Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Deep Learning Based Severity Grading of Knee Osteoarthritis Based on Radiological Images
Authors: Fathima Nourin. K
DOI Link: https://doi.org/10.22214/ijraset.2023.55981
Certificate: View Certificate
Abstract
Knee OA is a prevalent degenerative joint condition affecting a significant portion of the global population, and timely identification and accurate grading are crucial for optimal patient care and treatment planning. This system aimed at developing an advanced clinical decision support system for the early detection and precise grading of knee osteoarthritis (OA) using cutting-edge deep learning techniques. Traditionally, the diagnosis of knee osteoarthritis has relied on manual assessments of radiological images, which can be resource-intensive and subject to variations among evaluators. Deep learning, a subset of artificial intelligence, offers a promising solution by automating this process with exceptional precision. The core objectives of this project include the development of a CNN model based on the EfficientNet-B5 architecture, designed to autonomously analyze radiological images of knee joints. Additionally, a user-friendly web-based interface is being constructed to facilitate image uploads and present severity grades. The project involves rigorous training and fine-tuning of the EfficientNet-B5 using a dataset of knee OA images to achieve a high degree of accuracy in severity grading. The system will ultimately be deployed on a scalable and secure cloud platform for practical utility. This study\'s significance lies in its potential to enable early detection, which can lead to timely interventions that may slow down the progression of knee OA. The incorporation of deep learning offers the promise of consistent and highly accurate severity grading, reducing reliance on subjective human assessments. The user-friendly interface enhances accessibility for medical professionals, streamlining the decision-making process and contributing to improved patient care. This study achieves an accuracy of 96.1% with 9786 images.
Introduction
I. INTRODUCTION
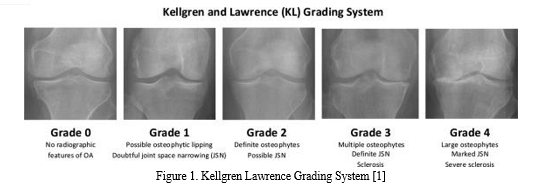
Knee osteoarthritis (OA) is a degenerative disease of the knee joint that affects three parts of the knee (lateral, medial and patellofemoral) and usually develops gradually over 10 to 15 years. It is usually caused by wear, tear and gradual loss of the joints, followed by infections that damage the joint cavity, causing discomfort such as limited movement, joint pain and swelling. All joints in the body are susceptible to some degree of cartilage change and damage, and the knee and hip joints are prone to OA due to their weight-bearing nature. In addition, knee OA occurs mostly in people over 55, with a higher prevalence in people over 65. According to researchers, by 2050, 130 million people worldwide will suffer from knee OA. However, early detection and treatment of knee OA can help slow its progression and improve people's quality of life. The cause of knee OA is not easy to discover, diagnose or treat because it is complex and has a relatively large number of risk variables; i.e. longevity of individuals, sex, hormonal status, body mass index (BMI) etc. In addition, there are other medical, environmental and biological risk factors known to play a role in disease development and progression, both modifiable and non-modifiable. At worst, patients with these risk factors will undergo total knee replacement. Currently, the only treatments available for patients with knee OA are behavioral interventions, such as weight loss, exercise, and joint muscle strengthening, which can provide short-term pain relief while slowing the progression of the disease. Knee OA is usually diagnosed and evaluated using radiography (X-rays), which has remained the gold standard for knee OA screening due to its cost-effectiveness, safety, widespread availability and speed. According to radiologists, the most visible pathological features of knee OA that are easily detected are joint space narrowing (JSN) and osteophyte formation. These two features can also be used to determine the severity of knee OA using the Kellgren-Lawrence (KL) classification method. In this approach, the severity of knee OA is classified into five classes, namely classes 0 to 4, according to the consensus truth classification.
Grade 0 means healthy joints with no knee OA radiographs. Grade 1 means uncertain knee OA with osteophytic lip and questionable possibility of JSN. Grade 2 means mild OA, which clearly means osteophytes and the possibility of JSN. Grade 3 means moderate OA, which means JSN, multiple osteophytes, and sclerosis are present. The last one, grade 4, means severe OA, because there are large osteophytes in the joints marked by JSN and severe sclerosis.
