Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Diabetes Mellitus: Types, Complications and Ways of Treatment
Authors: Jia Jaison
DOI Link: https://doi.org/10.22214/ijraset.2025.66842
Certificate: View Certificate
Abstract
The primary aim of this study is to investigate the types of Diabetic diseases, their characteristics and impact on lives, further complications and ways of treatment. Diabetes is a dangerous disease in which the body cannot properly control the amount of sugar in your blood because it does not have enough insulin. Also known as Diabetes mellitus (DM), it is the most common manifestation of glucose metabolism alterations, and it is both a cardiovascular risk factor and a cardiovascular disease per se. It is associated with long-term micro and macrovascular complications. There are several different types of diabetes: Type 2 Diabetes, Type 1 Diabetes, Diabetes Insipidus, and Gestational Diabetes. This paper evaluates all ranges of the diseases and provides an important array of information with regard to Diabetes.
Introduction
I. INTRODUCTION
A. Overview of Diabetes Mellitus
Diabetes is a chronic, ever-present disease that places the patient in a unique position of self-care, requiring daily decisions and the ability to solve problems when they arise [8]. Diabetes mellitus is a condition of chronically elevated blood glucose concentrations which give rise to its main symptom of passing large quantities of sweet–tasting urine (diabetes from the Greek word meaning ‘a siphon’, as the body acts as a conduit for the excess fluid, and mellitus from the Greek and Latin for honey) [1]. Patients with diabetes mellitus may exhibit classic symptoms (polyuria, polydipsia, polyphagia), or more commonly, they can be asymptomatic [2]. Diabetes screening may detect an early asymptomatic phase [2]. The fundamental underlying abnormality is a net (relative or absolute) deficiency of the hormone insulin. [1] Insulin is essentially the only hormone that can lower blood glucose [1]. Diabetes mellitus is diagnosed by identifying chronic hyperglycemia [1]. The World Health Organization (WHO) and the American Diabetes Association (ADA) use a fasting plasma glucose (FPG) of 7 mmol/L or higher to define diabetes [1].
B. Type 1 Diabetes
Type 1 diabetes occurs in the setting of insulin deficiency [2]. It accounts for 5% of diagnosed diabetes cases [2]. The underlying mechanism is destruction of insulin-producing pancreatic beta cells, which can be autoimmune-mediated, idiopathic, or acquired [2]. Autoimmune-mediated type 1 diabetes mellitus can result from a combination of genetic, environmental, and autoimmune factors [2]. Type 1 diabetes mellitus (T1DM) is a disorder of glucose homeostasis characterized by autoimmune destruction of the insulin-producing pancreatic b-cell that progressively leads to insulin deficiency and resultant hyperglycemia [10]. If left untreated, insulin deficiency leads to progressive metabolic derangement, with worsening hyperglycemia, ketoacidosis, starvation, and death [10]. In an effort to restore and maintain euglycemia, treatment attempts to mimic the action of the native b-cell by exogenously replacing insulin and includes frequent monitoring of blood glucose levels [10]. Autoimmune-mediated type 1 diabetes has classically been considered a disease of children and young, thin adults, although it can occur at any age or BMI range [2]. The initial presentation can range from modest elevations in plasma glucose levels to diabetic ketoacidosis (DKA) [2]. The time course for β cell destruction is also variable, although it is frequently more rapid in children compared with adults [2].
New-onset T1DM presents in the majority of pediatric patients with the classic symptoms of polyuria and polydipsia (69%) and somewhat less frequently with polyphagia and weight loss (33%) [1]. Patients and families usually report the duration of symptoms as lasting 1 to 2 weeks, but sometimes several months [1]. Often, these symptoms become more apparent after an episode of enuresis or with the emergence of nocturia [1]. Patients frequently have vague complaints, such as fatigue, and may note blurred vision [1]. Lifelong insulin therapy is the first-line treatment for type 1 diabetes [2]. Physiologic insulin therapy, also known as intensive insulin therapy, is the ideal insulin regimen as it attempts to mimic the actions of normal pancreatic beta cells [2].
