mofetil has replaced azathioprine and allowed for considerably lower dosages of glucocorticoid therapy. Before islet xenotransplantation is widely used, there are a number of problems that need to be seen, including possible stability of genes in transgenic pigs and ethical issues.
b) Pancreatic Islet Transplantation
Pancreas contains groupings of cells known as pancreatic islets, also called islets of langerhans. The organ known as the pancreas produces hormones that aid in the body's digestion and use of meals. Numerous cell types, including beta cells, that produce the insulin, are found in islets. Insulin regulates blood glucose, commonly known as blood sugar, and aids in the body's utilization of glucose for energy.
Islets are removed from pancreas of a deceased donor in order to perform an islet transplant. The islets are refined, transformed, and given to a different individual. The beta cells in these islets start producing, releasing insulin as soon as they are transplanted. It has been projected by researchers that transplantation of islets will enable individuals with type-1-diabetes to avoid needing insulin injections every day.
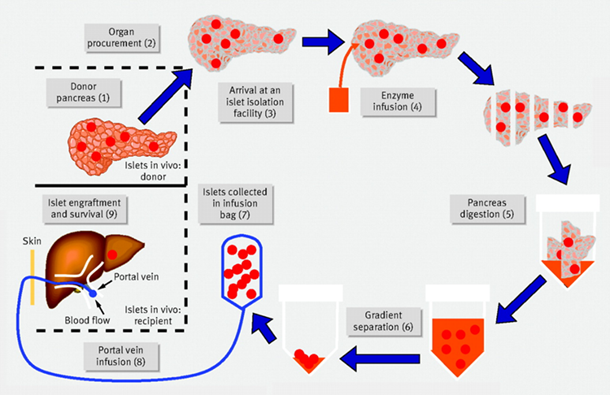
Figure 7: Langerhans Islet Transplantation
Surgical islet transplantation offers an appealing option because it involves a simple infusion of islets into the liver through a catheter. Before research by the Edmonton group (Berney et al., 2000), successful islet transplantations were rare. In 2000, a medical protocol was released showing successful glucose control without insulin injections after islet cell transplantation in Types 1 Diabetes (T1D) patients who received the treatment (Shapiro et al., 2000). The Edmonton Protocol revamped the islet transplantation method by eliminating steroids from the immune suppression regimen, opting instead for a combination of daclizumab, sirolimus, and tacrolimus or transplanting a larger islet mass, averaging around 11,000 islet equivalents per kilogram of body weight.
Islet transplantation aims to provide sufficient healthy cells to manage blood sugar levels, eliminating the need for insulin injections. It additionally enhances glucose regulation and helps prevent the risks associated with low blood sugar emergencies.
???????B. Ayurvedic Approach
According to Ayurveda, diabetes mellitus is termed as madhumeha, this implies that urine passed by diabetic patients is sweet, like honey, hence the name (Vikaspedia Domains, n.d.). Diabetes results from an imbalance of the kapha (earth and water) dosha. Being a kapha dominant person doesn't automatically indicate diabetes. However, if the kapha dominance isn't properly balanced through a healthy diet and lifestyle, it can eventually develop into diabetes.
Texts on Ayurveda that discuss treating diabetes with Ayurveda also mention "Dhatu Paka Janya Vikruti." It’s the detrimental impact of elevated sugar levels on various body tissues. A propensity to inherit the illness is also mentioned in the literature. According to Ayurveda, Ojas is the essence of life; if it is lost, all body processes lose their strength. Diabetes is frequently referred to as Ojomeha, or the loss of Ojas via urine, because Ojas is excreted with urine in this condition.
Ayurvedic treatment for diabetes views diabetes as a metabolic disorder caused by an imbalance in the body’s doshas i.e., Vata (air), Pita (fire), and Kapa (earth and water).
1) Ayurvedic Herbs
Ayurveda employs a variety of herbs and herbal blends to enhance general health and helps manage blood sugar levels. Herbs that are frequently employed to control diabetes include:
a) Acacia arabica (Babul)
Acacia arabica can also referred as babul or Indian Arabic gum tree, is a type of tree endemic to the Indian subcontinent, portions of Africa, and the Middle East. The plant is an antidiabetic agent by performing as secretagogue to release insulin. Because of its possible advantages in the treatment of diabetes, Acacia arabica has been used in Ayurveda. Due to its hypoglycaemic qualities, which are ascribed to substances like tannins and flavonoids, studies indicate that this plant may help control blood sugar levels. Better insulin sensitivity is another benefit of Acacia arabica, which is important for diabetics. Its abundance of antioxidants may shield pancreatic beta cells from stress (oxidative), assisting in generation of insulin. Moreover, its ability to reduce inflammation may aid in the fight against insulin resistance-related inflammation. Blood sugar regulation is indirectly supported by Acacia arabica's digestive assistance, which is likewise appreciated in Ayurveda. Acacia arabica provides a comprehensive strategy for managing diabetes because of its possible benefits for weight management (Modak et al., 2007).

Figure 8: Acacia arabica
b) Aloe vera and Aloe barbadensis
Aloe, a famous plant, has been used as an all-rounder medicine. There are two primary products that can be isolated from the plant i.e., latex and gel. Gel, also known as aloe latex or “aloe juice,” is the mucilage or pulp of the leaves; it is bitter and yellow coloured fluid that emerges from the pericyclic tubules immediately below the leaf's outer rind. Aloe vera gel has the potential to reduce blood sugar levels. However, it's crucial to use it cautiously because Aloe vera and its active component stimulate the production and/or release of insulin from cells in the pancreas (Ajabnoor et al., 19902), (Modak et al., 2007).

Figure 9: Aloe barbadensis
(Keerthana et al., n.d.)
c) Azadirachta indica (Neem)
Neem potentially benefits in regulating blood sugar, improving insulin sensitivity, and protecting pancreatic beta cells make it a viable plant for managing diabetes. Neem leaves contain bioactive substances that may improve cells' uptake of glucose and reduce blood sugar. Furthermore, neem's antioxidant and anti-inflammatory qualities may lessen the frequency and intensity of diabetes symptoms like neuropathy and retinopathy. Because lipid profiles are indicated by LDL and total cholesterol levels, neem may also help with weight management attempts. For diabetics who want to better manage their condition, neem may be helpful in their treatment regimen (Biswas et al., 2002), (Modak et al., 2007).

Figure 10: Azadirachta indica
(Tharindu et al., 2022)
d) Momordica charantia (bitter gourd)
In Asian countries, Momordica charantia is widely employed as an antidiabetic and an antihyperglycemic medicine. Studies have shown that extracts from various parts of plants, including fruit pulp, seeds, leaves, and whole plants, can lower blood sugar levels in animals. In humans and langurs, a polypeptide extracted from the fruit, seeds, and tissues of Momordica charantia has been found to have a significant hypoglycaemic effect when administered subcutaneously (Khanna et al., 1981). Traditionally, this plant has been used in medicine to manage blood sugar levels (Modak et al., 2007).

