Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Decoding Tomorrows Biotechnology: AI and Machine Learning Unveiled
Authors: Vijay P. Thikare, Prutha S. Pathak
DOI Link: https://doi.org/10.22214/ijraset.2024.58430
Certificate: View Certificate
Abstract
This review paper explores the impact of AI and ML on biotechnology, focusing on drug discovery, protein prediction, automation, and ethics.AI and ML are transforming drug discovery by automating tasks, identifying new drug targets, and designing more effective drugs. AI-powered languages and functional genomics are being used to accelerate drug discovery processes. AI tools such as AlphaFold are being used to predict protein structures and functions. Biotechnology automation is being used to improve efficiency in laboratory settings. The use of AI and ML in biotechnology raises ethical and regulatory concerns. It is important to find the potential risks and benefits of this technology before it is widely adopted.
Introduction
I. INTRODUCTION
In 1955, the term "Artificial Intelligence" first appeared in print. The power of Artificial Intelligence (AI) is making a significant impact in biotechnology, effectively addressing a diverse array of challenges. These problems include things like Drug Discovery, Drug Safety, functional and structural genomics, proteomics, metabolomics, pharmacology, pharmacogenetics, and pharmacogenomics Fig1. 1 AI is involved in all these areas and more, making a big difference in biotechnology. The pace of innovation in the biotechnology industry is quickening, and biotechnology companies are beginning to recognize the value that AI can bring to their entire business, in the form of accelerated R&D, analysis of humongous databases, effective decision-making, and cost-effectiveness. Biotechnology has long been at the forefront of scientific breakthroughs, from genetic engineering to personalized medicine. However, the complex nature of biological systems and the vast amount of data generated present significant challenges for traditional analytical approaches. This is where AI and ML step in, offering powerful tools to extract valuable insights and patterns from these complex datasets. Over the past decade, Artificial Intelligence (AI) has contributed substantially to the resolution of various medical problems, including cancer 2. AI's development and application relies on digital technology, especially digital computers. Digital transformation refers to using digital tech to fundamentally change how entities like companies, research institutions, and universities operate 1. In biotech, this means introducing new tech and methods to enhance research speed, accuracy, and product development. This transformation speeds up AI integration by offering big data access and automating tasks, boosting efficiency and precision.

II. OVERVIEW OF AI AND ML
Artificial intelligence (AI) is currently a hot topic due to its widespread popularity (such as ChatGPT). When biotechnology and AI evolve together, unheard-of new potential solutions become available 1. This can aid in solving numerous global issues and advance significant Sustainable Development Goals. Food security, health and well-being, clean water, clean energy, responsible consumption and production, combating climate change, life below the ocean, protecting, restoring, and promoting sustainable use of terrestrial ecosystems, sustainably managing forests, preventing desertification, halting and reversing land degradation, and halting biodiversity loss are some examples of current priorities. Today, AI is pervasive in the biological sciences 4.
Machine learning (ML) refers to the part of research on AI that seeks to provide knowledge to computers through data and observations without being explicitly programmed 5. Machine learning has been utilized in biology for a number of decades, but its significance has continuously increased to the point that it is now utilized in almost all biological disciplines. Machine learning encompasses the practice of constructing predictive models from data and uncovering meaningful clusters within datasets. It strives to replicate human pattern recognition, but in an unbiased manner, through computational methods. This field becomes invaluable when dealing with extensive datasets or intricate data with numerous attributes, circumstances where human examination is impractical. Additionally, it serves the purpose of automating data analysis to create a consistent and efficient workflow 3. Machine learning algorithms frequently employed in practice include linear and logistic regression, Artificial Neural Networks (ANN), Support Vector Machines (SVM), and tree-based methods. These individual models can then be combined with one another using ensemble learning, a methodology which leverages the power of multiple weak classifiers to achieve optimal overall performance 6.