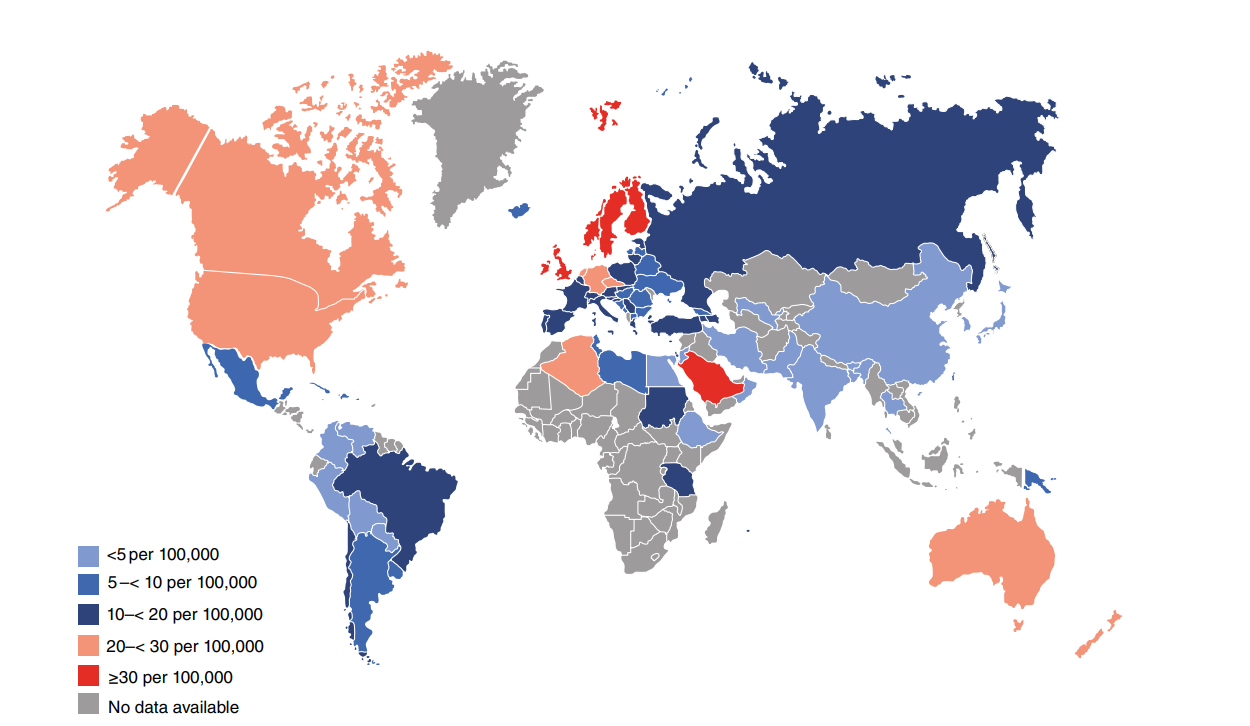
Fig. 1 - Age and gender standardized incidence rates per 100,000 population per year for type 1 diabetes in children and adolescents aged 0–14 years (2019).
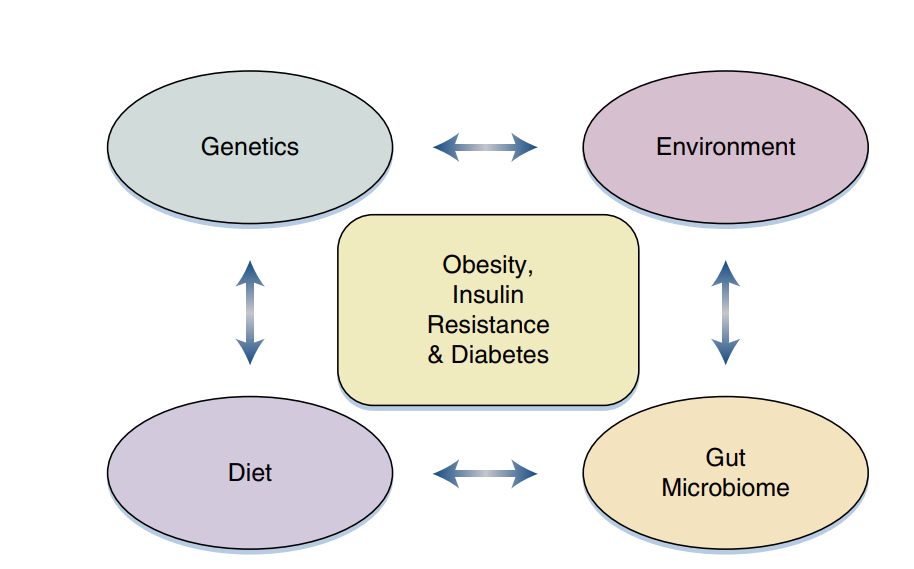
Fig 2 – Criteria for Diagnosis of Diabetes.