Figure 11: Momordica charantia
(Kazi S, 2014)
e) Trigonella foenum graecum
Trigonella foenum graecum seeds have a modern impact to control sugar levels. It is widely distributed throughout India, and these seeds are typically one of the main ingredients in Indian spices. In both rats and humans, isolated islet cells released more insulin in response to glucose stimulation when a new amino acid from fenugreek seeds, was added (Sauvaire et al., 1998). The seed intake enhances blood sugar control and helps normalize muscle and liver function in people with diabetes. It also reduces the activity of specific enzymes involved in glucose metabolism in the liver and kidneys (Gupta et al., 1999). This plant also shows antioxidant activity (Ravikumar et al., 1999), (Dixit et al., 2005), (Modak et al., 2007).

Figure 12: Trigonella foenum graecum
(Moradi et al., 2013)
f) Pterocarpus Marsupium
Pterocarpus marsupium Roxb. (Leguminosae) plant is used as a medicine in the Ayurvedic tradition, specifically as a "rasayana," which refers to a class of substances believed to promote rejuvenation and overall health (Halagappa et al., 2010). Pterocarpus marsupium is a deciduous tree, commonly found in the hilly areas of India. Its leaves are shed seasonally. Studies have shown that a specific part of the tree, called the flavonoid fraction, has the ability to restore the functionality of beta cells (Chakravarthy et al., 1980). Pterocarpus marsupium exhibits antioxidant properties, potentially counteract harmful free radicals, mitigating cell and tissue damage caused by oxidation. These effects contribute to its protective benefits on pancreatic beta cells along with overall health in individuals with diabetes (Modak et al., 2007).

Figure 13: Pterocarpus marsupium
(Mahamood M, 2020)
g) Tinospora cordifolia (Guduchi)
commonly called guduchi, is a large, hairless vine native to the Menispermaceae family. It's commonly found in India and is used in Ayurvedic medicine (Stanely et al., 2001), (Prince et al., 1998), (Mathew et al., 1997). The plant's extract, whether in an alcoholic or water-based form, has been shown to lower blood sugar levels and improve glucose tolerance when taken daily (Gupta SS, 1967), (Modak et al., 2007).

Figure 14: Tinospora cordifolia
(Kumar et al., 2017)
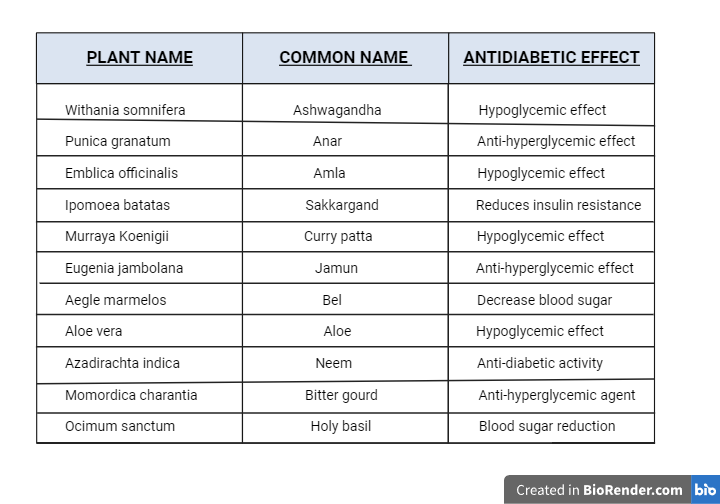
Table 2: Ayurvedic plants and their effect
2) Dietary Modifications
Ayurveda places a strong emphasis on dietary and lifestyle modifications (Ahmad, 2021) as a long-term treatment for diabetes, as these can significantly improve a diabetic patient's quality of life.
- Aim to consume fewer calories than you burn each day. Incorporate plenty of vegetables and greens into your regular meals.
- Include more fibrous foods in your diet and reduce your intake of foods high in carbohydrates.
- Eat a well-balanced diet that includes a moderate number of protein-rich foods, such as meat, fish, eggs, and beans and a moderate number of cereal grains, such as bread, pasta, rice, and oatmeal
- Reduce consumption of fatty and fried food choices.
- Consuming whole grains can stabilize blood sugar levels, enhance the body's ability to use insulin, improve the function of insulin-producing cells in the pancreas, increase the release of insulin. For diabetics, incorporating whole grains into their diet can have beneficial effects. This includes consuming wheat-based products (bread, pasta), low-fat crackers, cereals with minimal sugar, and brown rice.
- Limit eating to regular mealtimes and refrain from unnecessary snacking, especially during passive activities like watching television.
- Regular consumption of garlic and onions is recommended for managing sugar levels. Garlic contains allicin, a compound that helps lower blood sugar levels. Additionally, onions are naturally low in sugar, making them a good choice for individuals looking to control their blood sugar.
- Skim milk-based cheese and yogurt are acceptable for consumption.
- Refrain from consuming sugary fruits such as pineapple, grapes, and mangoes. Instead, incorporate low glycemic index fruits into your diet. These include Indian gooseberry, apples, peaches, pears, oranges, guava, and Jambul fruit.
- Adding Bengal gram, also known as chickpeas or "Chana," to your meals can be beneficial. Research indicates that it may improve the body's ability to regulate blood sugar levels and the removal of waste products through urine.
- Create a diabetes-friendly flour, combine 1 part barley, 1-part black chickpeas, and 4 parts whole-wheat flour. Use this flour mixture to make chapattis, pancakes, or bread to help you control your diabetes through dietary choices.