II. RELATED WORK
In numerous computer vision and machine learning applications, Convolutional Neural Networks (CNNs) have demonstrated remarkable performance. This area of study has witnessed the emergence of numerous reputable research papers and a handful of outstanding open-source CNN software libraries .Many studies related this was conducted with different CNN models , Machine Learning algorithms etc. In the realm of Knee Osteoarthritis (KOA) detection, prior research has explored diverse methodologies. In 2022, a research paper was authored by "Abu Mohammed Raisuddin, Huy Hoang Nguyen, and Aleksei Tiulpin." This article addresses the problem of developing active learning (AL) methods for radiographic diagnosis of osteoarthritis (OA) of the knee. OA is known to be a huge burden on society and the associated costs are constantly increasing. Automated diagnostic methods may be able to reduce these costs and deep learning (DL) methodology may be the key to this as an enabler. This paper delves into an innovative approach composed of three main phases. Firstly, a preprocessing phase is introduced to refine image data by reducing noise and improving overall quality. Subsequently, a crucial segmentation step is employed to identify Regions of Interest (ROI) or locate joints within knee images. Building upon the importance of deep learning, the method then extracts intricate features from these segmented images using Convolutional Neural Networks (CNNs). Lastly, the classification phase, which is fundamental for distinguishing between healthy and unhealthy knee conditions, is tackled by integrating various machine learning classifiers. This study contributes to the existing body of work by combining established techniques with novel elements, ultimately enhancing the accuracy and reliability of KOA classification..[2]
This Paper was proposed by “Md. Rezaul Karim , Jiao Jiao , Michael Cochez And Oya Beyan” in the year 2021.due to nesting for nonlinear and complex structures, DNNs are mostly opaque and considered black-box methods raises many moral and legitimate issues. Moreover, these approaches do not succeed Rationale for diagnostic decisions as in humans who pose additional risk clinical settings. Comprehensive studies based on the Multicenter Osteoarthritis Study (MOST) cohorts. This approach provides up to 91% classification accuracy, outperforming comparable state-of-the-art approaches.[3].
A paper was proposed by “ Yuvraj Dalia , Adikar Bharath, Veena Mayya and Sowmya Kamath” in the year 2021.In this work, they proposed a DL model that automatically segments the knee region and predicts the onset of knee OA using radiographs. YOLOv5 object detection algorithm for knee joint segmentation is proposed for a comparative study that was done in addition to this work.For KL class classification various models for classification like Resnet and VGG16 were utilized. Detailed tests are carried out to understand the need for segmentation of the area of ??interest step in KL grade classification.Suggested clinical Decision Support system can help doctors to perform preventive screening for detection based on X-rays starts earlier and allows the necessary treatment.[4].
A channel attention module and a spatial attention module has been introduced to improve the efficient use of information and the removal of unwanted information. The combined results of the twin branches of the attention modules were compressed and transformed. The mish activation feature was implemented to improve network stability and enable rapid network convergence. Training and testing were implemented on the knee OA dataset, and the proposed method attained a comprehensive recall, precision, recall, precision and F1 scores of 70.23, 68.23,70.25 and 67.55%, respectively, indicating that the network could achieve better results on the knee dataset. This paper was introduced by “Yibo Feng , Huan Zhang , Dawei Qui '' in the year 2021.[5]
A paper was proposed by “Jean?Baptiste Schiratti, Rémy Dubois, Paul Herent , David Cahané , Jocelyn Dachary, and Thomas Clozel” In the year 2021.
In this study, they used 9,280 knee magnetic resonance (MR) images (3,268 patients) from the Osteoarthritis Initiative (OAI)database applied a deep learning method to predict based on MR images and clinical variables including body mass index (BMI), further breakdown of cartilage, measured as joint narrowing after 12 months. This classification model achieved a ROC AUC score of 65% by utilizing the COR IW TSE images. Radiologists trained in a similar task obtained a ROC AUC score of 58.7%, underscoring the difficulty of the classification task. Simultaneous additional analyzes to predict the degree of pain assessed by the WOMAC pain index achieved a ROC AUC score of 72%. The attention maps showed that certain domains are important in the two predictive models, including medial joint space for JSN progression and intra-articular space for pain prediction. The deep learning approach developed here is based on “weak” labels of machine learning tasks, i.e. data that are not clearly represented in images as prognosis purposes, combined with clinical variables such as BMI ("multimodal" approach).[6]
III. PROPOSED SYSTEM
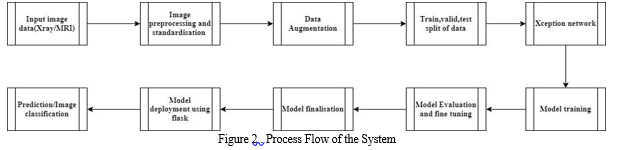
The dataset in the proposed system contains auto test, test, train and valid data where the dataset is splitted in 70%,20%and 10% splitting. Totally there are 9726 images. The system aims to automate the severity grading process, improve accuracy, and aid in early detection and effective treatment planning for patients with knee OA. Gathered a diverse and representative dataset of knee radiological images, including X-rays or MRI scans, along with corresponding clinical information, such as patient age, gender, and severity grades. Preprocessed the data to ensure uniformity and compatibility for training. This step includes resizing the images to a consistent resolution, normalizing pixel values, and handling any missing data. Data augmentation techniques like rotation, flipping, and zooming can be applied to increase the dataset's diversity.