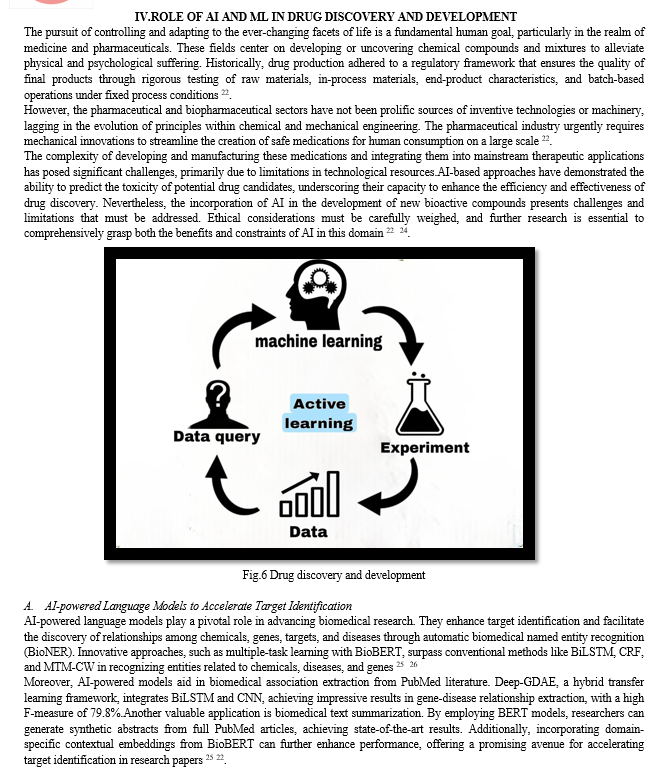
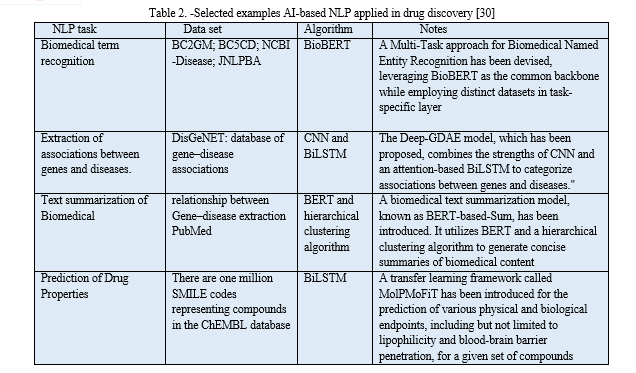
B. Challenges and Limitation
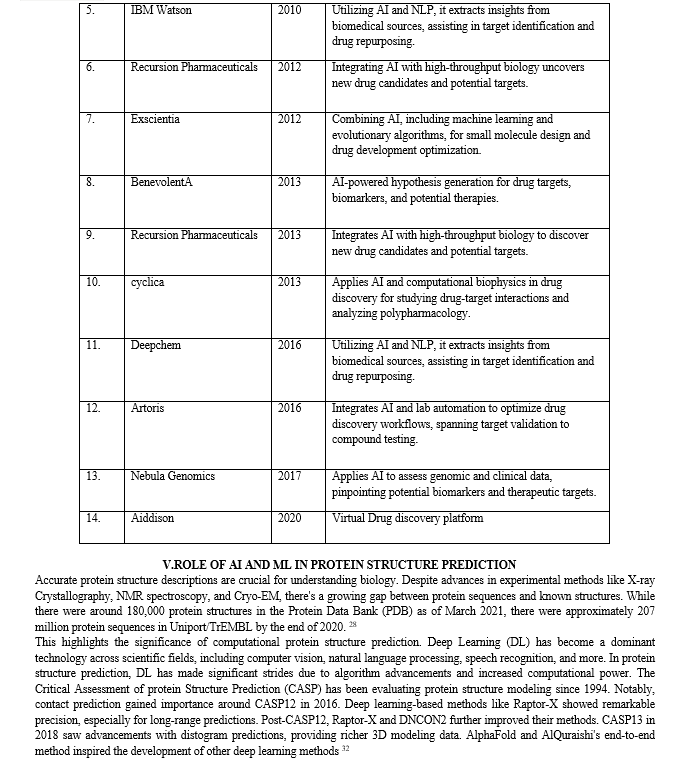
While AI holds significant promise in drug discovery, it brings forth several challenges and limitations that warrant consideration. Among these challenges, the availability of suitable data stands out as a primary concern. AI-driven approaches typically necessitate a substantial volume of data for effective training24. In numerous instances, the accessible data may be limited in quantity or marred by low quality and inconsistency, which can adversely impact result accuracy and reliability. Another formidable challenge arises from ethical considerations associated with AI applications, which may give rise to concerns regarding fairness and bias, as discussed in the subsequent section. For instance, if the data used to train machine learning algorithms exhibit bias or fail to represent the entire spectrum, the resulting predictions may be inaccurate or unjust. Ensuring ethical and equitable deployment of AI in the development of new therapeutic compounds emerges as a crucial imperative 27
Several strategies and approaches can be deployed to surmount the hurdles confronted by AI in the realm of chemical medicine. One such approach is data augmentation, involving the generation of synthetic data to supplement existing datasets. This serves to augment the quantity and diversity of training data for ML algorithms, thereby enhancing result accuracy and dependability. Another strategy is the adoption of explainable AI (XAI) methods, aiming to furnish interpretable and transparent rationales for the predictions made by ML algorithms. This approach aids in addressing concerns related to bias and fairness in AI-driven methodologies while promoting a deeper comprehension of the underlying mechanisms and assumptions behind these predictions 24.