- To maintain low blood sugar levels, refrain from consuming potatoes and sweet potatoes, taro (Colocasia), yams, fresh grains and pulses (e.g., beans, lentils) and whole yogurt (due to its high fat content)
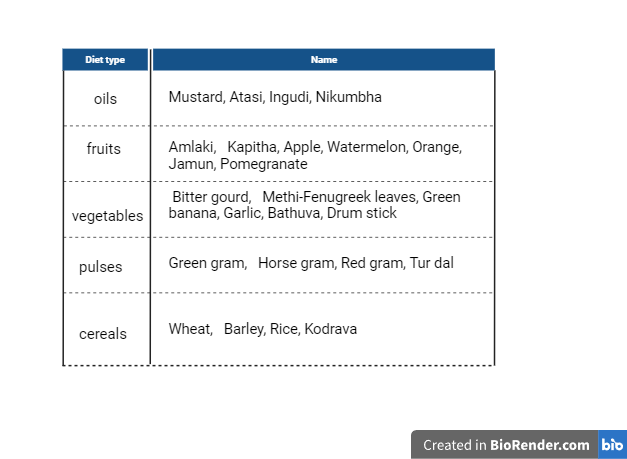
Table 3: Diet plan
3) Panchakarma Treatment for Diabetes
Panchakarma, an Ayurvedic treatment for diabetes, is tailored to individual needs based on their physical makeup, present imbalances, and overall health. This comprehensive approach aims to balance and restore health through detoxification techniques. The duration of Panchakarma therapy ranges from 7 to 15 days, depending on the specific treatments selected by the Ayurvedic practitioner. Here are some Panchakarma treatments that may be considered beneficial for managing diabetes:
a) Vamana
This therapy involves therapeutic vomiting using herbal medicine and is generally recommended for individuals with excess Kapha dosha. It helps to eliminate toxins from the upper respiratory and digestive tracts. Vamana can help improve digestion, reduce body weight, and balance blood sugar levels. Toxins are naturally removed, promoting tissue renewal, rejuvenating the body and mind. Additionally, it alleviates symptoms commonly associated with elevated Kapha, such as excessive urination and murky urine.
b) Virechana
In ayurvedic treatment for Diabetes; Virechana is a purgation therapy that primarily targets Pitta dosha and is beneficial for individuals with diabetes associated with excess heat and inflammation. It helps cleanse the liver and intestines, promoting better digestion and metabolism. Virechana involves the consumption of oral medication to expel toxins through the anal passage. This process rids the body of surplus toxins located in the middle region, boosting appetite, enhancing physical strength, and promoting overall vitality.
c) Basti
Basti, or medicated enemas, can be beneficial for balancing Vata dosha and managing diabetes. It involves the use of herbal oils and decoctions to cleanse the colon and nourish the body. Basti treatments may help regulate bowel movements and improve insulin sensitivity. Specific medications are administered via the anus to remove accumulated toxins and waste products. This method is primarily used in a disease known as "vat Pradhan prameh" for 16 days (kala Basti). Two types of enemas, Niru and anuvasan, can be given on alternating days.
There are several and noteworthy advantages to using Panchakarma as an Ayurvedic treatment for diabetes. Improved glycemic control is one of the main benefits of Panchakarma, since it helps control insulin secretion, improve glucose metabolism, and improves the body's ability to utilize glucose efficiently. As a result, people with diabetes have improved glycemic control. Panchakarma also treats the underlying cause of the illness, providing a thorough method of managing diabetes. Moreover, Panchakarma therapies may decrease insulin resistance by enhancing insulin sensitivity in cells, a condition that is frequently seen in people with type-2-diabetes. Additionally addressing emotional, mental, and spiritual imbalances, Panchakarma therapy's holistic approach promotes general wellbeing and reduces the risks associated with diabetes, including respiratory issues, constipation, and neuropathy.
4) Yoga Practices
Yoga's benefits extend to managing diabetes by addressing its underlying causes. Diabetes often stems from stress and excess weight. By incorporating yoga into your routine, you not only relieve stress through meditation, but also control weight gain by reducing fat accumulation.
Many yoga practices such as Pranayama, Surya namaskar, Balasana, Vajrasana, Sarvangasana, Halasana, and Dhanu asana are beneficial in managing diabetes, but it's crucial to assess a patient's health, risk factors, and personal needs before recommending a practice plan. Based on this assessment, yoga with varying intensities (low or high) can be tailored to meet their individual requirements (Raveendran et al., 2018), (Chaudhary et al., 2020).
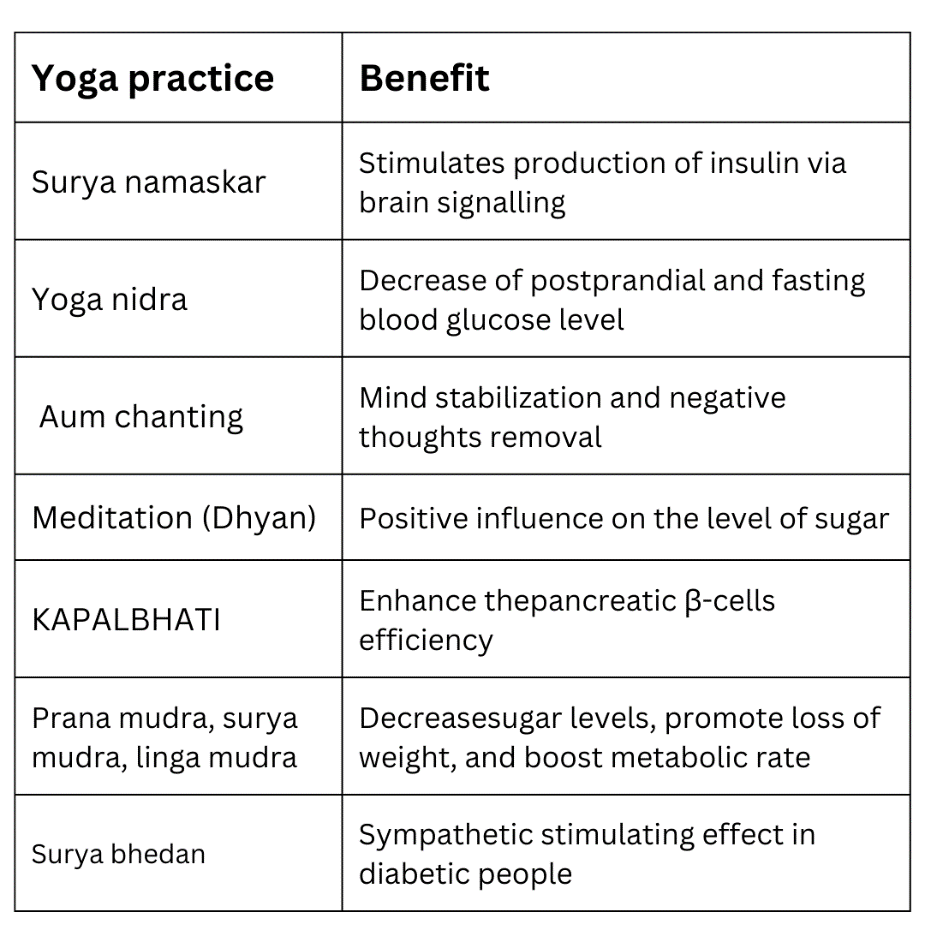
Table 4: Yoga practices
5) Herbal Formulations
You can use herbal products for the regular treatment of diabetes, but only with your doctor's advice.