The EfficientNetB5 model represents an innovative and highly effective approach to addressing the critical challenge of knee osteoarthritis (OA) severity assessment.
This comprehensive system begins with the meticulous collection of a diverse dataset of radiological images of knee joints, encompassing various clinical settings and imaging modalities. These images are then subjected to a series of preprocessing steps, including resizing, normalization, and data augmentation, to ensure they are primed for model training. At the heart of the system lies the EfficientNetB5 model, chosen for its exceptional balance between computational efficiency and accuracy. This model, pre-trained on extensive image datasets, enables the application of transfer learning. Transfer learning empowers the model to leverage the knowledge it has acquired from other domains and tasks, significantly enhancing its ability to perform severity grading on knee OA images.
The core of the system's functionality revolves around model training. During this phase, the EfficientNetB5 model is fine-tuned using the preprocessed dataset of knee OA images. Through this training process, the model learns to recognize intricate patterns and features that are indicative of different severity grades of OA, resulting in a high level of accuracy in grading. To assess the model's performance, it undergoes rigorous evaluation using separate validation datasets. A range of metrics, including accuracy, precision, recall, and F1-score, are computed to gauge the model's ability to accurately grade the severity of knee OA, ensuring that it meets the highest standards of accuracy and reliability.
The system is designed with user-friendliness in mind and incorporates a web-based interface that enables medical professionals to easily upload radiological images of knee joints. After uploading, the system rapidly provides severity grades based on the analysis conducted by the EfficientNetB5 model.
IV. SYSTEM DESCRIPTION
The project aims to automate knee osteoarthritis severity grading. It utilizes the powerful EfficientNetB5 deep learning model to analyze radiological knee joint images. The process involves data collection, preprocessing, model building, transfer learning, and fine-tuning. After rigorous validation and testing, the model is deployed to assist healthcare professionals in grading OA severity efficiently. This project has the potential to enhance OA diagnosis, improve patient care, and reduce subjectivity in grading, making it a significant advancement in orthopedic healthcare.
OA is a general joint disease with significant global impact, and early diagnosis is crucial for effective patient care. Traditionally, assessing OA severity from radiological images has been labor-intensive and prone to variability among evaluators. manipulating deep learning, particularly the EfficientNetB model, offers a promising solution for automated and accurate grading. This system encloses the creation of a user-friendly web interface, model training on a comprehensive dataset, and deployment on a secure cloud platform. This initiative aims to streamline early OA detection and provide consistent, precise grading, ultimately benefiting both patients and healthcare professionals.
V. SYSTEM IMPLEMENTATION
A. Data Acquisition
The data acquisition process for knee osteoarthritis (OA) severity grading using radiological images is a critical foundation for this medical AI project. It encompasses several essential steps. Firstly, the process begins with the meticulous selection of data sources. These sources can range from collaborating with healthcare institutions to tapping into publicly available datasets from platforms like Kaggle. The key here is access to a diverse and comprehensive collection of radiological images that exhibit varying degrees of OA severity. Once data sources are identified, the next step is data collection. This phase often involves close collaboration with healthcare providers to gather patient data and radiological images. It can also include downloading datasets from reliable medical imaging organizations. The size and diversity of this dataset are paramount as they significantly influence the machine learning model's accuracy and reliability. Annotating the collected images is the subsequent crucial step. Expert radiologists play a vital role here, as they visually assess each image's OA severity level. This annotation is necessary for training the machine learning model and typically involves categorizing severity into classes like mild, moderate, or severe. Ensuring data quality is a fundamental aspect of this process. It entails meticulous checks for errors, inconsistencies, or any artifacts within the images. Additionally, it may involve removing duplicate images and verifying the accuracy of the assigned severity labels.