It is essential to recognize that current AI-based approaches cannot substitute traditional experimental methods and the expertise of human researchers. AI can provide predictions based on available data, but the subsequent validation and interpretation of results necessitate human intervention. Nevertheless, the synergy between AI and traditional experimental methods has the potential to enhance the drug discovery process. By harnessing the predictive capabilities of AI in conjunction with the expertise and experience of human researchers, it becomes possible to optimize drug discovery and expedite the development of new pharmaceuticals 24 27.
C. Functional Genomics in AI
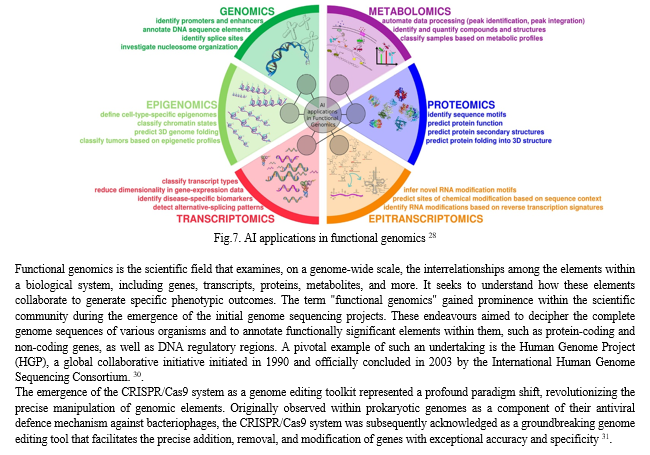
In recent decades, scientific development has shifted from evaluating individual genes or a small set of genes to investigating thousands of genes at the same time. Various scientific disciplines contribute to the generation of extensive biological data, each named based on its primary focus.
These disciplines encompass the study of an organism or system's DNA information content (genomics), the reversible modifications that DNA can undergo (epigenetics), the RNA transcripts produced from a genome (transcriptomics), the collection and dynamics of RNA modifications (epitranscriptomics), the translational products derived from protein-coding transcripts (proteomics), and the metabolites found in a specific organism or system under various physiological and pathological conditions at a given time 28.
In 1981, the inaugural full genome sequence of a eukaryotic organelle, specifically the human mitochondrion spanning 16.6 kilobases in length, was successfully deciphered. This evolution marks a shift from examining discrete units of inheritance to investigating an organism's complete genome. The field of genomics, originally focused on deciphering DNA sequences (the specific order of nucleotides within a DNA fragment), has swiftly broadened its scope. It now encompasses a more functional dimension, delving into the study of gene and protein expression profiles as well as their respective functions 29.