C. Type 2 Diabetes [T2DM]
Type 2 diabetes mellitus accounts for most (90%-95%) diagnosed diabetes cases [2]. Type 2 diabetes generally has an insidious onset of prolonged asymptomatic hyperglycemia [2]. Patients with type 2 diabetes may present first with macrovascular or microvascular changes [2]. The risk of heart disease and stroke is commonly stated to be two to four times higher, and that of lower-extremity amputation is approximately 20 times higher for people with diabetes than for those without diabetes [8]. Life expectancy is reduced by approximately 10 years in people with diabetes, and the hazard ratio for death is most pronounced in those having diabetes under age 55 years [8]. Although type 2 diabetes is often considered an adult disease, the incidence is increasing along with obesity rates in children and adolescents [2]. Insulin resistance from obesity in the setting of relative insulin deficiency contributes to the development of type 2 diabetes in the majority of patients [2]. The degree of hyperglycemia depends on the extent of beta-cell function, which can decline over time [2]. Residual insulin production from the β cells is insufficient to control glucose adequately, but it is able to suppress lipolysis for most persons with type 2 diabetes [2]. Under extreme metabolic stress, such as an illness, some patients with type 2 diabetes cannot suppress lipolysis and present with DKA [2]. Progression of the pathophysiologic abnormalities underlying T2DM typically leads to increasing difficulty in controlling hyperglycemia over time [8]. At the same time, an increasing burden of medical illness, both diabetes related and from other causes, adds to potential risks and may limit potential benefits of intensive therapy [8]. By 10 years after diagnosis, progression of the disorder commonly leads to a need for multiple and often injected therapies, more personalized choices of therapies, and greater reliance on treatments with less well-understood long-term benefits versus risks [8]. How to provide these more complex and time-consuming services is one of the dilemmas of health system management [8]. In general, the best results are obtained by access to a specialized team approach [8].
The diagnosis of diabetes rests on demonstration of hyperglycemia [8]. Because plasma glucose concentrations range as a continuum, the criteria are based on estimated thresholds for the complications of diabetes [8]. The primary endpoint used to evaluate the relationship between glucose levels and complications is retinopathy [8].
 Fig 3 – Genetics, the environment, diet, and the gut microbiome are among many interacting factors influencing the development of obesity, insulin resistance, and diabetes.
Fig 3 – Genetics, the environment, diet, and the gut microbiome are among many interacting factors influencing the development of obesity, insulin resistance, and diabetes.
D. Diabetes Insipidus (DI)
Diabetes insipidus (DI) DI is a rare disease with inability to concentrate urine, so that large amount of dilute urine is passed (usually >4 litres per day) throughout the 24 hours [3]. It is called central if due to depressed ADH arginine vasopressin (AVP) secretion, causing increased free water diuresis and reduced free water reabsorption) [3]. It is called nephrogenic if there is impaired response by the kidney to the action of ADH [3]. The polyuria persists after fasting, which serves as a good screening test [3]. Despite of increased plasma osmolality, the urine is dilute (ratio of urine to plasma osmolality is <2:1, and DI is generally excluded if the ration is >2:1) [3]. Psychiatric patients, who compulsively drink huge quantities of water, have similar features, but maintain the power to concentrate urine once subjected to water deprivation [3]. Patients with DI will continue to pass diluted urine after a 12 hour fast [3]. On the other hand, inappropriate ADH syndrome, is associated with over-secretion of ADH (reverse of DI) due to a variety of chest, neurological, and drug causes that results in dilution of the blood with low sodium, potassium, and urea [3]. Adults present with polyuria, polydipsia, and thirst [5]. Children with DI may present with failure to thrive, polydipsia, and enuresis [5]. The patient may have other features of underlying pathology, including headache, visual disturbance, or other endocrinopathy [5].
Adipsic diabetes insipidus (ADI) is a rare clinical situation which occurs when damage to osmoreceptors in the anterior hypothalamus results in failure to respond to rising plasma sodium with appropriate fluid intake [5]. It is associated with chronic hypernatraemia and is associated with significant morbidity and mortality [5]. The commonest cause of adipsic DI is surgical clipping of aneurysms of the anterior communicating aneurysm, following subarachnoid hemorrhage [5]. It has also been associated with extensive surgery for craniopharyngioma, TBI, neurosarcoidosis, and congenital causes [5]. Patients are susceptible to hypernatraemia, even when ambulant, but are particularly vulnerable during acute illness, particularly when vomiting occurs [5].