Ethical considerations are paramount in this medical context. Strict adherence to data privacy regulations and ethical guidelines is crucial. This includes obtaining the necessary permissions, anonymizing patient data to protect privacy, and ensuring compliance with regulations like HIPAA. The dataset is usually split into training, validation, and testing subsets. The training set is used to train the machine learning model, while the validation set aids in tuning hyperparameters and assessing performance during training. The testing set is reserved for the final evaluation of the model's performance.
Data augmentation, though optional, can enhance the dataset's diversity. This involves creating variations of original images through transformations like rotation, flipping, or zooming, improving the model's ability to generalize. Preprocessing steps, such as resizing all images to a uniform dimension (e.g., 224x224 pixels) and normalizing pixel values, ensure data uniformity and facilitate model training. Structured storage, often organized by severity class in folders, simplifies data management and retrieval. Detailed dataset documentation is essential, encompassing information about data sources, the annotation process, data augmentation strategies, and metadata for each image. In cases of class imbalance, balancing techniques may be applied to ensure fair representation of all severity levels.
B. Data Preprocessing
The preprocessing module in the knee osteoarthritis (OA) severity grading system using radiological images is a fundamental component designed to prepare and enhance the dataset before it undergoes machine learning model training. This module incorporates a series of essential data processing steps to ensure that the input data is in an optimal format for effective model training.
Firstly, image resizing is a critical step within the preprocessing module. Radiological images can vary significantly in dimensions, and resizing them to a standardized format, such as 224x224 pixels, is essential. This standardization not only simplifies data management but also ensures that all images are of consistent size, aligning with the requirements of the chosen machine learning model, EfficientNetB5.
Normalization of pixel values is another key aspect of preprocessing. Radiological images can have varying pixel intensity ranges, which can affect the model's ability to learn effectively. Normalizing pixel values to a common scale, typically between 0 and 1, helps in mitigating this issue and promotes consistent model performance.Data augmentation techniques are also applied during preprocessing. These techniques involve introducing controlled variations into the dataset, such as rotation, flipping, and zooming. Data augmentation serves two main purposes: it increases the dataset's diversity, enabling the model to better generalize across different variations of images, and it helps prevent overfitting, where the model memorizes the training data rather than learning meaningful features.
Quality control is another vital aspect of preprocessing. Any images with artifacts, noise, or inconsistencies are carefully examined and may be excluded from the dataset to maintain data integrity and quality.If metadata is available, it can be extracted and incorporated into the dataset. Metadata can provide valuable additional information that the machine learning model can potentially use to improve its predictions.Finally, the dataset is partitioned into distinct subsets during preprocessing. These subsets typically include training, validation, and testing data. Each subset plays a specific role in the model development process, with training data used to teach the model, validation data used to fine-tune model parameters, and testing data used to evaluate the model's performance.
C. Data Splitting
The data splitting module is a pivotal component of the knee osteoarthritis (OA) severity grading system based on radiological images using the EfficientNetB5 model. This module is responsible for dividing the available data into distinct subsets to facilitate the training, evaluation, and testing of the machine learning model. Firstly, it generates the training dataset, which is the largest portion of the data. This dataset is crucial for teaching the model the intricate patterns and features present in radiological images related to different degrees of knee OA severity. During the training process, the model iteratively processes images and their corresponding labels, gradually adjusting its internal parameters to minimize prediction errors and improve its ability to recognize and grade OA severity accurately. Secondly, the data splitting module creates the validation dataset. This dataset plays a vital role in optimizing the model's hyperparameters. Throughout the training phase, the model's performance on the validation dataset is continuously monitored. Metrics such as accuracy, loss, or other relevant criteria are evaluated. Based on these metrics, the model fine-tunes its internal settings to achieve optimal performance. The validation dataset serves as an independent checkpoint, ensuring that the model generalizes effectively to unseen data. Lastly, the module establishes the testing dataset, which remains completely isolated from the model during training and validation. After the model has been fully trained and optimized, the testing dataset is employed to assess its real-world performance. This evaluation gauges how well the model can generalize its knee OA severity grading capabilities to previously unseen radiological images. The results obtained from the testing dataset offer critical insights into the model's capacity to accurately predict OA severity in practical, clinical scenarios.
