Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Early Classification of Emphysema in CT Scans Using Deep Learning
Authors: R. Jyoshna , B. Jyothi, P. Sandhya, T. Karthik Yadav , Sk Mahabob Vali, K. Sneha Priya , R. Karthik , Sacheena Kumbara
DOI Link: https://doi.org/10.22214/ijraset.2024.63296
Certificate: View Certificate
Abstract
Learning about Lung Diseases and their characterization is one of the most interesting research topics in recent years. With the various uses of medical images in hospitals, pathologies, and diagnostic centers, the size of the medical image datasets is also expanding expeditiously to capture the diseases in hospitals. Though a lot of research has been done on this particular topic still this field is confusing and challenging. There are lots of techniques in literature to classify medical images. The main drawback of traditional methods is the semantic gap that exists between the low-level visual information captured by imaging devices and high-level semantic information perceived by a human being. The difficulty of querying and managing the large datasets leads to a new mechanism called deep convolutional neural network. Chronic Obstructive Pulmonary Disease (COPD) is a prevalent respiratory disorder characterized by airflow limitation, often caused by emphysema. Early detection and classification of emphysema severity play a crucial role in effective treatment and management. Deep learning techniques have recently achieved an impressive result in the field of computer vision along with Medical Engineering. Our aim was to determine whether participant-level emphysema pattern could predict impairment and mortality when classified by using a deep learning method.
Introduction
I. INTRODUCTION
A substantial number of disorders that threaten the global population are lung-related. Cardiovascular complications like chronic obstructive pulmonary disease (COPD), asthma, fibrosis and pneumonia are impacting the foremost portion of society globally. Emphysema, which has a major risk factor for mortality worldwide, is one crucial constituent of COPD. The
disease can cause breathing difficulties due to the massive growth of the respiratory tract. In specific, emphysema is sub classified into CLE, paraseptic emphysema (PSE) and PLE .For instance, CLE is generally related to smoking and PSE is not always related to the major signs or neurological disabilities. As a result, it is essential to classify and quantify emphysema. The CT scan has been deemed to be the foremost specific scanning technology to identify emphysema, classify its category and assess its seriousness. In CT, emphysema’s categories contain different radiology appearances. Over the past few decades, several algorithms are suggested for classifying the emphysema in lung CT images. Such algorithms are categorized into supervised and unsupervised models. The goal of unsupervised algorithms is to find new emphysema categories, which go beyond the common categories rec organized on autopsy. A generative framework has been constructed to find the disease categories within emphysema and find patient groups, which are represented via different distributions of those categories. On the other hand, supervised algorithms concentrate on categorizing the typical emphysema categories detected on autopsy, which include various pathophysiologic significances. The variances in CT scans can impact the efficiency of classifying the emphysema; therefore, it is required to develop a model that is highly strong to those variabilities.
II. PROBLEM STATEMENT
The primary objective of this project is to develop an advanced predictive model capable of accurately classifying emphysema patterns in CT (Computed Tomography) images, with the ultimate goal of facilitating early detection and differentiation of emphysema severity. particularly by employing the DenseNet121 architecture, this study aims to overcome these challenges and enhance the accuracy of emphysema classification. DenseNet121 is chosen for its proven efficiency in feature extraction and classification tasks, making it well-suited for the complexities of medical image analysis. Through meticulous training and validation processes, the model is expected to learn intricate patterns and subtle variations indicative of different emphysema patterns, such as Clear Lung Emphysema (CLE), Patchy Lung Emphysema (PLE), Predominantly Subpleural Emphysema (PSE), and Non-Emphysematous (NT) regions.
Furthermore, accurate classification can aid in developing personalized management strategies tailored to individual patients, optimizing their quality of life and clinical outcomes.