E. Hyperglycemia In Pregnancy (HIP)
According to WHO and the International Federation of Gynecology and Obstetrics (FIGO), hyperglycemia in pregnancy (HIP) can be classified as either gestational diabetes mellitus (GDM) or diabetes in pregnancy (DIP) [4]. GDM is diagnosed for the first-time during pregnancy and may occur anytime during pregnancy (most likely after 24 weeks) [4]. DIP applies to pregnant women who have previously known diabetes or have hyperglycemia that was first diagnosed during pregnancy and meets WHO criteria of diabetes in the non-pregnant state [4]. DIP may also occur at any time during pregnancy, including the first trimester [4]. It has been estimated that most (75–90%) cases of DIP are GDM [4].
Besides those women with hyperglycemia early in pregnancy, GDM arises in women with insufficient insulin secretory capacity to overcome the diminished action of insulin (insulin resistance) due to hormone production by the placenta [4]. Risk factors for GDM include older age, overweight and obesity, previous GDM, excessive weight gain during pregnancy, a family history of diabetes, polycystic ovary syndrome, habitual smoking and a history of stillbirth or giving birth to an infant with a congenital abnormality [4]. GDM is more common in some ethnic groups [4]. GDM usually exists as a transient disorder during pregnancy and resolves once the pregnancy ends [4]. However, pregnant women with hyperglycemia are at higher risk of developing GDM in subsequent pregnancies [4].
In addition, the relative risk of developing type 2 diabetes is particularly high at 3–6 years after GDM and at less than 40 years of age [4]. The increased risks remain markedly elevated thereafter [4]. Considering the high risk of early onset type 2 diabetes and the fact that early onset type 2 diabetes predisposes to high CVD (cardiovascular disease) risk, any lifestyle intervention should be started within three years after the index pregnancy in order to achieve the maximum benefit for the prevention of diabetes [4].
Babies born to mothers with GDM also have a higher lifetime risk of obesity and developing type 2 diabetes themselves [4]. Women with hyperglycemia detected during pregnancy are at greater risk of adverse pregnancy outcomes [4]. These include high blood pressure and a large baby for gestational age (termed ‘macrosomia’), which can make a normal birth difficult and hazardous, with the baby more prone to fractures and nerve damage [4]. Identification of hyperglycemia in pregnancy combined with good control of blood glucose during pregnancy can reduce these risks [4]. Women of child-bearing age who are known to have diabetes prior to pregnancy should receive pre-conception advice, higher dose folic acid treatment, medication review, intensive diabetes management and a planned approach to pregnancy [4]. All women who have HIP – be it GDM, previously undiagnosed HIP or existing and known diabetes – require optimal antenatal care and appropriate assistance in postnatal management [4].
Women with hyperglycemia during pregnancy may be able to control their blood glucose levels through a healthy diet, moderate exercise and blood glucose monitoring [4]. Interaction with healthcare professionals is important to support their self-management and also to identify when medical (e.g., prescription of insulin or oral medications) or obstetric intervention is needed [4].
F. Maturity-Onset Diabetes of the Young (MODY)
The recently published WHO report on the classification of diabetes mellitus lists a number of ‘other specific types’ [of diabetes] including monogenic diabetes and what was once termed ‘secondary diabetes’ [4]. Monogenic diabetes, as the name implies, results from a single gene rather than the contributions of multiple genes and environmental factors as seen in type 1 and type 2 diabetes [4]. Monogenic diabetes is much less common and represents 1.5–2% of all cases, though this may well be an underestimate [4]. It is often misdiagnosed as either type 1 or type 2 diabetes [4].
These monogenic forms present a broad spectrum, from neonatal diabetes mellitus (sometimes called ‘monogenic diabetes of infancy’), maturity onset diabetes of the young (MODY) and rare diabetes associated syndromic diseases [4]. Although rare, these can serve as ‘human knockout models’ providing insight into diabetes pathogenesis [4]. From a clinical perspective, the exact diagnosis of the monogenic forms of diabetes is important because in some instances therapy can be tailored to the specific genetic defect [4]. Further distinction between the fourteen different sub-types of MODY leads not only to differences in clinical management but different predictions of complication risk [4]. In recent years, with the accumulation of whole genome genetic studies, an increasing number of monogenic forms of diabetes is being discovered thus the true prevalence of these types may be underestimated [4].
MODY, the commonest cause of monogenic β-cell dysfunction, is estimated to be the underlying cause in 0.5–1% of all patients with diabetes [5]. A typical ‘MODY patient’ has a young age of onset (<45 years, frequently <25 years), an autosomal dominant (AD) family history of diabetes, absence of autoimmune markers, absence of insulin resistance, and remains C-peptide positive even if insulin-treated [5]. Mutations in a number of different genes have been associated with the MODY phenotype; however, in clinical practice, the vast majority of MODY cases are due to heterozygous mutations in genes encoding the enzyme glucokinase (GCK) and the nuclear transcription factors hepatocyte nuclear factor 1α (HNF1A), hepatocyte nuclear factor 4α (HNF4A), and hepatocyte nuclear factor 1β (HNF1B) [5].