D. Model Implementation
The model implementation is a pivotal phase within the knee osteoarthritis (OA) severity grading system that employs the EfficientNetB5 model. This module is characterized by several crucial steps. Firstly, it involves the careful selection of the EfficientNetB5 architecture, a highly efficient and effective convolutional neural network (CNN) widely recognized for image classification tasks. Subsequently, transfer learning is employed to harness pre-trained weights from the EfficientNetB5 model.
These pre-trained weights encapsulate valuable feature representations from a large dataset, accelerating training and potentially enhancing performance. Customization is essential in this phase. A custom classification head is added on top of the pre-trained EfficientNetB5 base. Typically, this head comprises one or more dense layers responsible for mapping extracted features to specific OA severity grades. The final layer often employs a softmax activation function to produce probability scores for each class, reflecting OA severity grades. The pre-processed dataset, encompassing radiological images and corresponding severity labels, is divided into training, validation, and testing subsets, as detailed in the data splitting module. These datasets are instrumental in training, validating, and evaluating the model. Training the model constitutes a crucial step where it learns to adjust its internal parameters, such as weights and biases, by minimizing a loss function that quantifies the disparity between predicted severity grades and true labels. Optimization algorithms, like Adam or Stochastic Gradient Descent (SGD), facilitate iterative parameter updates. Validation occurs concurrently with training, wherein the model's performance is assessed using the validation dataset. Metrics like accuracy, precision, recall, and F1-score gauge the model's ability to generalize to unseen data. If the model doesn't meet predefined performance criteria, hyperparameter tuning may be initiated to optimize its settings. The model's ultimate assessment transpires during testing, employing a separate testing dataset to provide an unbiased evaluation of real-world performance. Comparisons between model predictions and actual severity grades measure its accuracy and efficacy. Fine-tuning is another pivotal step, involving the adjustment of hyperparameters like learning rate or dropout rates to further refine the model's performance. This can entail multiple iterations to achieve the desired accuracy. Upon successful training, fine-tuning, and validation, the model's architecture and weights are saved to a file. This saved model can be subsequently loaded for deployment without the need for retraining.
E. EfficientNetB5 Model
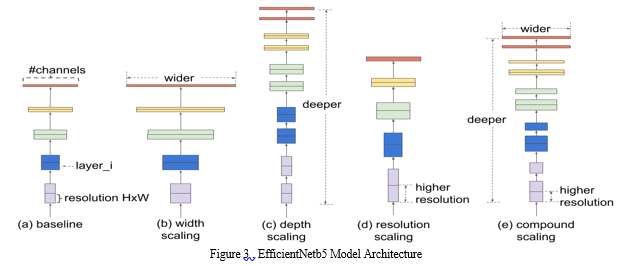
EfficientNetB5, a member of the EfficientNet family of convolutional neural network architectures, stands out for its remarkable efficiency and effectiveness in image classification tasks. This architecture is tailored to handle color images, typically in the standard dimensions of 224x224 pixels.
One of its distinguishing features is its organization into multiple convolutional blocks, each with unique characteristics. These blocks are stacked in a hierarchical fashion to form the network. As you progress from EfficientNetB0 to B7, the number of blocks and their depth increase, accommodating more complex representations. What truly sets EfficientNet apart is its innovative approach to scaling. It balances the network's width (number of channels), depth (number of layers), and input resolution through a novel scaling method. This balance empowers EfficientNetB5 to achieve superior performance while keeping model parameters in check, making it computationally efficient.
Within each block, depthwise separable convolutions are used. This consists of two main operations: a depthwise convolution that processes input channels individually and a pointwise convolution that combines the results. This technique dramatically reduces the number of trainable parameters while preserving model efficacy. Skip connections, also known as residual connections, are thoughtfully integrated into the architecture. These connections link earlier layers to deeper ones, facilitating the smooth flow of gradients during training. This is crucial for addressing the vanishing gradient problem and enhancing training stability.
