Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Design and Application of Ohmic Heating to Study the Effect on Tomato Puree
Authors: Aruna Singh, Moses Paul M , S Joy Daniel, Indusree R, Aslam Abdullah
DOI Link: https://doi.org/10.22214/ijraset.2025.66201
Certificate: View Certificate
Abstract
Ohmic heating is a non-conventional method of heating as an alternative to the existing heating techniques. Tomato is an abundant vegetable used widely and about 16% is lost as waste. To reduce waste, alternate products like puree, jam, juice can be made. Lycopene is a heat sensitive antioxidant present in tomatoes. The present study is done to design ohmic heating to treat tomato puree and study the effect on lycopene content. The study also tries to understand the correlation between the heating rate and various other parameters, such as voltage gradient, conductivity, and temperature. The voltage was varied between 10 and 30 V/cm, with a 5 V/cm interval. The time taken to reach a temperature of 98 ? varied from 20 to 140 s for 10 V/cm to 30 V/cm respectively, with a minimum reduction of 12% in lycopene content at 30 V/cm.
Introduction
I. INTRODUCTION
The tomato is the edible berry of the plant Solanum lycopersicum, commonly known as a tomato plant. Every year, a considerable amount to the tune of 16% produce of tomatoes is dumped as waste. Reducing waste and converting it into a value-added product is always a viable commercial option. The tomato berry can be used to make puree, juice, lycopene extract (an antioxidant), and so on. Heating is a traditional and widely used treatment for food processing and preservation. The demand is always for high-energy efficient, minimum treatment, and cost-effective heating technologies that do not compromise the safety and quality of the products. Ohmic Heating (OH). A large number of potential applications exist for OH, including blanching, dehydration, evaporation, pasteurization, sterilization, fermentation, extraction, etc. [18]. OH, is a thermal processing technique where passing electric current generates heat at a very rapid rate. This is also known as joule heating or electro-conductive heating [5]. The food behaves like a resistor [9] and gets heated up in the process. OH, is also referred to as electro-heating or electro-conductive heating. The idea of OH dates to 1897. The treatment of OH was first introduced in the late 1920s as a popular commercial technique, referred to as the “electro pure” process [2]. OH, prepares products with higher quality, particularly with respect to retention of flavour, nutrients, and integrity. OH, is better suited to treat viscous and particulate foods because of its mechanism of uniform volumetric heating, which reduces internal temperature gradients [19]. Permeable materials usually have shorter conductivity. Meaty, muscle cuts are shown to have more conductivity [18]. Unlike conventional heating, OH achieves the process treatment temperature in seconds and is hence energy efficient [23]. OH, treatment is also reported to increase shelf life [17]. This technology reduces thermal damage to food for a variety of reasons, including the fact that heating and cooling are immediate, avoiding overprocessing [21]. As with any other processing, the absorption of energy may result in some inevitable degradation of food components. However, on a larger scale, this degradation is within acceptable limits, or much less relative. Ohmic heating is gaining renewed interest from the scientific community, and continuous plants are set up to treat foods [10]. Researchers have proven that OH can preserve the nutritional content better than conventional heating, which tends to destroy sensitive nutrients [1]. Moreover, OH extracts valuable compounds like phenols and carotenoids, which possess antioxidant properties, from tomato by-products like peels and seeds during tomato processing. This methodology not only reduces waste but also generates additional revenue streams for farmers [6]. [14] described OH as claiming higher levels of bioactive compounds, such as lycopene, in tomatoes due to its minor thermal effects.
The present study was undertaken to design and fabricate a laboratory OH unit to treat 100 mL of tomato puree: 1) to study the effect of voltage gradient, conductivity, and time-temperature; and 2) to study the effect on tomato puree's lycopene content.
II. MATERIALS AND METHODS
A. Design of an Ohmic Heating Unit
The ohmic heating unit essentially consists of a conductive chamber, electrodes, a variable-voltage transformer, and measuring digital meters for temperature, pH, and voltage.