III. LITERATURE REVIEW
The proliferation of computers, smartphones, and other Internet- enabled gadgets leaves the world vulnerable to cyber assaults. A plethora of malware detection methods have arisen in response to the explosion in malware activity. When trying to identify malicious code, researchers use a variety of big data tools and machine learning techniques. Traditional machine learning-based malware detection approaches have a considerable processing time, but may effectively identify newly emerging malware. An enhanced CNN-based model [1] has been developed to categorize the Interstitial Lung Disease (ILD) from CT scans. Initially, the scan patches were acquired from the annotated Region-Of-Interests (ROIs), and they were spa tially related or invariant to their overall intra-slice CT regions. Afterward, features with their classes were fed to the weakly supervised training-based CNN to categorize the ILD tags. But, if similar patches have many disease classes, then it was complex to classify the ILD. Automated DL-based categorization of centrilobular emphysema from CT scans [18] was developed. The CNN was integrated into the long short-term memory (LSTM) network to predict the topic-level values from CT images. Also, the Fleischner framework was used to handle the training data. But, it requires more memory to train the data and causes an overfitting problem. Automatic categorization of emphysema from weakly labeled high-resolution CT lung images [2] has been designed. First, the COPD patient’s CT images were col lected and the lung parenchyma in these images was rep resented via the textual features. Also, these images were trained by miSVM (multiple instances SVM) and multiple instance learning by embedded instance selection (MILES). On the other hand, the training samples were not adequate and the data imbalance problem has occurred. An improved pneumonia prediction model [3] was suggested based on machine and DL algorithms. First, the dataset comprising a group of stroke people with and without pneumonia was obtained. After, the time-aware characteristics have been obtained and provided to the linear regression, SVM, XGBoost, multi-linear perceptron and recurrent neural network to categorize pneumonia. However, many kinds of lung disorders were needed for categorization. The lung diseases were recognized and categorized from the CT images [4] by extracting the Haralick texture characteristics, Zernike’s moments, Gabor and Tamura surface characteristics. After that, these features were fed to the improvised crow search grey wolf and cuttlefish algorithms to decide the best attribute subset. Also, K-nearest neighbor, SVM, random forest (RF) and decision tree classifiers have been used to categorize COPD. But, it has high computation time complexity. A new framework [4] has been suggested to split tree like structures via adjacent data and introduced a graph-cut scheme for guaranteeing the spatial and directional dependability of the constructed subtrees. Also, an RF classification was conducted to categorize the pulmonary artery vein by predicting the correlation between lung syndromes and lung tissue patterns. An enhanced method [5] has been developed to cate gorize various classes of lung diseases from CT images using the ensemble of Riesz and deep characteristics. Pri marily, the discriminative parametric pulmonary surface patterns were trained by the Riesz descriptions. After, the attributes from deep CNNs were determined by adjusting the inception V3 model. Such learned descriptions were fused in a softmax layer to analyze the lung tissues. Segmentation and classification of lung parenchyma [6] were designed based on the CNN structure. Initially, a clustering scheme was applied to automatically create a dataset for training the CNN. After, a k-means clustering scheme was performed to segment the CT slices into image patches. Moreover, a cross-shaped proof, a size-junction, simultaneous testing and a patch development were utilized for generating the resultant dataset. At last, this dataset was fed to the CNN to categorize COPD. But, the dimension of patches was constant, and its effect on the efficiency of CNN was not analyzed. A level of accuracy in the medical systems using an open dataset was analyzed [7] by the DL. First, the spectrogram attributes and labels of the annotated lung images were extracted. Then, such attributes were applied to the 2D-CNN model to categorize lung diseases. But, the training data were not sufficient. A two-phase method was recommended [8] to visualize the COPD growth at the extent of unorganized medical files. First, a 4-layer DL structure was created, which exploits a certain RNN to discover the inaccurate period-lapse parts. After, a tem poral image was created by the irregular period-lapse parts to categorize the conditions of COPD. But, it needs more memory for training.
IV. METHODOLOGY
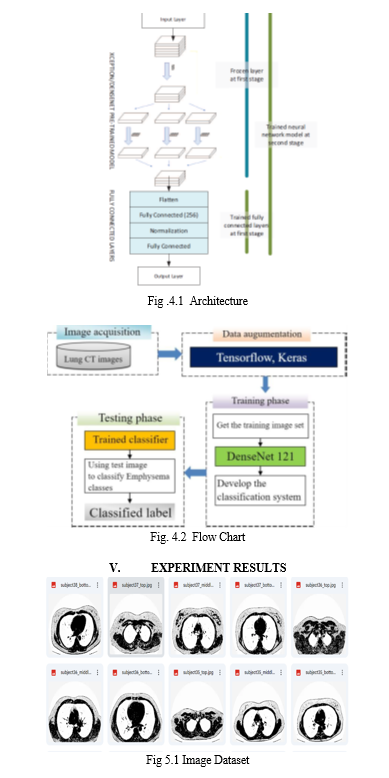
The research methodology process will be explained in this section. The general overview of the research methodology is shown in Fig.4.1.
A. Modules Used Are
- TensorFlow: TensorFlow is a machine learning framework developed by Google, used for building and deploying machine learning models, especially deep learning models.
- Pillow (PIL): Pillow is a Python Imaging Library (PIL) fork that provides capabilities for opening, manipulating, and saving various image file formats.