Machine Learning (ML) has gained extensive traction across various "omics" disciplines, particularly in fields marked by the generation of substantial datasets and intricate processes influenced by the collaborative engagement of diverse factors. Key applications encompass the prediction of DNA regulatory segments, elucidation of cellular morphology and spatial arrangements, discernment of links between phenotypic traits and genotypic makeup, categorization of DNA methylation and histone modifications, revelation of biomarkers, detection of transcriptional enhancers, facilitation of cancer diagnosis, and exploration of evolutionary mechanisms 28 29. (Fig.5)
In the contemporary landscape of biological research, marked by remarkable advancements in sequencing technologies and our deepening understanding of the intricate nature of DNA, this notion has been broadened to encompass the entire array of DNA sequences within a cell or organism. This expansion accounts for factors like the number of copies of the fundamental set of chromosomes, known as ploidy, and incorporates the genetic material derived from extra nuclear organelles like mitochondria 28.

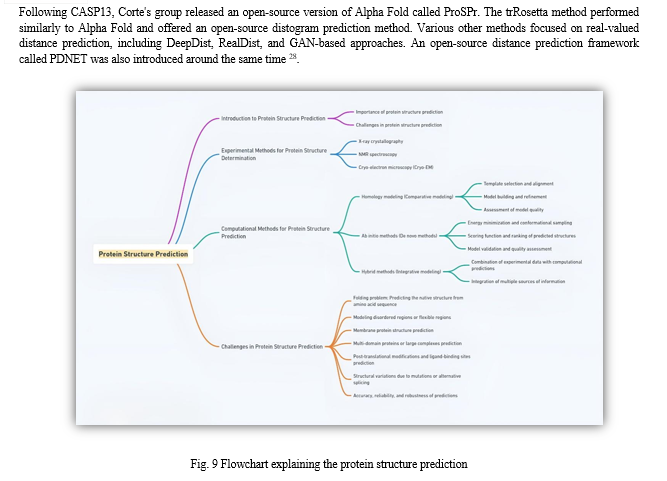
A. Alpha Fold Network for Prediction
Alpha Fold significantly enhances the precision of protein structure prediction through the incorporation of innovative neural network architectures and training methodologies rooted in the evolutionary, physical, and geometric constraints inherent in protein structures. Notably, this work introduces a novel architectural framework for the simultaneous integration of multiple sequence alignments (MSAs) and pairwise features. It also introduces a fresh output representation coupled with an associated loss function, which collectively facilitate the achievement of accurate end-to-end structure prediction. 33
B. Working of Alpha Fold Network
The Alpha Fold network conducts direct predictions of the 3D coordinates of all heavy atoms within a given protein by leveraging primary amino acid sequences and aligned sequences from homologous proteins as input data. The methodological intricacies pertaining to the inputs, encompassing database utilization, construction of multiple sequence alignments (MSAs), and template utilization, are detailed elsewhere. Notably, the core architecture and training procedures are provided in Supplementary Methods.
The Alpha Fold network operates through two primary stages. First, the network's core, referred to as the "trunk," processes the inputs through iterative layers of an innovative neural network module, denoted as the "Evoformer." This process yields two essential outputs: an Nseq × Nres array, which encapsulates the processed MSA, and an Nres × Nres array, representing residue pairs. The MSA representation is initially seeded with the raw MSA data, albeit with specific considerations for handling exceptionally deep MSAs, as elaborated in Supplementary Methods. The Evoformer blocks encompass both attention-based and non-attention-based components.
It is demonstrated in the "Interpreting the neural network" section that the Evoformer blocks give rise to a concrete structural hypothesis early in their processing and continually refine it. Notably, the Evoformer block introduces novel mechanisms for interchanging information within the MSA and pair representations, facilitating direct inference of spatial and evolutionary relationships 34
Following the trunk, the network includes a "structure module" that introduces an explicit 3D structure, incorporating rotation and translation parameters for each protein residue in the form of global rigid body frames 33. These representations are initialized in a rudimentary state, with all rotations set to identity and positions set to the origin. However, they swiftly evolve and refine, ultimately yielding a highly accurate protein structure replete with atomic precision. Key innovations within this segment of the network involve the breaking of chain structures to enable simultaneous local refinement of all structural components. Additionally, a novel equivariant transformer is introduced to allow implicit reasoning about unrepresented side-chain atoms, complemented by a loss term that accords significant importance to the orientational fidelity of residues.