The chamber was fabricated from an 8-mm-thick acrylic sheet to the pre-optimized dimensions of 10x3x5 (LxBxH) with a lid. Among the choices of material for electrodes, stainless steel (SS316) was selected because of its cost and electrical conductivity.
The electrodes were fixed to the sides of the chamber with a distance of 3 cm between them. The source of electric current was a variable-type voltage transformer, which can be used to vary the input voltage to the cells between 10V/cm and 140V/cm. (Fig. 1)
A thermocouple that can withstand a temperature of 300 °C was used as a temperature sensor. A digital multimeter was used for other measurements of current density, output voltage, and resistance. A portable pH meter was also used. The total setup was fixed on an insulating surface for safety purposes.
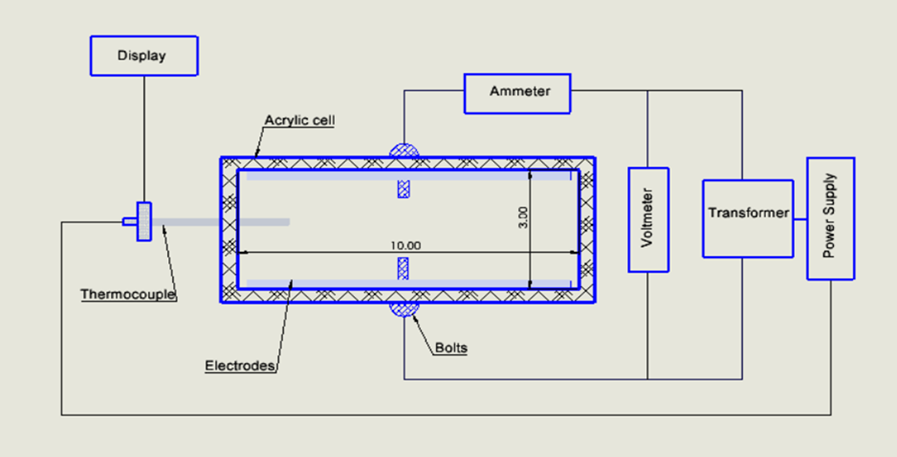
Fig. 1 Schematic representation of the ohmic heating Unit
The experiments were designed to subject the puree to 5 different voltage gradients (10, 15, 10, 25, 30 V/cm). 3 different time intervals were selected for each voltage gradient. The puree was poured into the ohmic heating cell and the transformer switched on to set the desired voltage. Once the time limit is reached the setup is switched off.
The samples were then analysed for lycopene content. Lycopene was extracted using hexane:ethanol:acetone (2:1:1) (v/v) mixture [8]. The absorbance of samples was determined at 503 nm by spectrophotometer. Calculation of the lycopene levels in the hexane extracts were calculated according to,
Lycopene (mg/kg fresh wt.) = A503 x 171.7/ W
where A503 is the absorbance at 503nm, W is the weight in grams of tomato puree added.
The electrical conductivity was calculated using the cell dimensions, voltage and current by the following equation,
Where A is the area of cross-section of the sample in the cell (in m2), V is the voltage reading, I is the electric current and L, the distance between the electrodes which was 0.03m in this case. The area of contact between the sample and electrode pair which reduces due to reduction in water content of the tomato puree, is calculated as follows,
Therefore, the electrical conductivity equation is changed into,
The percentage change of pH was determined by the equation,
The energy for evaporation was estimated by the following equation [3],
Where Es is the energy consumption (J). The energy loss is the summation of the heat loss for physical, chemical and electrochemical changes of tomato samples i.e., heat needed for heating the cell and electrodes, the heat loss by natural convection to the surrounding, and the electrical energy that was not converted into heat energy.
Where ms the mass of the sample in kg, mw is the mass of water evaporated in kg, Cps is the specific heat capacity of the tomato (KJ/kg C), λ is the latent heat of vaporization of water (J/kg), and t is the heating time (s).