- cv2: OpenCV (Open Source Computer Vision Library) provides functions for image processing, computer vision tasks, and machine learning algorithms related to image analysis.
- Numpy: NumPy is a fundamental package for scientific computing with Python, providing support for multidimensional arrays, matrices, mathematical functions, and more.
- Scikit-learn: Scikit-learn is a machine learning library in Python that offers tools for data mining, analysis, and modeling, including a wide range of machine learning algorithms. matplotlib: Matplotlib is a plotting library for Python that provides functions for creating static, animated, and interactive visualizations, widely used for data visualization and analysis.
- Keras: Keras is a user-friendly deep learning library in Python. It offers a simple interface for building neural networks and includes utilities for data preprocessing and model evaluation. It's widely used for training deep learning models and supports data augmentation through the ImageDataGenerator class.
B. Methods and Algorithms
- Convolutional Neural Networks (CNNs): Convolutional Neural Networks (CNNs) are specialized deep learning models tailored for analyzing and categorizing visual data, such as images. Comprising numerous layers, they include convolutional layers, pooling layers, and fully connected layers. Convolutional layers employ convolution operations to extract distinctive features from input images by systematically applying small filters (kernels) across the image, detecting patterns and features. Pooling layers subsequently reduce the spatial dimensions of the obtained feature maps, extracting key features. Finally, fully connected layers process the flattened feature maps to generate predictions, often incorporating activation functions like ReLU (Rectified Linear Unit) to introduce non-linearities.
- Data Augumentation: Data augmentation encompasses the application of various transformations, including rotation, flipping, scaling, and cropping, to input images. Through the integration of these transformed images into the training dataset, data augmentation enhances its diversity, thereby aiding the model in better generalization to novel data. This technique proves especially beneficial when dealing with constrained training datasets, as it effectively amplifies the dataset's effective size without the need for additional labeled samples. Commonly employed methods for data augmentation in image classification tasks include random rotation, horizontal flipping, and random scaling.
- DenseNet: DenseNet represents a distinct CNN architecture distinguished by its dense interconnections among layers. Within DenseNet, each layer establishes direct connections with every other layer in a feed-forward fashion, forming dense connections within a dense block. These interconnections foster effective feature reuse and facilitate enhanced gradient flow during training, effectively mitigating challenges like vanishing gradients. Renowned for their capacity to discern intricate patterns and features from images, DenseNet architectures, such as DenseNet-121, have demonstrated remarkable efficacy in various image classification tasks. Specifically, DenseNet-121 denotes a variant of DenseNet comprising 121 layers, striking a favorable balance between model complexity and performance.
- Ensemble Methods: Ensemble methods refer to strategies where predictions from multiple individual models are amalgamated to enhance overall performance and resilience. Typical ensemble techniques comprise averaging predictions, stacking, and boosting. These methods harness a variety of models trained with distinct architectures, initializations, or hyperparameters to capture complementary insights and mitigate overfitting risks. Notably, ensemble methods excel when individual models showcase diverse behaviors and errors, as they can collectively rectify individual model shortcomings, thereby bolstering effectiveness.

VI. FUTURE ENHANCEMENT
In the realm of medical image analysis, particularly concerning lung diseases like Chronic Obstructive Pulmonary Disease (COPD) and emphysema, there exists a burgeoning interest in leveraging deep learning techniques for classification and prediction tasks. While traditional methods have made strides, they often grapple with the semantic gap between low-level visual information from imaging devices and high-level semantic understanding needed for accurate diagnosis. Deep convolutional neural networks (CNNs) have emerged as a promising solution to bridge this gap, showcasing remarkable results in computer vision and medical engineering. Looking ahead, future enhancements could encompass a multifaceted approach. Firstly, integrating multi-modal data sources such as CT scans, X-rays, and clinical records could provide a more holistic view of the disease. Transfer learning techniques may aid in adapting pre-trained models to specific lung disease classification tasks, potentially reducing the need for extensive annotated datasets. Moreover, incorporating methods for uncertainty estimation, explainability, and interpretability is crucial for building trust in these models among healthcare professionals.
Longitudinal analysis could offer insights into disease progression over time, while advanced data augmentation and synthesis techniques could enhance model generalization. Additionally, exploring semi-supervised and weakly supervised learning approaches may leverage large amounts of unlabeled or weakly labeled data, making the development process more cost-effective. Ultimately, collaboration with medical experts and rigorous validation through clinical trials are paramount to ensuring the clinical relevance and efficacy of these deep learning models in improving patient outcomes and healthcare delivery.
VII. ACKNOWLEDGEMENT
An endeavor over a long period can be advice and support of many well wishers. We take this opportunity to express our gratitude and appreciation to all of them.