G. Neonatal Diabetes
Affects ~1 in 100,000–150,000 live births [5]. Diabetes diagnosed before 6 months is likely to be one of the monogenic subtypes of neonatal diabetes, rather than T1DM [5]. A genetic diagnosis is found in over 80% of cases and diagnosis could change management at any age [5]. Neonatal diabetes may be transmitted by AD or AR inheritance and may lead to either isolated diabetes or diabetes as part of a complex syndrome [5]. Where neonatal diabetes is the 1st presentation of a rare syndrome, identifying the cause may guide future clinic management or inform prognosis [5].
H. Mitochondrial Diabetes
Mitochondrial diabetes has a similar prevalence to the rarer forms of MODY and is another important diagnosis not to miss [5]. Caused by Maternal inheritance [5]. Commonly associated with sensorineural deafness (MIDD: maternally inherited diabetes and deafness) [5]. Clinical features include myopathy, cardiomyopathy, macular retinal dystrophy (which may be detected at retinal screening), CNS disease, and renal disease [5].
Clinical phenotype can be variable within the same family, due to heteroplasmy (variable distribution of abnormal mitochondria) [5]. Clinical management should include cardiology assessment (interval echocardiography), audiology, and clinical genetics advice [5]. Lack of access to insulin, misdiagnosis or delayed diagnosis of type 1 diabetes, results in diabetic ketoacidosis, a common cause of death in children and young people with diabetes [4].
II. COMPLICATIONS
A. Hyperglycemic Hyperosmolar State - HHS
Acute diabetes complications, resulting from extremes of blood glucose levels are common in type 1 diabetes and can occur, with certain medications, in type 2 diabetes and other forms of the condition as well [4]. They can lead to permanent neurological consequences or death [4]. If the initial diagnosis of type 1 diabetes is delayed, it typically presents with a build-up of ketones in the body, or diabetic ketoacidosis (DKA) [4]. It will also appear if blood glucose control is sub-optimal [4]. DKA is a complex metabolic disorder that requires expert guidelines-based management [4]. With such care, outcomes are usually satisfactory, but deaths can still occur, particularly if cerebral oedema develops [4]. Hyperosmolar hyperglycemic state (HHS) used to be called hyperosmolar non?ketotic hyperglycemic coma, or HONK, but is now termed HHS because mild ketosis can be present and because not all patients are comatose [1]. It The hyperglycemic hyperosmolar state (HHS) can also occur in people with type 2 diabetes [4]. The onset of HHS can be insidious but it can progress to profound dehydration and electrolyte losses, with a risk of other complications [4]. Accurate diagnosis and careful treatment are required to achieve good clinical outcomes. Although there are multiple precipitating causes, infections are the most common [4]. Up to 20% of people with HHS do not have a previous diagnosis of diabetes [4]. These are no universally agreed diagnostic criteria, but all patients are very unwell presenting with high osmolality, very high blood glucose concentrations, and severe dehydration [1]. The elderly, the chronically ill and institutionalized populations are at increased risk for HHS [4]. Overall mortality for HHS is estimated at 5–20% [4]: 10 times higher than that for diabetic ketoacidosis [4].
B. DED: Diabetic eye disease
Diabetic eye disease (DED) is a much-feared complication of diabetes, consisting predominantly of diabetic retinopathy (DR), diabetic macular oedema (DMO), cataract and glaucoma, but also double vision and inability to focus [4]. In most countries, DR is acknowledged to be one of the leading causes of blindness in the working age population with devastating personal and socioeconomic consequences, despite being potentially preventable and treatable [4]. Based on an analysis of 35 studies worldwide carried out between1980 and 2008, the overall prevalence of any DR in people with diabetes using retinal images was estimated to be 35% with vision threatening DR present in 12% [4]. DR prevalence increased with duration of diabetes in both type 1 and type 2 diabetes, and was associated with deteriorating glycemic control and the presence of hypertension [4]. Early diagnosis and timely treatment of diabetic retinopathy can prevent sight impairment and blindness [4]. Optimized blood glucose and blood pressure management complemented by screening for diabetic retinopathy can reduce the impact of diabetic eye disease [4]. Internationally agreed standards for screening methods and diagnostic criteria are required to make meaningful comparisons of diabetic retinopathy prevalence between countries, regions and ethnic groups [4].