The efficiency of heating system is evaluated by Specific Performance Coefficient (SPC) that is mathematically defined as below,
B. Sample Preparation
With visual observation, the tomatoes brought in from local vendors were selected for uniform color. Tomatoes with a uniform diameter (4 ± 0.2 cm) were selected and blanched to remove skin. After the seeds had been removed, the tomatoes were pureed. They were also analyzed for particle size (0.1204 mm) distribution using the standard wet sieve analysis procedure. The particle size distribution of tomato concentrates, and juice has a great influence on rheological properties.
C. Sample for Pasteurization
To evaluate the effects of OH on Pasteurization, the streak plate technique is utilized, where a puree sample consists of a loopful of puree that is spread onto an agar plate to ensure that individual cells are far apart from each other.
For OH pasteurisation, the samples were aseptically collected in a sterile vessel immediately after processing. The determination of total plate count (TPC) was conducted using Plate Count Agar (PCA). The PCA medium supports the growth of all aerobic microorganisms. The Petri plates for each treated sample were assessed at 2-week intervals for a total of 6 weeks. The specimens were placed in an incubator and kept at a temperature of 37? for a duration of 48 hours. The results were quantified as the logarithm of the number of colony forming units per millilitre of juice (log CFU/mL).
III. RESULTS AND DISCUSSION
A. Electrical Conductivity
Electrical conductivity is the most critical parameter to be tested in an ohmic heating setup. The electrical conductivity of biological tissue increases when it is heated as a result of an increase in the ionic mobility [16] which in turn causes an increase in current as observed in Fig. 2. The current passed through sample may change the structure of the cell wall components due to absorption of energy by the tissues, expulsion of gas bubbles, and lowering of viscosity [22]. This phenomenon of gas bubble formation was noted when the temperature of the sample was near 86°C thus increasing the resistance to flow of current [7], as the bubbles were non-conductive. The increase in the electrical conductivity with temperature and attributed this property to reduced drag that aided the movement of ions. This finding line up with the results of the study, which show that heat increases electrical conductivity of biological tissues, including tomato puree, because of reduced drag that facilitates ion flow [12].