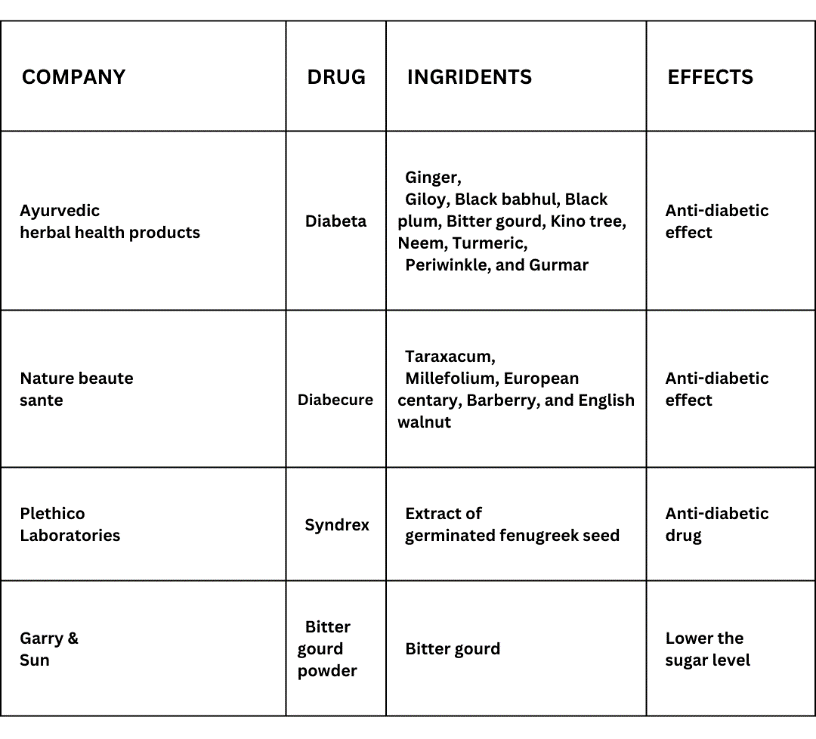
a) Syndrex
Plethico Laboratory produces a drug that includes extracts from fenugreek seeds, which have been utilized in traditional medicine for over a millennium. Current research involves investigating the drug's antidiabetic effects through animal studies and experiments with cultured islet cells. Syndrex is basically the extract of germinated fenugreek seed (Chaudhary et al., 2020).
b) Bitter Gourd Powder
Powdered bitter gourd is marketed by Garry and Sun. It reduces sugar-levels in the blood and urine. It purifies the blood and boosts the body's ability to fight off diseases. Bitter gourd has several beneficial medical properties. It has laxative, antidotally, antipyretic, tonic, palatable, stomachic, and ant bilious properties.
Native African and Asian remedies also contain bitter gourd. Particularly, bitter gourd is used in medicines to treat diabetes. Bitter glycosides, saponins, alkaloids, oils, phenolics, reducing sugars, polypeptides, sterols, free acids, and 17 amino acids are among the substances it contains.
A crystalline product known as p-insulin is also present. Apart from its properties as a treatment for haemorrhoids, it also tightens tissues, improves digestion, stimulates menstruation, supports liver function, eliminates intestinal worms, and purifies the blood. Notably, it also has blood sugar-lowering effects (Chaudhary et al., 2020).
c) Diabeta
Ayurvedic Cure's DIABETA formulation, available as capsules, is intended to lower blood sugar levels. that combines a number of scientifically validated anti-diabetic ingredients with strong immunomodulators, antihyperlipidemic, stress-relieving and liver-protecting compounds. Diabeta's ingredients are backed by modern research and clinical trials. It contains the following ingredients like Ginger, Giloy, Black babul, Black plum, Bitter gourd, Kino tree, Neem, Turmeric, Periwinkle and Gurmar (Chaudhary et al., 2020).
d) Dia-Care
Admark Herbals Ltd.'s product focused on regulating blood sugar levels: Dia-Care is advertised as having the ability to treat both Type 1 and Type 2 diabetes effectively in 3 months and to cure the conditions in 18 months.
Individuals on insulin will ultimately become independent of it. There are six phases to the entire treatment, each lasting ninety days. A tea spoon's amount of powder, or around 5 grams, it is mixed with half a glass of water, blended thoroughly, and left to rest for the night.
On an empty stomach, drink only the water, not the muddy particles upon waking at morning. Water is added to the leftover medicine, which is then kept for the entire day and taken as directed i.e., half an hour before dinner. It is natural and free of side effects (Chaudhary et al., 2020).
e) Gurmar Powder
Garry and Sun's gurmar powder is a natural supplement that helps manage blood sugar levels. It works by blocking the absorption of sugars in the intestines, preventing sharp increases in blood sugar.
Gurmar also supports the metabolism in the kidneys, liver, and muscles. It helps lower blood sugar and stimulates insulin production.
When applied to the tongue, it neutralizes the taste of sweetness, reducing sugar cravings and excessive sugar consumption in people with diabetes.
Its effects, which last for one to two hours, deaden the sweet and bitter tastes of foods. In addition to these benefits, it increases heart contractions, promotes urine production and regulates biochemical processes in the liver, kidneys, and muscles (Chaudhary et al., 2020).
 ???????
???????
Table 5: Herbal drugs
???????C. Future approach
1) Stem Cell Replacement Therapy
Stem cell-based therapies are becoming closer to reality (Chen et al., 2020), perhaps providing a remedy by replenishing or replacing Beta cells that are no longer present or are deteriorated. However, beta cells derived from stem cells must also be protected from damage caused by the body's own immune system, indicating immunomodulation to some extent will be required even with the development of a practical stem cell treatment for type 1 diabetes, except if a fully functioning capsule that can withstand the immune system is developed (Coppieters et al., 2020). In this situation, long-term survival of transplanted beta cells will also be made possible by improved preventative measures or treatment plans.
2) Regeneration of β cells
Despite continued autoimmunity, the attendance of βcells in netizens with long-lasting type 1 diabetes suggests that fresh βcell production may be occurring (Meier et al., 2005). Currently a contentious goal, specifically regenerating these βcells offers an alternative approach to avert type-1-diabetes. Thus, ongoing research is being done on the regeneration of βcells. Recent work has shown that cells that can develop into either the pancreas or other organs may be distinguished as well as islet β cells can be replicated. According to one study, there is only one adult pancreas precursor in mice that can develop into cells that resemble islet βcells (Seaberg et al., 2004). According to a different study, during adulthood and following pancreatectomy in mice, before existing βcells rather than stem cells are the primary source of arising βcells (Dor et al., 2004).
???????D. Ongoing Clinical trials on type 1 diabetes
1) Glucagon-like peptide 1 receptor agonists (GLP-1 RAs)
In individuals with t1d, GLP-1 RA process is an important adjunctive treating choice that can help reduce insulin dosages, promote weight loss, and improve HbA1c levels marginally without raising the risk of hypoglycaemia. Individuals who are overweight, have detectable C-peptide, or are unable to reach glycemic targets without hypoglycaemia have been shown to benefit most from GLP-1 RA treatment.