Both within the structure module and across the entire network, the principle of iterative refinement is consistently reinforced. This involves the recurrent application of the final loss function to network outputs, followed by their recursive feeding back into the same network modules. This iterative refinement process, termed "recycling," akin to concepts employed in computer vision, significantly enhances predictive accuracy with only marginal additional training time 34
VI. BIOTECHNOLOGY AUTOMATION
Automation has consistently embodied technological advancements across various fields, including industrial biotechnology. The key feature of incorporating automation into production processes lies in the replacement of manual tasks with mechanized tools. These automated tools facilitate superior process control and enable rapid optimization with heightened accuracy. They offer several advantages, such as accelerating the pace of data generation while substantially reducing inconsistencies stemming from human errors. 35.
Automation enhances safety by eliminating human distractions, fatigue, and exposure to hazardous substances like carboxylic acids. It also reduces contamination risks in fermentation environments. On an industrial scale, automation simplifies regulatory compliance, improving quality assurance and reducing supply chain risks. 36.Biotechnology demands efficient production and innovation due to growing demand. Increased automation is essential, but it's lower compared to traditional sectors like automobiles. This is because biotech requires flexibility, individualized production, and sterility, along with strict safety regulations. Automation lowers production costs, boosts throughput, and ensures safety, particularly in high-wage countries. It also expedites the transition from research to clinical applications. 37.
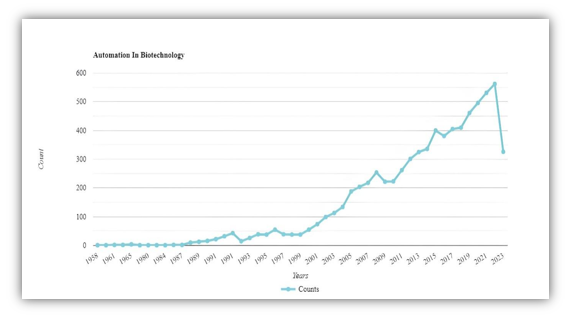
Conventional biological laboratories typically have equipment at automation level five, with static workstations designed for specific tasks such as centrifugation, PCR thermo cycling, or spectrophotometry. In biotechnology, some systems reach automation level seven, like fully automated facilities producing cell cultures such as StemCellFactory and StemCellDiscovery. These systems utilize robotic arms on linear axes for autonomous movement between bench top stations, performing reagent dispensing and handling. 35
To further increase automation, labs must interconnect existing equipment physically or digitally or acquire new devices capable of complex tasks. This presents a technical challenge due to integrating a variety of complex operations and device interfaces. Additionally, the presence of multiple equipment suppliers and limited standardization in software, lab ware, and consumables complicates this issue. 37 35
A. Benefits of Laboratory Automation
Automation improves reproducibility through three key mechanisms: reducing human-induced variability, increasing data generation speed, and decreasing contamination risk. Human-induced variability is common in research, arising from differences in how tasks are performed by individuals. Automation mitigates some of this variability by executing repetitive tasks consistently. It also accelerates data generation and allows for testing a wider range of variables, enhancing the chances of reproducibility. Additionally, automation minimizes contamination risks associated with human involvement and environmental exposure during manual handling. Efficiency in manufacturing means achieving a high production rate with fewer resources. Automation boosts production rates and reduces resource usage, leading to increased profits 37 35.