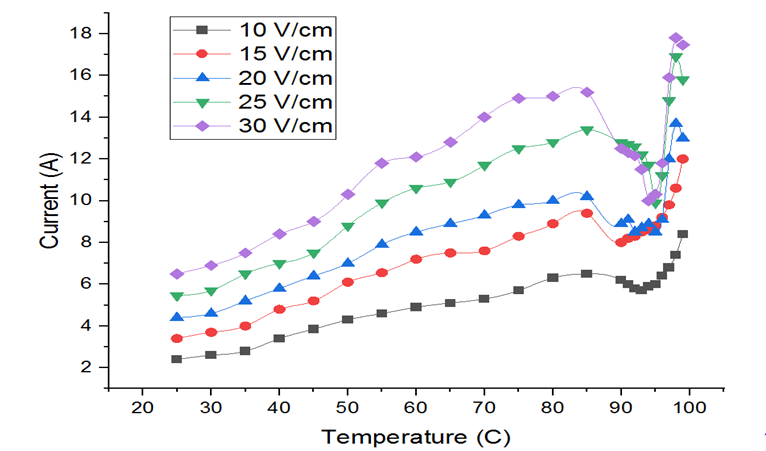
Fig. 1 Current as a function of temperature at varied voltage gradient
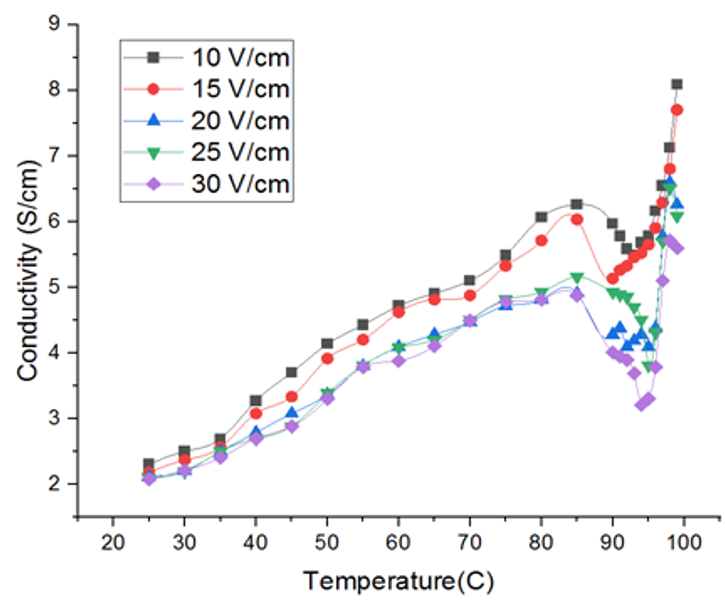
Fig. 3 Electrical conductivity profile during ohmic heating at different voltage gradients
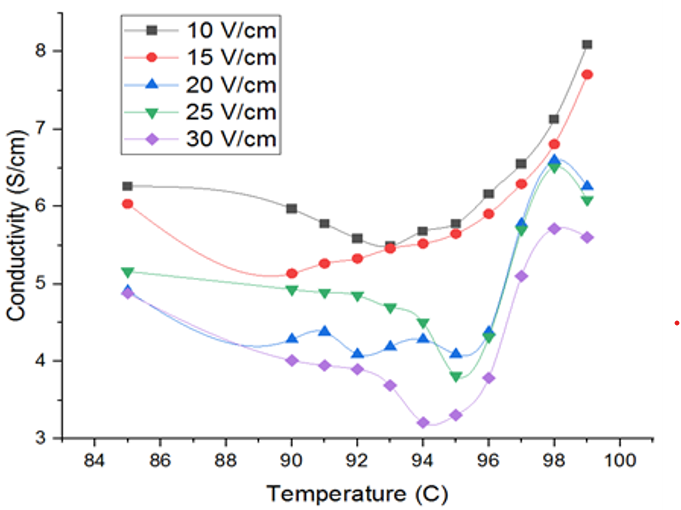
Fig. 4 Electrical conductivity profile at T >85
Further, in the plot of conductivity vs temperature, four stages of heating were observed in Fig. 3; a rapid increase during heating up period, followed by a steady increase. As the sample approaches its boiling point, the electrical conductivity reduces due to the formation of vapour. A drop in the electrical conductivity was noted when the temperature reached 92-95ºC in Fig. 4. The concentration of the tomato sample arises as the water evaporates, increasing electrical conductivity as a result. Comparatively high number of vapour bubbles are formed during the boiling motion. A reduction in electrical conductivity at this point, which shows that the heating has stopped, implies that the electrodes are not completely in touch with the sample. Electrical conductivity drops as voltage gradient rises, possibly as an outcome of greater voltage gradient-induced rates of vapour generation. Latest OH study supports this behaviour. For instance, [13] showed that similar structural shifts and ionic mobility initiate blended citrus juice's electrical conductivity to rise with temperature during OH. Additionally, [11] on Spirulina platensis have revealed that compounding OH with enzymatic treatment improves the extraction of bioactive compounds, which further highlights the significance of understanding and controlling electrical conductivity during OH processes. Additionally, an increase in viscosity due to loss of water may also reduce conductivity. The electrical conductivity values of the tomato samples were within the general ranges of 0.01 to 10 S/m for biological materials.
B. Temperature and Conductivity
A quadratic form is used for describing the relationship between electrical conductivity and temperature,
σ = AT2 + BT + C
Where A, B and C are the constants of regression, and T (ºC) is temperature of sample during ohmic heating at any time t.