GLP-1 RAs may be used to treat Diabetes Mellitus (T1DM) in the ways listed below:
When insulin is not enough to provide T1DM patients with optimal glycemic control, GLP-1 RAs may occasionally be utilized as an adjuvant medication. To raise blood glucose levels and perhaps lower the amount of insulin needed, GLP-1 RAs work by boosting insulin synthesis and blocking glucagon release. In addition, GLP-1 RAs may reduce the total daily insulin dose required by T1DM patients in order to preserve glycemic control. Reduced blood sugar fluctuations and a decreased risk of hypoglycaemia could arise from this reduction.
In addition, people with T1DM who have trouble controlling their weight or who are overweight may find that GLP-1RAs help with mass management. Unlike several other diabetic medicines, GLP-1RAs are linked with mass-loss rather than gain. A number of GLP-1 RAs have also demonstrated benefits for the cardiovascular system, such as a reduction in the risk of cardio-vascular procedures in individuals with T-2-DM. Those who are more susceptible to cardiovascular disease may want to consider these benefits, even if more research is required to confirm them in T1DM patients. It is important to keep in mind that drugs that stimulate GLP-1 receptors may cause digestive system problems such as feeling sick, throwing up, or having loose bowel movements. This is especially true when the dosage is changed or a new medicine is started. When considering GLP-1 RA therapy, people with type 1 diabetes should be aware of these potential side effects and consult with a doctor about the same. Individualized treatment plans should take into account factors such as cardiovascular risk, weight management objectives, glycemic control, and tolerability.
2) Provention Bio’s Tzield (teplizumab)
In order to deactivate T lymphocytes, teplizumab targets T cells, which have a cell surface protein called CD3, by binding to it. These T cells contain lymphocytes that mistakenly react against the pancreas's beta cells. This process is thought to be partially agonistic. In peripheral blood, it causes a rise in the percentage of worn-out CD8+ T cells and regulatory T cells. Teplizumab's development for T1D prevention and treatment was then pursued by Provention Bio, who purchased all of the drug's rights in 2018. Teplizumab was given the orphan drug designation from the FDA to treat T1D with recent onset. Additionally, teplizumab was given the EMA's PRIority MEdicines (PRIME) title for the same indication as well as the FDA's Breakthrough Therapy denomination for the avoidance or delay of clinical T1D in those who are at risk.
3) Diamyd Medical’s gamma-aminobutyric acid (GABA)
The FDA has given special priority to Diamyd, a treatment for newly diagnosed type 1 diabetes (T1D) in patients with a specific genetic marker (HLA DR3-DQ2). Diamyd aims to enhance blood sugar management in these patients. Diamyd is an immunotherapy that targets certain antigens with the goal of maintaining natural insulin production. Based on information from several trials showing Diamyd's ability to alter immune system reactions against pancreatic beta cells, the designation was given.
4) Imcyse’s IMCY-0098
In type 1 diabetes, the body's immune system mistakenly attacks and kills insulin-producing cells in the pancreas. As a result, the pancreas becomes unable to produce insulin. A new drug called IMCY-0098 is being tested. This drug aims to prevent the immune system from attacking these cells. It works by creating new immune cells that can identify and destroy the harmful immune cells causing the damage to the pancreas.
5) ProKidney’s renal autologous cell therapy (REACT)
REACT, a personalized cell therapy, is created using stem cells (SRCs) derived from kidney tissue samples obtained from each patient. ProKidney processes these tissue samples, isolating and expanding the SRCs. The selected SRCs are then combined to create a cryopreserved solution or a gelatin-based gel containing 100 million cells per milli-litre. This cell mixture is then shipped to the designated clinical facility for administration.
6) Jakpot T1D
Abrocitinib and ritlecitinib are in a new class of autoimmune treatments called Janus kinase (JAK) inhibitors. The U.S. Food and Drug Administration (FDA) has authorized the use of abrocitinib for the treatment of eczema. Ritlecitinib is being studied as a treatment for several autoimmune diseases, including alopecia, ulcerative colitis, Crohn’s disease, vitiligo, and rheumatoid arthritis.
Scientists think that the drugs abrocitinib and ritlecitinib may soothe the overactive immune system's attack on beta cells in the pancreas.
Beta cells are responsible for producing insulin, which helps control blood sugar levels. Even a small amount of insulin production can keep blood sugar levels stable, reducing the chances of serious health issues down the road.
If either or both treatments are successful, these JAK inhibitors may be studied in earlier stages of T1D to see if they can prevent or delay clinical diagnosis.
7) T1D relay
TrialNet administered rituximab-pvvr followed by abatacept to individuals (ages 8-5) to determine if the combined application enhances insulin output who were newly diagnosed with type 1 diabetes (T1D). Each therapy has a history of safety and effectiveness. Rituximab-pvvr is FDA granted in treating several auto-immune disorders, including Rheumatoid Arthritis (RA). Abatacept is FDA-approved to treat adult Rheumatoid Arthritis, as well as Juvenile Idiopathic Arthritis (JIA) in children as young as six.
In a recent study published in March 2023, the use of abatacept altered the immune response and maintained insulin production over a one-year period. However, abatacept did not prevent the progression from the early stage of type 1 diabetes (T1D) to the final stage before diagnosis, despite being the study's objective.
While this is the first T1D study to test rituximab-pvvr followed by abatacept, this sequence is used by clinicians to treat other autoimmune diseases. By adding abatacept after rituximab-pvvr, researchers predict more people with T1D will experience prolonged beta cell function during and possibly after treatment.