In research labs, automation enhances researchers' efficiency by producing more experiments without needing constant human intervention 35. It saves researchers time, allowing them to focus on other tasks and parallel experiments. Automation also supports quick adjustments and the "fail fast, fail often" approach in pharmaceutical development. Precise reagent dispensing reduces material usage, further improving efficiency. Automation plays a vital role in applied research labs developing therapies. Integrating automation early improves product quality, speeds up lab-scale to manufacturing transition, and accelerates commercialization for clinical use. 35
B. Limitation of Automation
While laboratory automation offers numerous benefits, there are notable limitations to consider. Incorrect implementation can lead to reduced efficiency and error propagation. Variability between automated systems and potential obsolescence pose challenges. Automation may also hinder innovation by limiting protocol alterations and could impact the workforce, particularly those focused on repetitive tasks. Additionally, both vendors and researchers should avoid exaggerating automation's capabilities, as it still requires careful operation, maintenance, and human input for experimental design and analysis. Acknowledging these limitations is essential for effective automation adoption and trust-building between commercial vendors and academic institutions. 35
VII. ETHICAL AND REGULATORY CONSIDERATION
The implementation of AI-driven explanation and fairness declarations within practical applications presents a multifaceted challenge. It necessitates a comprehensive examination that encompasses diverse factors, including the typology of explanations, the extent of fairness integration, and the contextual scenarios wherein AI-informed decision-making is employed. To optimize user confidence and foster the perception of equity in AI-influenced decision-making processes, it becomes imperative to tailor the specification of explanation formats and the degree of fairness articulation within the user interface of intelligent applications 38
Numerous pressing research questions in the AI field await exploration. Firstly, we must ensure that AI systems are developed and employed in ways aligning with ethical and societal standards, while safeguarding fundamental human rights and values. Second, it's imperative to ensure AI systems remain impartial, preventing the perpetuation or worsening of existing biases and discrimination. Furthermore, establishing transparency and explainability in AI systems is crucial to foster trust among users and stakeholders. Equally important is strengthening the security of AI systems to proactively mitigate risks to individuals and organizations. In addition, creating an inclusive environment for the development and operation of AI systems that welcomes diverse perspectives is vital.
Moreover, addressing the ethical and societal implications of emerging technologies like artificial general intelligence, machine learning, and autonomous systems is a complex challenge. Formulating and implementing effective policies, regulatory frameworks, and governance structures specific to AI is another key area of inquiry. To tackle these issues, promoting dialogue and collaboration among researchers, policymakers, industry stakeholders, civil society representatives, and other relevant actors is essential. Lastly, raising awareness and understanding of AI ethics, fairness, and trust among both the general public and those engaged in AI system design, development, and utilization is of utmost importance. 1
User trust over fairness and explanations graph

VIII. EMERGING TRENDS AND FUTURE DIRECTION
The future directions of AI and ML in biotechnology are highly promising, with these transformative technologies poised to revolutionize various aspects of the biotech industry. These advancements leverage the capabilities of AI and ML algorithms to process vast datasets, recognize intricate patterns, and provide predictions that surpass human capacity for manual analysis. This technological synergy is propelling biotechnology research into new realms, particularly in drug discovery, personalized medicine, and beyond.
In the realm of drug discovery, AI and ML are on the brink of a breakthrough. These technologies facilitate the identification of novel drug targets, the creation of innovative pharmaceutical compounds, and the forecasting of drug interactions within the human body. This expedites drug development, significantly enhancing its efficiency and effectiveness.11
Another compelling frontier for AI and ML in biotechnology lies in personalized medicine. By scrutinizing patient data, AI and ML can pinpoint biomarkers crucial for predicting individual responses to diverse treatments. This personalization ensures that patients receive treatments tailored to their unique needs, optimizing therapeutic outcomes.8
Moreover, AI and ML are extending their influence into various other biotechnological domains, including gene editing. These technologies contribute to the design of more precise and efficient gene-editing tools, revolutionizing genetic research. In agricultural biotechnology, AI and ML are instrumental in developing crop varieties resilient to pests and diseases, promising to enhance global food security. Furthermore, in the realm of biomaterials, AI and ML-driven design processes are yielding innovative biomaterials with superior properties, suitable for use in medical devices and an array of applications.
In summary, AI and ML are ushering in an exciting era for biotechnology, driving progress in drug discovery, personalized medicine, gene editing, agricultural biotechnology, and biomaterials. These technologies hold the potential to reshape the industry, offering more efficient and tailored solutions to longstanding challenges.