TABLE I
Constants of regression of electrical conductivity with temperature
|
Voltage gradient (V/cm) |
T<90 |
|||||
|
A |
B |
C |
R2 |
|||
|
10 |
-3.1879*10-4 |
0.1001 |
-0.1729 |
0.9848 |
||
|
15 |
-5.2383*10-4 |
0.1189 |
-0.7364 |
0.9794 |
||
|
20 |
-5.9923*10-4 |
0.1138 |
-0.6424 |
0.9779 |
||
|
25 |
-2.8943*10-4 |
0.0847 |
-0.064 |
0.9802 |
||
|
30 |
-5.9791*10-4 |
0.1132 |
-0.693 |
0.9723 |
||
|
Voltage gradient (V/cm) |
T>90 |
|||||
|
A |
B |
C |
R2 |
|||
|
10 |
0.0693 |
-12.894 |
605.208 |
0.9938 |
||
|
15 |
0.0409 |
-7.4779 |
347.393 |
0.9718 |
||
|
20 |
0.0677 |
-12.519 |
583.006 |
0.9769 |
||
|
25 |
0.0682 |
-12.759 |
600.463 |
0.9823 |
||
|
30 |
0.0739 |
-13.760 |
644.328 |
0.9801 |
||
Regression constants for electrical conductivity of tomato samples under different voltage gradient are given in the Table I. The high values of R2 are indicative of good fitness of empirical relationship to represent the variation in electrical conductivity with temperature of tomato samples. In the present study, the quadratic relation of conductivity with temperature was found to adequately represent the behaviour of tomato puree under OH.
C. Heating Rate
The effectiveness of the OH depends on the rate of heating. As seen in Fig. 5, the heating rate of tomato samples increased with an increase in voltage gradient. The resistance of the puree to the electric current generated heat and the temperature increased from 25 °C to the boiling point temperature of 96-98 °C linearly. The rate of heating was low for 10V/cm at 0.5409ºC/s and high for 30V/cm at 1.622 ºC/s for the voltage gradient considered. Higher the voltage gradient higher is the rate of heating. The effect of voltage gradient and electrical conductivity on the heating rate of tomato samples is in good agreement with the findings.
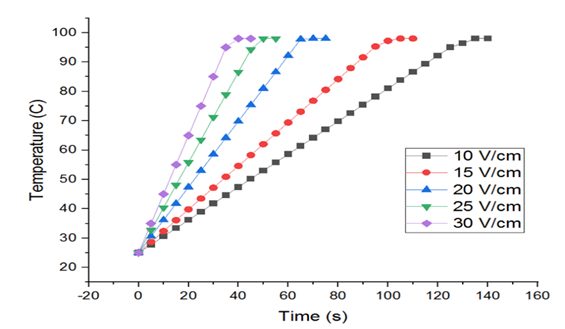
Fig. 5 Variation of temperature at different voltage gradients
D. pH
pH is an indicator of the changes that the sample undergoes after OH. Table II shows that the change in pH decreases with an increase in the voltage gradient. The maximum increase of pH was 2.9% at 10 V/cm. At higher voltage gradient of 30 V/cm, the heating rate (0.56 °C/s) is high, thus the time taken for the sample to heat up from 25 to 98 °C is short and more moisture is removed. The detected change in pH is limited (0.67%) because the reaction time is short. At low voltage gradient of 10 V/cm, the heating rate is 0.29°C/min. This lower heating rate results in a higher residence time; leads to a higher change in pH (2.9 %). At high electrical power and salt concentration, a notable loss in buffering capacity was observed. This is in line with the study by [10] that electrical conductivity and voltage gradients have similar effects on pH shifts when enhancing OH applications for pectin methylesterase inactivation in orange juice. Also, [13] demonstrated that quick heating at higher voltages minimalizes major chemical reactions and, as a result, pH changes in the samples. During OH, major reaction i.e., hydrolysis and corrosion reactions between the electrodes and the electrolyte solution may result in changes of pH [7]. The effect on pH was limited because the change is mostly <10% in most of the other studies [4].