III. SUMMARY
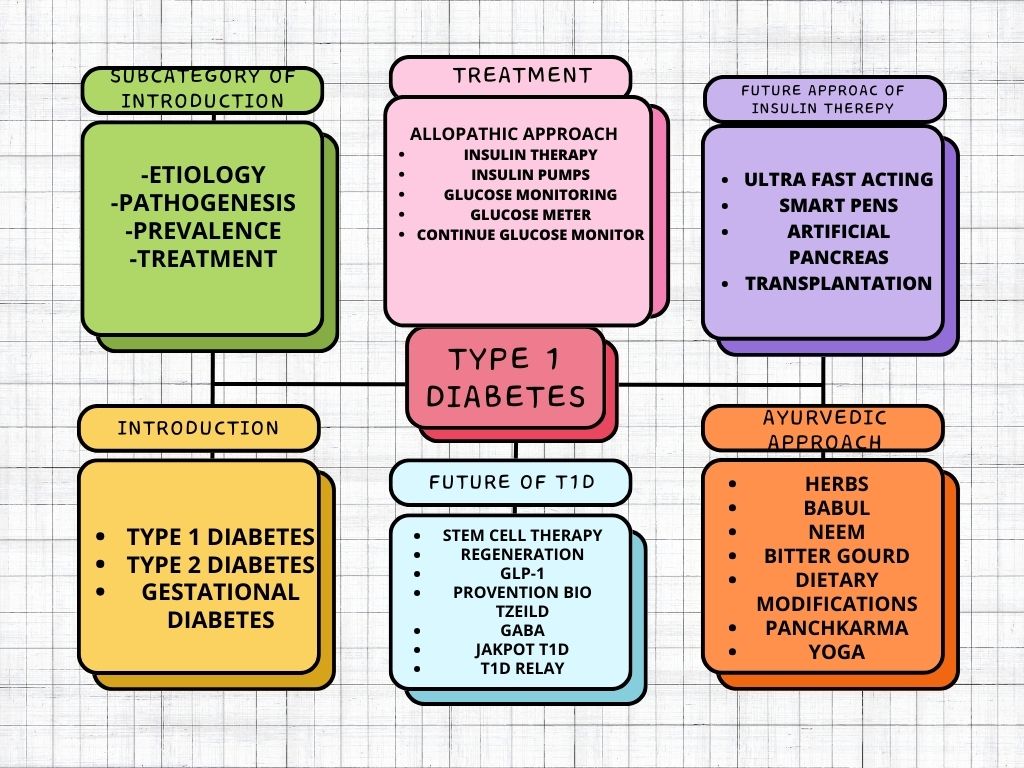 ???????Figure 15: Summary
???????Figure 15: Summary
References
[1] Aguirre, F., Brown, A., Cho, N. H., Dahlquist, G., Dodd, S., Dunning, T., & Whiting, D. (2013). IDF diabetes atlas. [2] Ahlqvist, E., Storm, P., Käräjämäki, A., Martinell, M., Dorkhan, M., Carlsson, A., ... & Groop, L. (2018). Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. The lancet Diabetes & endocrinology, 6(5), 361-369 [3] Ahmad, S. (2021, May 28). Ayurvedic Medicines for Diabetes: Food, Diet & Natural Tips. Possible. https://possible.in/ayurvedic-medicines-for-diabetes.html [4] Ajabnoor, M. A. (1990). Effect of aloes on blood glucose levels in normal and alloxan diabetic mice. Journal of ethnopharmacology, 28(2), 215-220. [5] Barnett, R. (2018). Type 1 diabetes. The Lancet, 391(10117), 195. [6] Bekiari, E., Kitsios, K., Thabit, H., Tauschmann, M., Athanasiadou, E., Karagiannis, T., ... & Tsapas, A. (2018). Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. bmj, 361. [7] Berney, T., & Ricordi, C. (2000). Islet cell transplantation: the future?. Langenbeck\'s archives of surgery, 385, 373-378. [8] Biswas, K., Chattopadhyay, I., Banerjee, R. K., & Bandyopadhyay, U. (2002). Biological activities and medicinal properties of neem (Azadirachta indica). Current science, 1336-1345. [9] Boughton, C. K., & Hovorka, R. (2019). Is an artificial pancreas (closed?loop system) for type 1 diabetes effective?. Diabetic Medicine, 36(3), 279-286. [10] Bowman, M. A., Leiter, E. H., & Atkinson, M. A. (1994). Prevention of diabetes in the NOD mouse: implications for therapeutic intervention in human disease. Immunology today, 15(3), 115-120. [11] Burke, G. W., Ciancio, G., & Sollinger, H. W. (2004). Advances in pancreas transplantation. Transplantation, 77(9), S62-S67. [12] Cengiz, E., Xing, D., Wong, J. C., Wolfsdorf, J. I., Haymond, M. W., Rewers, A., ... & T1D Exchange Clinic Network. (2013). Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatric diabetes, 14(6), 447-454. [13] Chakravarthy, B. K., GUPTA, S., Gambhir, S. S., & Gode, K. D. (1980). PANCREATIC BETA-CELL REGENERATION-A NOVEL ANTIDIABETIC MECHANISM OF PTEROCARPUS MARSUPIUM, ROXB. Indian journal of pharmacology, 12(2), 123-127. [14] Chaudhary, P., Kotnala, A., Negi, N., & Janmeda, P. (2020). Ayurvedic approach: a natural way to cure diabetes (Madhumeha). Vigyan Varta, 1(4), 12-15. [15] Chen, S., Du, K., & Zou, C. (2020). Current progress in stem cell therapy for type 1 diabetes mellitus. Stem cell research & therapy, 11(1), 275. [16] Cheng, M. (2015). Islet Xeno/transplantation and the risk of contagion: local responses from Canada and Australia to an emerging global technoscience. Life Sciences, Society and Policy, 11, 1-23. [17] Cohen, O., Filetti, S., Castañeda, J., Maranghi, M., & Glandt, M. (2016). When intensive insulin therapy (MDI) fails in patients with type 2 diabetes: switching to GLP-1 receptor agonist versus insulin pump. Diabetes Care, 39(Supplement_2), S180-S186. [18] Contreras, J. L., Xie, D., Mays, J., Smyth, C. A., Eckstein, C., Rahemtulla, F. G., ... & Eckhoff, D. E. (2004). A novel approach to xenotransplantation combining surface engineering and genetic modification of isolated adult porcine islets. Surgery, 136(3), 537-547. [19] Coppieters, K., Winkel, L., & von Herrath, M. (2020). Long-term viability through selective permeability. Nature Biomedical Engineering, 4(8), 763-764. [20] DCCT Research Group. (1991). Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The American journal of medicine, 90(4), 450-459. [21] Diabetes Control and Complications Trial Research Group. (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England journal of medicine, 329(14), 977-986. [22] Dixit, P., Ghaskadbi, S., Mohan, H., & Devasagayam, T. P. (2005). Antioxidant properties of germinated fenugreek seeds. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 19(11), 977-983. [23] Dor, Y., Brown, J., Martinez, O. I., & Melton, D. A. (2004). Adult pancreatic ?-cells are formed by self-duplication rather than stem-cell differentiation. Nature, 429(6987), 41-46. [24] Ekhlaspour, L., Mondesir, D., Lautsch, N., Balliro, C., Hillard, M., Magyar, K., ... & Russell, S. J. (2017). Comparative accuracy of 17 point-of-care glucose meters. Journal of diabetes science and technology, 11(3), 558-566. [25] Ekser, B., & Cooper, D. K. (2010). Overcoming the barriers to xenotransplantation: prospects for the future. Expert review of clinical immunology, 6(2), 219-230. [26] Foulis, A. K., McGill, M., & Farquharson, M. A. (1991). Insulitis in type 1 (insulin?dependent) diabetes mellitus in man—macrophages, lymphocytes, and interferon?? containing cells. The Journal of pathology, 165(2), 97-103. [27] Freckmann, G., Schmid, C., Baumstark, A., Rutschmann, M., Haug, C., & Heinemann, L. (2015). Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197: 2003, ISO 15197: 2013, and current FDA recommendations. Journal of diabetes science and technology, 9(4), 885-894. [28] Groth, C. G. (1995). Transplantation of porcine fetal pancreas to diabetic patients. The Lancet, 345(8951), 735. [29] Gruessner, A. C., & Sutherland, D. E. (2005). Pancreas transplant outcomes for United States (US) and non?US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clinical transplantation, 19(4), 433-455. [30] Guarnotta, V., Vigneri, E., Pillitteri, G., Ciresi, A., Pizzolanti, G., & Giordano, C. (2018). Higher cardiometabolic risk in idiopathic versus autoimmune type 1 diabetes: a retrospective analysis. Diabetology & metabolic syndrome, 10, 1-8. [31] Gupta, D., Raju, J., & Baquer, N. Z. (1999). Modulation of some gluconeogenic enzyme activities in diabetic rat liver and kidney: effect of antidiabetic compounds. [32] Haidar, A., Legault, L., Matteau-Pelletier, L., Messier, V., Dallaire, M., Ladouceur, M., & Rabasa-Lhoret, R. (2015). Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. The lancet Diabetes & endocrinology, 3(8), 595-604. [33] Halagappa, K., Girish, H. N., & Srinivasan, B. P. (2010). The study of aqueous extract of Pterocarpus marsupium Roxb. on cytokine TNF-? in type 2 diabetic rats. Indian journal of pharmacology, 42(6), 392-396. [34] Hering, B. J., Wijkstrom, M., Graham, M. L., Hårdstedt, M., Aasheim, T. C., Jie, T., ... & Schuurman, H. J. (2006). Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nature medicine, 12(3), 301-303. [35] Home, P. D., & Alberti, K. G. M. M. (1982). The new insulins: Their characteristics and clinical indications. Drugs, 24, 401-413. [36] Kalra, S., Kalra, B., & Sharma, A. (2010). Prevalence of type 1 diabetes mellitus in Karnal district, Haryana state, India. Diabetology & metabolic syndrome, 2, 1-3. [37] Karges, B., Schwandt, A., Heidtmann, B., Kordonouri, O., Binder, E., Schierloh, U., ... & Holl, R. W. (2017). Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. Jama, 318(14), 1358-1366. [38] Kazi, S. (2014). Use of traditional plants in diabetes mellitus. Int J Pharm, 4(4), 283-9. [39] Keerthana, S., Charumathy, M., Gangadhar, L., & ES, A. COMPARISION OF IN VITRO AND EX VIVO EXPERIMENTAL ANALYSIS OF ALOE VERA EXTRACTS ON THE ANTIOXIDANT AND ANTIMICROBIAL ACTIVITY. [40] Khanna, P., Jain, S. C., Panagariya, A., & Dixit, V. P. (1981). Hypoglycemic activity of polypeptide-p from a plant source. Journal of Natural Products, 44(6), 648-655. [41] Klonoff, D. C., Parkes, J. L., Kovatchev, B. P., Kerr, D., Bevier, W. C., Brazg, R. L., ... & Kohn, M. A. (2018). Investigation of the accuracy of 18 marketed blood glucose monitors. Diabetes Care, 41(8), 1681-1688. [42] Koike, C., Fung, J. J., Geller, D. A., Kannagi, R., Libert, T., Luppi, P., ... & Trucco, M. (2002). Molecular basis of evolutionary loss of the ?1, 3-galactosyltransferase gene in higher primates. Journal of Biological Chemistry, 277(12), 10114-10120. [43] Kumar, A., Kumar, S., Singh, M. K., & Tiwari, S. K. (2024). A comprehensive review on the chemical composition and pharmacological activities of ACACIA ARABICA. Intelligent Pharmacy. [44] Kumar, K. P., Azad, K., Zabeen, B., & Kalra, S. (2012). Type 1 diabetes in children: Fighting for a place under the sun. Indian journal of endocrinology and metabolism, 16(Suppl1), S1-S3. [45] Kumar, S., Dobos, G. J., & Rampp, T. (2017). The significance of ayurvedic medicinal plants. Journal of evidence-based complementary & alternative medicine, 22(3), 494-501. [46] Mathew, S., & Kuttan, G. (1997). Antioxidant activity of Tinospora cordifolia and its usefulness in the amelioration of cyclophosphamide induced toxicity. Journal of experimental & clinical cancer research: CR, 16(4), 407-411. [47] Meier, J. J., Bhushan, A., Butler, A. E., Rizza, R. A., & Butler, P. C. (2005). Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration?. Diabetologia, 48, 2221-2228. [48] Menon, P., Viramani, A., Shah, P., Raju, R., Sethi, A., Sethia, S., ... & Ahuja, M. M. (1990). Childhood onset diabetes mellitus in India: an overview. Int J Diabetes Dev Ctries, 10, 11-6. [49] Modak, M., Dixit, P., Londhe, J., Ghaskadbi, S., & Devasagayam, T. P. A. (2007). Indian herbs and herbal drugs used for the treatment of diabetes. Journal of clinical biochemistry and nutrition, 40(3), 163-173. [50] Moradi, N., & Moradi, K. (2013). Physiological and pharmaceutical effects of fenugreek (Trigonella foenum-graecum L.) as a multipurpose and valuable medicinal plant. Global journal of medicinal plant research, 1(2), 199-206. [51] Näntö-Salonen, K., Kupila, A., Simell, S., Siljander, H., Salonsaari, T., Hekkala, A., ... & Simell, O. (2008). Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. The Lancet, 372(9651), 1746-1755. [52] Newre, A. B., Jejurkar, A. S., Pagar, A. S., Gavali, A. S., & Kawde, R. M. (2023). A Comparative Study of Diabetic Treatment–Allopathy vs. Ayurveda. International Journal of Pharmaceutical Sciences, 1(10), 1-1. [53] Nguyen, C., Varney, M. D., Harrison, L. C., & Morahan, G. (2013). Definition of high-risk type 1 diabetes HLA-DR and HLA-DQ types using only three single nucleotide polymorphisms. Diabetes, 62(6), 2135-2140. [54] Nixon, R., Folwell, R., & Pickup, J. C. (2014). Variations in the quality and sustainability of long?term glycaemic control with continuous subcutaneous insulin infusion. Diabetic medicine, 31(10), 1174-1177. [55] Patterson, C. C., Karuranga, S., Salpea, P., Saeedi, P., Dahlquist, G., Soltesz, G., & Ogle, G. D. (2019). Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice, 157, 107842. [56] Pickup, J. C. (2012). Insulin-pump therapy for type 1 diabetes mellitus. New England Journal of Medicine, 366(17), 1616-1624. [57] Pickup, J. C. (2012). Insulin-pump therapy for type 1 diabetes mellitus. New England Journal of Medicine, 366(17), 1616-1624. [58] Prince, P. S. M., & Menon, V. P. (1999). Antioxidant activity of Tinospora cordifolia roots in experimental diabetes. Journal of ethnopharmacology, 65(3), 277-281. [59] Priya, G., & Kalra, S. (2018). A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin?. Diabetes Therapy, 9, 349-361. [60] Pterocarpus marsupium Roxb. by Muhsina Mahamood on 13 December 2020. (n.d.). India Biodiversity Portal. https://indiabiodiversity.org/observation/show/16248812 [61] Quirós, C., Giménez, M., Ríos, P., Careaga, M., Roca, D., Vidal, M., & Conget, I. (2016). Long?term outcome of insulin pump therapy: reduction of hypoglycaemia and impact on glycaemic control. Diabetic Medicine, 33(10), 1422-1426. [62] Ramachandran, A., Snehalatha, C., Khader, O. A., Joseph, T. A., & Viswanathan, M. (1992). Prevalence of childhood diabetes in an urban population in south India. Diabetes research and clinical practice, 17(3), 227-231. [63] Raveendran, A. V., Deshpandae, A., & Joshi, S. R. (2018). Therapeutic role of yoga in type 2 diabetes. Endocrinology and Metabolism, 33(3), 307. [64] Ravikumar, P., & Anuradha, C. V. (1999). Effect of fenugreek seeds on blood lipid peroxidation and antioxidants in diabetic rats. Phytotherapy research, 13(3), 197-201. [65] Robertson, R. P. (1999). Prevention of recurrent hypoglycemia in type 1 diabetes by pancreas transplantation. Acta diabetologica, 36(1), 3-9. [66] Sauvaire, Y., Petit, P., Broca, C., Manteghetti, M., Baissac, Y., Fernandez-Alvarez, J., ... & Ribes, G. (1998). 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes, 47(2), 206-210. [67] Seaberg, R. M., Smukler, S. R., Kieffer, T. J., Enikolopov, G., Asghar, Z., Wheeler, M. B., ... & van der Kooy, D. (2004). Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nature biotechnology, 22(9), 1115-1124. [68] Shah, V. N., Laffel, L. M., Wadwa, R. P., & Garg, S. K. (2018). Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes technology & therapeutics, 20(6), 428-433. [69] Shapiro, A. J., Lakey, J. R., Ryan, E. A., Korbutt, G. S., Toth, E., Warnock, G. L., ... & Rajotte, R. V. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New England Journal of Medicine, 343(4), 230-238. [70] Shapiro, A. J., Lakey, J. R., Ryan, E. A., Korbutt, G. S., Toth, E., Warnock, G. L., ... & Rajotte, R. V. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New England Journal of Medicine, 343(4), 230-238. [71] Skyler, J. S. (2018). Hope vs hype: where are we in type 1 diabetes?. Diabetologia, 61(3), 509-516. [72] Skyler, J. S., Krischer, J. P., Wolfsdorf, J., & Cowie, C. (2005). Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial-Type 1. Diabetes care, 28(5), 1068. [73] Slover, R. H., Tryggestad, J. B., DiMeglio, L. A., Fox, L. A., Bode, B. W., Bailey, T. S., ... & Kaufman, F. R. (2018). Accuracy of a fourth-generation continuous glucose monitoring system in children and adolescents with type 1 diabetes. Diabetes Technology & Therapeutics, 20(9), 576-584. [74] Sodhi, M. (2023, July 11). 5 Simple Tips To Choose The Right Blood Glucose Meter. PharmEasy Blog. https://pharmeasy.in/blog/5-simple-tips-to-choose-the-right-blood-glucose-meter/ [75] Sonnenberg, G. E., & Berger, M. (1983). Human insulin: much ado about one amino acid?. Diabetologia, 25, 457-459. [76] SS, G. (1967). Effect on fasting blood sugar level, glucose tolerance and adrenaline induced hyperglycemia. Indian J Exp Biol, 55, 733-745. [77] Stanely Mainzen Prince, P., & Menon, V. P. (2001). Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 15(3), 213-218. [78] Sutherland, D. E., Sibley, R. K., Xu, X. Z., Michael, A., Srikanta, A. M., Taub, F., ... & Goetz, F. C. (1984). Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Transactions of the Association of American Physicians, 97, 80-87. [79] Tharindu Madhuranga, Hewa Dikkumburage & Wijekumar, Praba & Samarakoon, Nirmani. (2022). Plants available in Sri Lanka with anti-viral potential: A review. [80] Torlone, E., Pampanelli, S., Lalli, C., Sindaco, P. D., Vincenzo, A. D., Rambotti, A. M., ... & Bolli, G. (1996). Effects of the short-acting insulin analog [Lys (B28), Pro (B29)] on postprandial blood glucose control in IDDM. Diabetes Care, 19(9), 945-952. [81] Trial, D. P. (2002). Effects of insulin in relatives of patients with type 1 diabetes mellitus. Diabetes prevention trial–type 1 diabetes study group. N. Engl. J. Med, 346, 1685-1691. [82] Tripathi, K. D. (2013). Essentials of medical pharmacology. JP Medical Ltd. [83] Vehik, K., Cuthbertson, D., Ruhlig, H., Schatz, D. A., Peakman, M., Krischer, J. P., & DPT-1 and TrialNet Study Groups. (2011). Long-term outcome of individuals treated with oral insulin: Diabetes Prevention Trial–Type 1 (DPT-1) oral insulin trial. Diabetes care, 34(7), 1585-1590. [84] vikaspedia Domains. (n.d.). https://vikaspedia.in/health/ayush/ayurveda-1/evidence-based-ayurvedic-practice/diabetes-madhumeha [85] Weinstock, R. S., Xing, D., Maahs, D. M., Michels, A., Rickels, M. R., Peters, A. L., ... & T1D Exchange Clinic Network. (2013). Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. The Journal of Clinical Endocrinology & Metabolism, 98(8), 3411-3419. [86] Yeh, H. C., Brown, T. T., Maruthur, N., Ranasinghe, P., Berger, Z., Suh, Y. D., ... & Golden, S. H. (2012). Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Annals of internal medicine, 336-347.
Copyright
Copyright © 2024 Divy Ankurkumar Patel, Dr. Jayvadan Vaishnav. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64214
Publish Date : 2024-09-11
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