Conclusion
The journey of AI and ML in life sciences has been marked by remarkable milestones, from early attempts at expert systems to the current sophisticated deep learning models. These technologies have revolutionized functional genomics by enabling the analysis of vast genomic datasets, deciphering genetic variations, and identifying potential therapeutic targets. In drug discovery and development, AI and ML have accelerated the identification of novel drug candidates, reduced development timelines, and enhanced precision medicine approaches. The automation of biotechnological processes has been streamlined through AI-driven robotics intelligent systems and reproducibility. Moreover, the prediction of protein structures has been greatly enhanced, opening doors to a deeper understanding of biological mechanisms and facilitating the design of novel proteins with diverse applications. The potential for personalized medicine and the discovery of new biomarkers will continue to push the boundaries of what is possible in the life sciences. Collaboration between interdisciplinary experts, including biologists, data scientists, and computational engineers, will be pivotal in harnessing the full potential of AI and ML in biotechnology. The future holds immense promise, with these technologies poised to drive groundbreaking discoveries, improve patient outcomes, and address some of the most pressing global health challenges
References
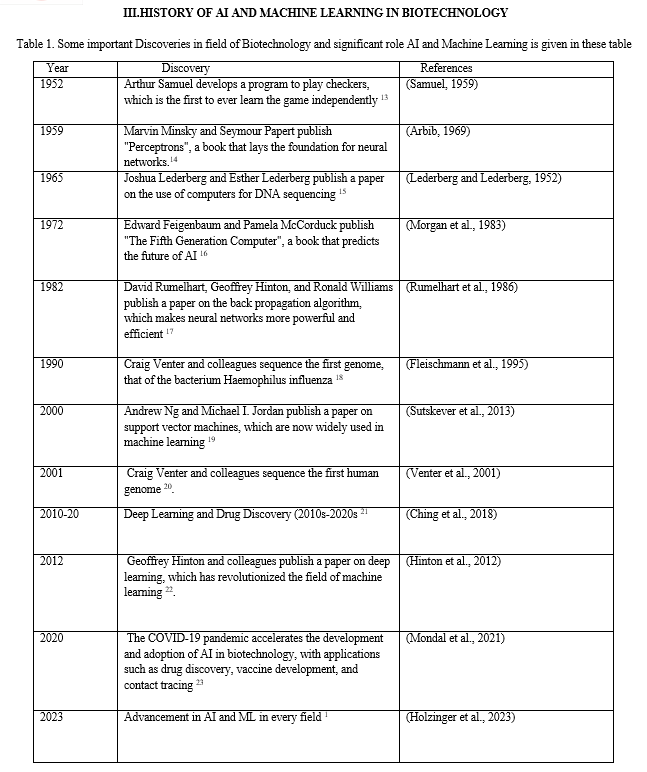
[1] Holzinger, A., Keiblinger, K., Holub, P., Zatloukal, K. & Müller, H. AI for life: Trends in artificial intelligence for biotechnology. N. Biotechnol. 74, 16–24 (2023). [2] Xiao, Q. et al. High-throughput proteomics and AI for cancer biomarker discovery. Adv. Drug Deliv. Rev. 176, 113844 (2021). [3] Hassoun, S. et al. Artificial Intelligence for Biology. Integr. Comp. Biol. 61, 2267–2275 (2021). [4] Tang, A. et al. Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology. Can. Assoc. Radiol. J. 69, 120–135 (2018). [5] Greener, J. G., Kandathil, S. M., Moffat, L. & Jones, D. T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 23, 40–55 (2022). [6] Al’Aref, S. J. et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur. Heart J. 40, 1975–1986 (2019). [7] Studies, C. PHYSIOLOGICAL PSYCHOLOGYl. (1950). [8] Choi, R. Y., Coyner, A. S., Kalpathy-Cramer, J., Chiang, M. F. & Peter Campbell, J. Introduction to machine learning, neural networks, and deep learning. Transl. Vis. Sci. Technol. 9, 1–12 (2020). [9] McBee, M. P. et al. Deep Learning in Radiology. Acad. Radiol. 25, 1472–1480 (2018). [10] Currie, G., Hawk, K. E., Rohren, E., Vial, A. & Klein, R. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J. Med. Imaging Radiat. Sci. 50, 477–487 (2019). [11] Lecun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015). [12] Nguyen, A., Yosinski, J. & Clune, J. Deep neural networks are easily fooled: High confidence predictions for unrecognizable images. Proc. IEEE Comput. Soc. Conf. Comput. Vis. Pattern Recognit. 07-12-June, 427–436 (2015). [13] Samuel, A. L. Eight-move opening utilizing generalization learning. (See Appendix B, Game G-43.1 Some Studies in Machine Learning Using the Game of Checkers. IBM J. 210–229 (1959). [14] Engelhart, M. D. & Moughamian, H. Book Reviews?: Book Reviews. Educ. Psychol. Meas. 28, 619–620 (1969). [15] LEDERBERG, J. & LEDERBERG, E. M. Replica plating and indirect selection of bacterial mutants. J. Bacteriol. 