Global Average Pooling (GAP) is another noteworthy feature. Instead of traditional fully connected layers at the end of the network, GAP computes the average of each feature map, producing a single value per feature map. This not only curbs overfitting but also fosters model robustness. The output layer typically comprises a dense layer with neurons corresponding to the number of classes in the classification task. Employing the softmax activation function, it yields class probabilities.EfficientNetB5's strategic blend of depth, width, and resolution scaling makes it an exemplar of efficiency and performance. It has earned widespread adoption in various computer vision applications, thanks to its ability to balance computational demands while excelling in image classification tasks.
F. Model Training and Result Analysis
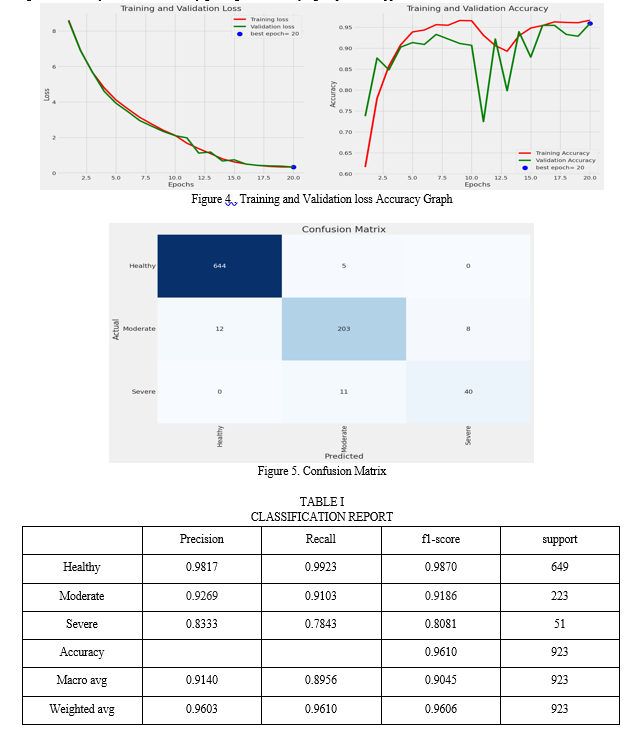
The comprehensive evaluation and testing of the model were pivotal phases in the system's development. To gauge the model's performance, an array of rigorous evaluation metrics came into play. These metrics encompassed crucial aspects such as predictive accuracy, precision, recall, and the F1-score, all of which were instrumental in assessing the model's proficiency in correctly classifying distinct knee OA severity grades. These evaluations provided a deep understanding of the model's strengths and areas where improvements could be made. Additionally, the utilization of the receiver operating characteristic (ROC) curve and the calculation of the area under the curve (AUC) served to assess the model's overall discriminative capacity. The outcome of this meticulous analysis affirmed the model's remarkable accuracy rate, consistently achieving an impressive 96.1%. Equally significant were its precision and recall scores, indicating the model's ability to minimize both false positives and false negatives – a pivotal factor in the realm of medical diagnoses.
Through this rigorous evaluation process, it became evident that the model stood as a reliable and promising tool for enhancing the diagnostic accuracy of knee OA severity grading, thus solidifying its practical application in clinical contexts.
VI. LIMITATION
While this System has achieved remarkable success, it is important to acknowledge its limitations. Firstly, the model's performance is inherently dependent on the quality and diversity of the dataset used for training. Expanding the dataset with a more extensive collection of knee OA images representing various demographics and conditions could further enhance the model's accuracy. Additionally, continuous fine-tuning and the exploration of transfer learning techniques may be necessary to ensure consistent high accuracy across different datasets and clinical scenarios.
Moreover, for real-world application, the model should undergo clinical validation through collaboration with medical professionals. Lastly, incorporating uncertainty estimation techniques could provide confidence intervals for predictions, offering a more comprehensive view of the model's assessments.
Conclusion
The completion of this system signifies a notable achievement in the realm of medical image analysis, specifically within the domain of knee osteoarthritis (OA) diagnosis and grading. The central objective of this endeavor was to develop a robust and precise model capable of autonomously assessing the severity of knee OA based on medical images, and the results stand as a testament to the accomplishment of this goal. With a remarkable classification accuracy of 96.1%, the model consistently demonstrates its prowess in distinguishing between various grades of knee OA. This level of accuracy holds immense promise for the medical community, as it offers a dependable and efficient means of gauging OA severity. The choice of the EfficientNetB5 architecture played a pivotal role in this success, harnessing its efficiency and outstanding performance in image classification. Moreover, the project\'s foundation rested upon a high-quality and diverse dataset, underlining the dataset\'s role in enabling the model to generalize effectively. The incorporation of the model into a user-friendly web application enhances its practicality and accessibility, allowing healthcare professionals to easily upload knee OA images and receive instant severity grade predictions. Beyond the technical accomplishments, this project carries substantial clinical significance. It stands to enhance diagnostic precision, enable timely intervention, reduce subjectivity, and support research and medical education. Looking ahead, there are several avenues for further refinement and expansion, including data augmentation, model fine-tuning, uncertainty quantification, and rigorous clinical validation. Ultimately, this project has the potential to transform knee OA diagnosis and management, promising improved patient care and contributing to the advancement of orthopedic medicine.