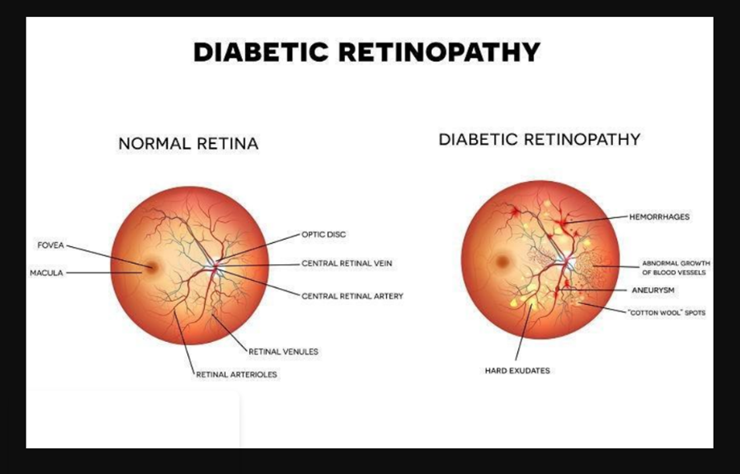
Fig 4 - Difference between a normal retina and one infected with Diabetic Eye Disease
C. Diabetes and Cardiovascular Diseases (CVD)
Diabetes and elevated blood glucose are associated with an approximate doubling of cardiovascular diseases risk [4]. The risk of cardiovascular diseases in people with diabetes can be reduced by lowering high blood pressure and high glucose levels, and using lipid lowering medications [4]. Elevated levels of blood glucose, and diabetes itself, lead to increased risk of CVD through multiple mechanisms, including insulin resistance, inflammation, endothelial dysfunction, and the toxic effects of glucose on microvasculature [4]. In addition, elevated blood glucose levels are associated with a common set of other underlying metabolic risk factors, including hypertension, dyslipidemia, and central obesity [4].
D. Diabetic Kidney Disease
Chronic kidney disease (CKD) in people with diabetes can result from diabetic nephropathy or can be the result of other associated conditions such as hypertension, polyneuropathic bladder dysfunction, increased incidence of relapsing urinary tract infections, or macrovascular angiopathy [4]. Diabetes, hypertension, or a combination of both, cause 80% of end-stage renal disease globally [4]. Diabetes, hypertension and CKD are highly interlinked [4]. In type 2 diabetes, hypertension often precedes CKD and contributes to the progression of nephropathy, whereas in type 1 diabetes, hypertension is more often a consequence of CKD [4]. Hyperglycemia induces hyperfiltration and morphological changes in the kidneys that ultimately lead to an increased urinary albumin excretion (albuminuria), podocyte damage and loss of filtration surface, hence the use of albuminuria and glomerular filtration as screening tests in this field is allowed [4]. The most effective strategy to reduce the impact of diabetic kidney disease is to prevent type 2 diabetes and, among those already affected by diabetes, to diagnose and treat CKD in its early Stages [4]. Screening for albuminuria, or glomerular filtration rate (GFR), is cost-effective in people with diabetes and hypertension [4]. Screening for albuminuria is recommended yearly after diagnosis of type 2 diabetes, and the same after the first five years in people with type 1 diabetes [4].
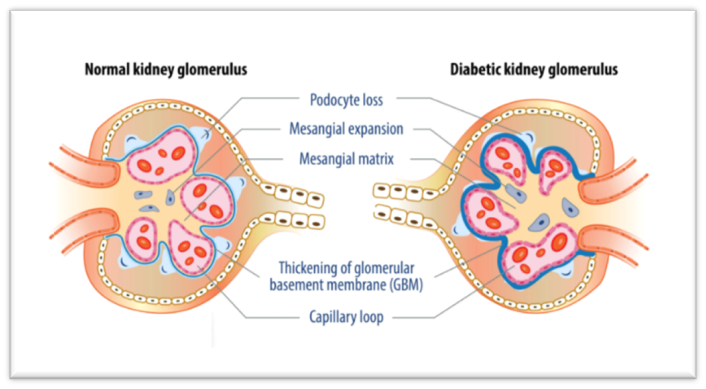
Fig 5 - Difference between a normal kidney glomerulus and a kidney glomerulus affected with Diabetic kidney disease
E. Nerve and/or vascular Damage and Diabetic Foot Complication
Peripheral neuropathy is the most common form of diabetes-related neuropathy [4]. It affects the distal nerves of the limbs, particularly those of the feet [4] It mainly alters the symmetrical sensory function causing abnormal feelings and progressive numbness [4]. These conditions facilitate the development of ulcers resulting from external trauma and/or abnormal distribution of the internal bone pressure (the so-called ‘diabetic foot’) [4]. Diabetic foot complications are severe and chronic [4]. They consist of lesions in the deep tissues associated with neurological disorders and peripheral vascular disease (PVD) in the lower limbs [4]. The reported prevalence of diabetes-related peripheral neuropathy ranges from 16% to as much as 87% with painful diabetes-related neuropathy reported in about 26% of adults with diabetes [4]. Diabetic foot and lower limb complications, which affect 40 to 60 million people with diabetes globally, are an important source of morbidity in people with diabetes [4]. Chronic ulcers and amputations result in a significant reduction in the quality of life and increase the risk of early death [4]. People with PVD have an increased risk of diabetic foot amputation, myocardial ischaemia and stroke, with long-term disability, and an increased risk of death [4]. Approximately 50% of people with PVD are asymptomatic, while 33% have atypical symptoms [4].