63, 399–406 (1952). [16] Morgan, M. G. & Wynne, B. Rationality and Ritual: The Windscale Inquiry and Nuclear Decisions in Britain. J. Policy Anal. Manag. 3, 156 (1983). [17] Rumelhart, D. E. & Hintont, G. E. Learning Representations by Back-Propagating Errors. Cogn. Model. 3–6 (2019) doi:10.7551/mitpress/1888.003.0013. [18] Fleischmann, R. D. et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science (80-. ). 269, 496–512 (1995). [19] Sutskever, I., Martens, J., Dahl, G. & Hinton, G. momentum?Nesterov accelerate?On the importance of initialization and momentum in deep learning. J. Mach. Learn. Res. 28, 1139–1147 (2013). [20] Craig Venter, J. et al. The sequence of the human genome. Science (80-. ). 291, 1304–1351 (2001). [21] Ching, T. et al. Opportunities and obstacles for deep learning in biology and medicine. Journal of the Royal Society Interface vol. 15 (2018). [22] Hinton, G. E., Srivastava, N., Krizhevsky, A., Sutskever, I. & Salakhutdinov, R. R. Improving neural networks by preventing co-adaptation of feature detectors. 1–18 (2012). [23] Mondal, M. R. H., Bharati, S. & Podder, P. Diagnosis of COVID-19 Using Machine Learning and Deep Learning: A Review. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 17, 1403–1418 (2021). [24] Blanco-González, A. et al. The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies. Pharmaceuticals 16, 891 (2023). [25] Liu, Z. et al. AI-based language models powering drug discovery and development. Drug Discov. Today 26, 2593–2607 (2021). [26] Giorgi, J. M. & Bader, G. D. Towards reliable named entity recognition in the biomedical domain. Bioinformatics 36, 280–286 (2020). [27] Tsuji, S. et al. Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for Alzheimer’s disease. Alzheimer’s Res. Ther. 13, 1–15 (2021). [28] Caudai, C. et al. AI applications in functional genomics. Comput. Struct. Biotechnol. J. 19, 5762–5790 (2021). [29] Lesk, A. CHAPTER 1. Introduction to genomics. Introd. To Genomics 823, 3–20 (2017). [30] Abdellah, Z. et al. Finishing the euchromatic sequence of the human genome. Nature 431, 931–945 (2004). [31] Bak, R. O., Gomez-Ospina, N. & Porteus, M. H. Gene Editing on Center Stage. Trends Genet. 34, 600–611 (2018). [32] Pakhrin, S. C., Shrestha, B., Adhikari, B. & Kc, D. B. Deep learning-based advances in protein structure prediction. Int. J. Mol. Sci. 22, (2021). [33] Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [34] Imaging, N. & Angeles, L. Auto-context and Its Application to High-level Vision Tasks and 3D Brain Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 32, 1744–1757 (2010). [35] Holland, I. & Davies, J. A. Automation in the Life Science Research Laboratory. Front. Bioeng. Biotechnol. 8, 1–18 (2020). [36] Wainaina, S. & Taherzadeh, M. J. Automation and artificial intelligence in filamentous fungi-based bioprocesses: A review. Bioresour. Technol. 369, 128421 (2023). [37] Solutions, E. & Developments, O. Existing Solutions and Ongoing Developments. (2021). [38] Angerschmid, A., Zhou, J., Theuermann, K., Chen, F. & Holzinger, A. Fairness and Explanation in AI-Informed Decision Making. Mach. Learn. Knowl. Extr. 4, 556–579 (2022).
Copyright
Copyright © 2024 Vijay P. Thikare, Prutha S. Pathak. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET58430
Publish Date : 2024-02-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