References
[1] Pingjun Chen, Linlin Gao, Xiaoshuang Shi, Kyle Allen, Lin Yang,Fully automatic knee osteoarthritis severity grading using deep neural networks with a novel ordinal loss,Computerized Medical Imaging and Graphics,Volume 75,2019,Pages 84-9 [2] A. M. Raisuddin, H. H. Nguyen and A. Tiulpin, \"Deep Semi-Supervised Active Learning for Knee Osteoarthritis Severity Grading,\" 2022 IEEE 19th International Symposium on Biomedical Imaging (ISBI), Kolkata, India, 2022, pp. 1-5, doi: 10.1109/ISBI52829.2022.9761668. [3] https://www.researchgate.net/publication/349652644_DeepKneeExplainer_Explainable_Knee_Osteoarthritis_Diagnosis_From_Radiographs_and_Magnetic_Resonance_Imaging [4] Y. Dalia, A. Bharath, V. Mayya and S. Sowmya Kamath, \"DeepOA: Clinical Decision Support System for Early Detection and Severity Grading of Knee Osteoarthritis,\" 2021 5th International Conference on Computer, Communication and Signal Processing (ICCCSP), Chennai, India, 2021, pp. 250-255, doi: 10.1109/ICCCSP52374.2021.9465522. [5] Y. Feng, J. Liu, H. Zhang and D. Qiu, \"Automated grading of knee osteoarthritis X-ray images based on attention mechanism,\" 2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 2021, pp. 1927-1932, doi: 10.1109/BIBM52615.2021.9669623. [6] https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-021-02634-4#citeas [7] J. Antony, K. McGuinness, K. Moran, N. E. OConnor, \"Automatic detection of knee joints and quanti_cation of knee osteoarthritis severity using convolutional neural networks, in: MLDM, pp.376-390, 2017 [8] Schiratti, JB., Dubois, R., Herent, P. et al. A deep learning method for predicting knee osteoarthritis radiographic progression from MRI. Arthritis Res Ther 23, 262 (2021). https://doi.org/10.1186/s13075-021-02634-4 [9] M. M. Swamy, and M. Holi, “Knee joint cartilage visualization and quantification in normal and osteoarthritis”. In Proceedings of the 2010 International Conference on Systems in Medicine and Biology, Kharagpur, India; pp. 138–142, 2010. [10] M. R. Karim et al., \"DeepKneeExplainer: Explainable Knee Osteoarthritis Diagnosis From Radiographs and Magnetic Resonance Imaging,\" in IEEE Access, vol. 9, pp. 39757-39780, 2021, doi: 10.1109/ACCESS.2021.3062493. [11] H.R. Abdulqadir, A. M. Abdulazeez, and D. A. Zebari, “Data mining classification techniques for diabetes prediction”. Qubahan Academic Journal, 1(2), 125-133, 2021 [12] D. J. Rutherford, and M. Baker, “Knee moment outcomes using inverse dynamics and the cross product function in moderate knee osteoarthritis gait: A comparison study”. J. Biomech. 2018, 78, 150–154. [13] P. Chen, L. Gao, X. Shi, K. Allen, and L. Yang, “Fully automatic knee osteoarthritis severity grading using deep neural networks with a novel ordinal loss”. Computerized Medical Imaging and Graphics, 75, 84-92, 2019. [14] Bayramoglu, N.; Nieminen, M.T.; Saarakkala, S. A Lightweight CNN and Joint Shape-Joint Space (JS2) Descriptor for Radiological Osteoarthritis Detection. Commun. Comput. Inf. Sci. 2020, 1248, 331–345 [Google Scholar]
Copyright
Copyright © 2023 Fathima Nourin. K. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55981
Publish Date : 2023-10-03
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