We owe our tribute to Dr. Thayyaba Khatoon(Head of Department) , for giving all of us such a wonderful opportunity to explore ourselves and the outside world to work on the real-life scenarios where the machine learning is being used nowadays.
We are very grateful to our project guide Prof. RAGIPATI KARTHIK, for the guidance ,inspiration and constructive suggestions that helped us in the development of this application. We also thank our parents and family at large for their moral and financial support in funding the project to ensure successful completion of the project .
Conclusion
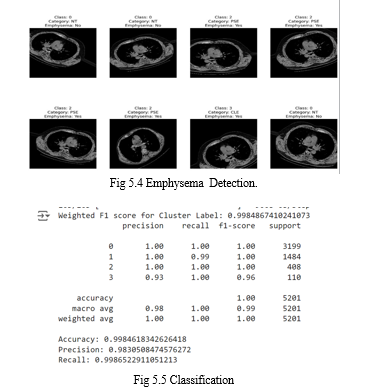
In conclusion, the emphysema detection project, leveraging deep learning techniques on CT scans, marks a significant advancement in respiratory health diagnostics. Through the fusion of deep learning algorithms and high-resolution CT imaging, the project has showcased remarkable efficacy in precisely identifying and characterizing emphysema-related anomalies within lung scans. This innovative approach not only aids in the early detection of emphysema but also offers insights into disease progression and severity. By harnessing the entirety of information provided by CT scans, including subtle structural changes and spatial distributions, the developed model demonstrates its potential to revolutionize clinical practice by providing accurate and timely diagnoses. Moving forward, continued refinement and validation of the deep learning models across diverse patient cohorts will be essential to ensure robust performance and widespread applicability in real-world healthcare settings. Ultimately, this project underscores the transformative impact of deep learning in advancing medical imaging and enhancing the management of respiratory conditions like emphysema.
References
[1] Gao M, Bagci U, Lu L, Wu A, Buty M, Shin HC, Xu Z (2018) Holistic classification of CT attenuation patterns for interstitial lung diseases via deep convolutional neural networks. Comput Methods Biomech Biomed Eng Imaging Vis 6(1):1–6 [2] Humphries SM, Notary AM, Centeno JP, Lynch DA (2018) Automatic classification of centrilobular emphysema on CT using deep learning: comparison with visual scoring. Image analysis for moving organ, breast, and thoracic images. Springer, Cham, pp 319–325 [3] Pino Pen˜a I, Cheplygina V, Paschaloudi S, Vuust M, Carl J, Weinreich UM, de Bruijne M (2018) Automatic emphysema detection using weakly labeled HRCT lung images. PLoS ONE 13(10):1–16 [4] Ge Y, Wang Q, Wang L, Wu H, Peng C, Wang J, Yi Y (2019) Predicting post-stroke pneumonia using deep neural network approaches. Int J Med Inform 132:1–32 [5] Gupta N, Gupta D, Khanna A, Rebouc¸asFilho PP, de Albu querque VHC (2019) Evolutionary algorithms for automatic lung disease detection. Measurement 140:590–608 [6] Jimenez-Carretero D, Bermejo-Pela´ez D, Nardelli P, Fraga P, Fraile E, Este´par RSJ, Ledesma-Carbayo MJ (2019) A graph-cut approach for pulmonary artery-vein segmentation in noncontrast CT images. Med Image Anal 52:144–159 [7] Joyseeree R, Ota´lora S, Mu¨ller H, Depeursinge A (2019) Fusing learned representations from Riesz filters and deep CNN for lung tissue classification. Med Image Anal 56:172–183 [8] Automated classification of emphysema using data augmentation and effective pixel location estimation with multi-scale residual network T. Manikandan1 • S. Maheswari2 [9] Tang C, Plasek JM, Zhang H, Kang MJ, Sheng H, Xiong Y, Zhou L (2019) A temporal visualization of chronic obstructive pul monary disease progression using deep learning and unstructured clinical notes. BMC Med Inform Decis Mak 19(8):1–9 [10] Liu H, Wang L, Nan Y, Jin F, Wang Q, Pu J (2019) SDFN: segmentation-based deep fusion network for thoracic disease classification in chest X-ray images. Comput Med Imaging Graph 75:66–73m
Copyright
Copyright © 2024 R. Jyoshna , B. Jyothi, P. Sandhya, T. Karthik Yadav , Sk Mahabob Vali, K. Sneha Priya , R. Karthik , Sacheena Kumbara. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63296
Publish Date : 2024-06-14
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