TABLE III
pH change with Voltage gradient
|
Voltage Gradient V/cm |
pH |
?pH % |
|
10 |
4.61 |
2.90 |
|
15 |
4.59 |
2.45 |
|
20 |
4.56 |
1.78 |
|
25 |
4.53 |
1.12 |
|
30 |
4.51 |
0.67 |
|
Fresh |
4.48 |
E. Energy Consumption
Energy consumption is primary in any process because this decides the feasibility. Fig. 6 suggests that the specific energy requirement of tomato samples at different voltage gradients increased with an increase in temperature. The energy loss also increased with time. The least energy consumption was for 10 V/cm at 36642J for 100mL puree. The Eloss values increased with an increase in voltage gradient. The specific energy loss obtained during heating for 30 V/cm voltage gradient level was 1.84-fold higher than 10 V/cm. This is due to increased current for higher voltage gradients shown in Fig. 3 & Table III.
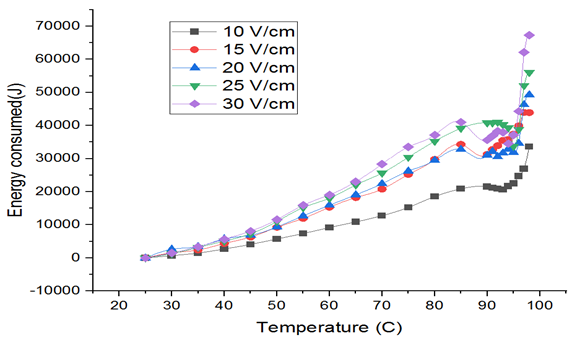
Fig. 6 Energy consumed v/s Temperature Plot
Another parameter that was used to evaluate the efficiency is the Specific Performance Coefficient (SPC). The SPC for 10 V/cm was higher compared to 30 V/cm indicating that heat required to heat the tomato puree sample was small at higher voltage [16]. SPC varied between 0.9471 and 0.5069 for 10 V/cm to 30 V/cm respectively. A similar trend was reported by [15] in their study on orange juice and apricot puree.
TABLE IIIII
Energy change with Voltage gradient
|
Voltage gradient (V/cm) |
Total energy (VI?t) (J/g) |
Energy loss (J) |
Specific energy loss (J/g) |
Specific Performance Coefficient (SPC) |
|
10 |
36642 |
8363 |
83.63 |
0.9471 |
|
15 |
43891 |
9736 |
97.36 |
0.7237 |
|
20 |
49276 |
10601 |
106.01 |
0.6555 |
|
25 |
55990 |
11213 |
112.13 |
0.5889 |
|
30 |
67274 |
16621 |
166.21 |
0.5069 |
F. Lycopene Content
Heat treatments can cause degradation in the lycopene which has important antioxidant effects. [20] reported 90% degradation in lycopene content in the concentrated tomato paste when the conventional pasteurization was applied. In the present study of OH, 76.45 mg/kg of lycopene degraded at the voltage gradient of 10 V/cm from the initial value of 232.9 mg/kg i.e., a 37.72% reduction. As the voltage gradient increased the degradation decreased to 12.2% at 30V/cm in Fig. 7. The lower thermal degradation at higher voltage gradient may be owing to short retention time. This suggests that with an increase in holding time the amount of lycopene degradation also increased.
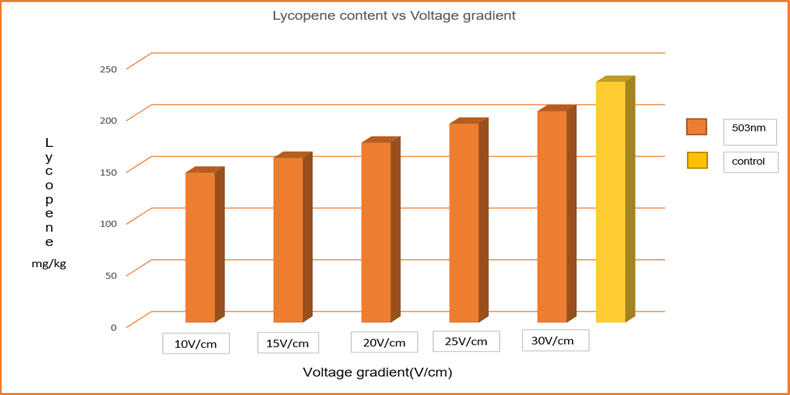
Fig. 7 Lycopene content at varied Voltage gradient
G. Effect of OH on Pasteurization
The OH is not only an effective volumetric heating technique but also effects pasteurization in liquid and slurry foods by destroying pathogenic organisms. It was evident from Table IV. Study states that when tomato puree is held in between 92 °C and 97 °C it is completely pasteurized with no change to Rheology, Colour or Texture of the sample. This was achieved by the experiments conducted at all the Voltage gradients in the present study. A storage study over a period of 6 weeks for the treated samples were done at an interval of 2 weeks. No microbial growth was observed for all samples upto 4 weeks. But at the end of 6 weeks samples treated with higher voltages (>20V/cm) showed microbial growth. This may be interesting to note that lower voltage is preferable as pasteurization. Though heating at higher voltage takes lesser time it is not favourable as deterioration of the pulp is observed.