F. Diabetes and Cancer
A greater risk of cancer has been detected among adults with type 2 diabetes and those with a high body mass index (BMI), with the strongest associations found for breast and endometrial cancer in women and colorectal and intrahepatic (liver) cholangiocarcinoma in both genders [5] The elevated cancer risk for these sites ranges from 20% higher risk (breast cancer) to a nearly two-fold greater risk (endometrial and intrahepatic cholangiocarcinoma) [5].Type 2 diabetes and high body mass index are associated with an increased risk of a number of common cancers, with high body mass index associated with almost twice as many cancers as diabetes [6].
III. TREATMENT
A. Insulin
Critically ill patients with type l diabetes mellitus will require insulin therapy upon admission to the hospital. [2] For critically ill patients with type 2 diabetes, intravenous insulin infusion therapy should be initiated when plasma glucose levels exceed 180 to 200 mg/dL (10-11. l mmol/L) [2]. Glucose goals on intravenous insulin are 140 to 200 mg/dL (7.8-11.1 mmol/L) with frequent bedside point-of care (POC) monitoring every 1 to 2 hours for insulin adjustments [2]. In noncritically ill patients, the ADA and AACE advocate a premeal glucose goal of less than 140 mg/ell (7.8 mmol/L) and random plasma glucose values less than 180 mg/clL (10 mmol/L) [2]. Therapy adjustments should be considered when plasma glucose levels are less than 100 mg/dL (5.6 mmol/L) and are necessary when glucose values fall below 70 mg/dL (3.9 mmol/L) to avoid continued hypoglycemia [2].
Treatment of hypoglycemia is twofold: immediate correction of hypoglycemia and prevention of future events [2]. If a patient is conscious, 15 to 20 g of a carbohydrate with glucose should be consumed [2]. Glucose tablets or glucose gel are ideal treatment regimens [2]. The blood glucose level should be checked again after 15 minutes, and consumption of 15 to 20 g of glucose should occur again if the hypoglycemia does not improve to greater than 70 mg/dL (3.9 mmol/L) [2]. Since the effects of the insulin or oral hypoglycemic agents are likely still present, a meal or snack should be consumed after the glucose has been corrected to avoid continued hypoglycemia [2]. Every patient with diabetes on medications associated with hypoglycemia should receive a prescription for a glucagon kit, which should be used when oral consumption of glucose is not possible or safe [2].
Insulin is synthesized in and secreted from the β cells within the islets of Langerhans in the pancreas [1]. The normal pancreas has about 1 million islets, which are derived embryologically from endodermal outgrowths of the fetal gut [1]. The islets can be identified easily with various histological stains with which the cells react less intensely than does the surrounding exocrine tissue [1]. Pancreatic islets vary in size from having a few dozen to several thousands of cells and they are scattered irregularly throughout the exocrine pancreas [1]. The main cell types of the pancreatic islets are β?cells that produce insulin, α?cells that secrete glucagon, δ?cells that produce somatostatin and PP?cells that produce pancreatic polypeptide [1]. Different islet cell types can be identified by various immunostaining techniques [1]. β?cells are the most numerous cell type and are located mainly in the core of the islet, while α and δ cells are located in the periphery [1]. Islet cells interact with each other through direct physical contact and through paracrine effects of their hormone products (glucagon stimulates insulin secretion and somatostatin inhibits insulin and glucagon.) [1].