TABLE IV
Microbial Growth of samples treated with OH at 10V/cm to V35/cm after 2, 4 and 6 weeks
|
Weeks |
Microbial growth of raw sample |
Sample treated with 10V/cm |
Sample treated with 15V/cm |
Sample treated with 20V/cm |
Sample treated with 25V/cm |
Sample treated with 30V/cm |
Sample treated with 35V/cm |
|
2 |
1.2 x 105 |
<1 |
<1 |
<1 |
<1 |
<1 |
<1 |
|
4 |
2.6 x 108 |
<1 |
<1 |
<1 |
<1 |
<1 |
<1 |
|
6 |
3.2 x 108 |
<1 |
<1 |
1.5 x 105 |
3.1 x 107 |
2.1 x 107 |
2.5 x 107 |
Conclusion
Ohmic heating is emerging as a viable alternative to conventional heating based on the energy consumption. The degradation of heat sensitive components of the food material subjected to ohmic heating is much less compared to other forms of heating. The tomato puree was studied for the feasibility of ohmic heating for a voltage gradient of 10V/cm to 30V/cm. The time taken to reach 98ºC varied between 35 s and 130s. Heating rate was faster for higher voltage gradient. The change in pH was within observed limits was observed. Degradation of lycopene was 12.2% for 30V/cm as against 37.7% for 10 V/cm. Pasteurization effect was observed for samples treated with lesser voltage upto 6 weeks as compared to treatment with >20V/cm. Further studies are required to quantify the extent of changes due to electroporation of the microbial load.
References
[1] Z. T. Alkanan, A. B. Altemimi, A. R. S. Al-hilphy, F. Cacciola, and S. A. Ibrahim, “Application and effects of ohmic-vacuum combination heating on the quality factors of tomato paste,” Foods, vol. 10, no. 12, p. 2920, Dec. 2021. [2] A. K. Anderson and R. Finkelstein, “A Study of the Electro-Pure Process of Treating Milk,” Journal of Dairy Science, vol. 2, no. 5, pp. 374–406, May 1919. [3] A. M. Assiry, “Application of ohmic heating technique to approach near-ZLD during the evaporation process of seawater,” Desalination, vol. 280, no. 1–3, pp. 217–223, Jul. 2011. [4] A. Assiry, S. K. Sastry, and C. Samaranayake, “Degradation kinetics of ascorbic acid during ohmic heating with stainless steel electrodes,” Journal of Applied Electrochemistry, vol. 33, no. 2, pp. 187–196, Feb. 2003. [5] L. P. Cappato et al., “Ohmic heating in dairy processing: Relevant aspects for safety and quality,” Trends in Food Science & Technology, vol. 62, pp. 104–112, Jan. 2017. [6] M. C. Coelho et al., “Tomato Processing By-Products Valorisation through Ohmic Heating Approach,” Foods, vol. 12, no. 4, p. 818, Feb. 2023. [7] H. Darvishi, M. H. Khostaghaza, and G. Najafi, “Ohmic heating of pomegranate juice: Electrical conductivity and pH change,” Journal of the Saudi Society of Agricultural Sciences, vol. 12, no. 2, pp. 101–108, Apr. 2013. [8] A. R. Davis, W. W. Fish, and P. Perkins-Veazie, “A rapid spectrophotometric method for analyzing lycopene content in tomato and tomato products,” Postharvest Biology and Technology, vol. 28, no. 3, pp. 425–430, Jun. 2003. [9] D. De Halleux, G. Piette, M.-L. Buteau, and M. Dostie, “Ohmic cooking of processed meats: Energy evaluation and food safety considerations,” Canadian Biosystems Engineering, vol. 47, p. 41, 2005. [10] A. Demirdöven and T. Baysal, “Effects of electrical pre-treatment and alternative heat treatment applications on orange juice production and storage,” Food and Bioproducts Processing, vol. 94, pp. 443–452, Sep. 2015. [11] P. Ferreira-Santos et al., “Sequential multi-stage extraction of biocompounds from Spirulina platensis: Combined effect of ohmic heating and enzymatic treatment,” Innovative Food Science and Emerging Technologies, vol. 71, 2021. [12] K. Gomathy, K. Thangavel, M. Balakrishnan, and R. Kasthuri, “Effect of Ohmic Heating on the Electrical Conductivity, Biochemical and Rheological Properties of Papaya Pulp,” Journal of Food Process Engineering, vol. 38, no. 4, pp. 405–413, Aug. 2015. [13] S. M. B. Hashemi, A. Gholamhosseinpour, and M. Niakousari, “Application of microwave and ohmic heating for pasteurization of cantaloupe juice: Microbial inactivation and chemical properties,” Journal of the Science of Food and Agriculture, vol. 99, no. 9, pp. 4276–4286, Jul. 2019. [14] S. M. B. Hashemi and R. Roohi, “Ohmic heating of blended citrus juice: Numerical modeling of process and bacterial inactivation kinetics,” Innovative Food Science and Emerging Technologies, vol. 52, pp. 313–324, Mar. 2019. [15] F. Icier and C. Ilicali, “Electrical conductivity of apple and sour cherry juice concentrates during ohmic heating,” Journal of Food Process Engineering, vol. 27, no. 3, pp. 159–180, Jul. 2004. [16] F. Icier and C. Ilicali, “Temperature dependent electrical conductivities of fruit purees during ohmic heating,” Food Research International, vol. 38, no. 10, pp. 1135–1142, Dec. 2005. [17] R. Ito, M. Fukuoka, and N. Hamada-Sato, “Innovative food processing technology using ohmic heating and aseptic packaging for meat,” Meat Science, vol. 96, no. 2, pp. 675–681, Oct. 2014. [18] N. Kaur and A. K. Singh, “Ohmic Heating: Concept and Applications—A Review,” Critical Reviews in Food Science and Nutrition, vol. 56, no. 14, pp. 2338–2351, Oct. 2016. [19] S. Leizerson and E. Shimoni, “Effect of ultrahigh-temperature continuous ohmic heating treatment on fresh orange juice,” Journal of Agricultural and Food Chemistry, vol. 53, no. 9, pp. 3519–3524, May 2005. [20] N. Manzo et al., “Degradation kinetic (D100) of lycopene during the thermal treatment of concentrated tomato paste,” Natural Product Research, vol. 33, no. 13, pp. 1835–1841, Jul. 2019. [21] M. Sakr and S. Liu, “A comprehensive review on applications of ohmic heating (OH),” Renewable and Sustainable Energy Reviews, vol. 39, pp. 262–269, Nov. 2014. [22] S. Sastry, “Ohmic heating and moderate electric field processing,” Food Science and Technology International, vol. 14, no. 5, pp. 419–422, Oct. 2008. [23] X. Tian, Q. Yu, W. Wu, and R. Dai, “Inactivation of microorganisms in foods by ohmic heating: A review,” Journal of Food Protection, vol. 81, no. 7, pp. 1093–1107, Jul. 2018.
Copyright
Copyright © 2025 Aruna Singh, Moses Paul M , S Joy Daniel, Indusree R, Aslam Abdullah. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66201
Publish Date : 2024-12-31
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