Insulin is synthesized in the β cells from a single amino acid precursor called proinsulin [1]. Synthesis begins with the formation of an even larger precursor, preproinsulin, which is cleaved by protease activity to proinsulin [1]. The gene for preproinsulin (and therefore the ‘gene for insulin’) is located on chromosomes [1]. Proinsulin is packaged into vesicles in the Golgi apparatus of the β cell; in the maturing secretory granules that bud off it, proinsulin is converted by enzymes into insulin and connecting peptide (C?peptide) [1]. Insulin and C?peptide are released from the β cell when the secretory granules are transported (‘translocated’) to the cell surface and fuse with the plasma membrane (exocytosis) [1]. Microtubules, formed of polymerized tubulin, probably provide the mechanical framework for granule transport, and microfilaments of actin, interacting with myosin and other motor proteins such as kinesin, may provide the mechanical force that propels the granules along the tubules [1].
B. Insulin Secretion in the Body
Glucose is the main stimulator of insulin release from the β cells, and insulin secretion occurs in a characteristic biphasic pattern – an immediate ‘first phase’ response that lasts only a few minutes, followed by a more gradual sustained ‘second phase’ [1]. The first phase of insulin release involves a small, readily releasable pool of granules fusing with the plasma membrane [1]. Of particular importance is the observation that first?phase insulin secretion is lost in patients with type 2 diabetes [1]. Various types of fuels, hormones, and neurotransmitters regulate insulin secretion [1]. Glucose is the most important regulator and glucose stimulates insulin secretion by mechanisms that depend upon the metabolism of glucose and other nutrients in the β cell [1].
A triggering pathway involves closure of ATP?sensitive potassium channels (KATP channels), cellular depolarization, an influx of calcium through voltage?dependent calcium channels and an increase in intracellular calcium concentration [1]. Simultaneously, a metabolic amplifying pathway augments the stimulatory effect of calcium on the exocytosis of insulin?containing granules. The second messenger cAMP is an important amplifier of insulin secretion triggered by Ca2+ elevation in the β cells [1].
Understanding the action profiles of different insulins is key to understanding when to take them and which insulin may be contributing to high or low glucose readings at any given time [5]. Physiological insulin replacement depends on dividing the insulin into basal (background) and bolus (mealtime/quick-acting) insulin [5]. In most patients, aim for a 50/50 split between basal and bolus insulin [5]. Background insulin is affected by weight, stress, exercise, alcohol [5]. Quick-acting insulin is adjusted, according to carbohydrate intake and exercise, and is also used to correct high readings [5]. Biosimilar insulins: Biological copies of analogue insulins made by other companies which may offer cost advantages, e.g. Abasaglar, Semglee (both insulin glargine), Lispro Sanofi (insulin Lispro) [5].
Conclusion
We explored the various types of diabetes, its’ serious effect on life and complications, and final treatment. Diabetes causes a dramatic change in the patients’ life and requires a healthy amount of mental and physical strength to get through. The most effective management of diabetes mellitus includes a disciplinary approach, including patient education and support, engaging patients in their care and decision making, lifestyle modifications with diet and exercise, reduced caloric intake for overweight and obese patients, and pharmacologic therapies when necessary to meet individual goals. Self-management for people with diabetes is an important part of successfully preventing or delaying diabetes complications in the future.
References
[1] Richard Donnelly (Author), Rudy Bilous (Author), Iskandar Idris (Author). Handbook of Diabetes, 5th Edition. [2] Cynthia A. Burns (Editor), MKSAP Endocrinology and Metabolism Paperback – 28 February 2019 [3] Mushtaq Haroon (Author), Sundus Maryium Husnain (Author), Diabetes and Endocrinology: Essentials of Clinical Practice Paperback. [4] (International Diabetics Federation) IDF DIABETES ATLAS: Ninth edition 2019 [5] Dr. Katharine Owen (Editor), Dr Helen Turner (Editor), Professor John Wass (Editor), Oxford Handbook of Endocrinology & Diabetes 4e (Oxford Medical Handbooks) [6] Piero Portincasa (Editor), Gema Frühbeck (Editor), Hendrik M. Nathoe (Editor), Endocrinology and Systemic Diseases 1st ed. 2021 Edition [7] Mijovic Kondejewski, Endocrinology Pocket Guide: Full illustrated [8] Shlomo Melmed (Author), Ronald Koenig (Author), Clifford Rosen MD (Author), Richard Auchus MD PhD (Author), Allison Goldfine MD (Author), Williams Textbook of Endocrinology 14th Edition, by [9] Amanda Ogilvy-Stuart (Author), Practical Neonatal Endocrinology (Cambridge Clinical Guides) 1st Edition [10] Justin M. Gregory, Daniel J. Moore and Jill H. Simmons, Pediatrics in Review 2013;34;203, Type 1 Diabetes Mellitus
Copyright
Copyright © 2025 Jia Jaison. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66842
Publish Date : 2025-02-05
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

