Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Ecotoxicological and Potential Health Risk Assess-ment in Dimna Canal, Interlinking Lentic and Lo-tic Ecosystem in Jamshedpur, India
Authors: Preeti Kumari, Puja Hansdah
DOI Link: https://doi.org/10.22214/ijraset.2023.55935
Certificate: View Certificate
Abstract
Heavy and Trace Elements (HTEs) pollution is a serious menace to the ecosystem. These elements are ubiquitous that can bioaccumulate and biomagnify in the food chain. This study aimed to assess the HTE pollution on different trophic level of an aquatic food chain of Dimna canal interlinking a lentic (Dimna Lake) and a lotic (Subarnarekha River) ecosystem. In this study, water, sediments, and biotic samples of molluscs (Limnaea stagnalis) and fish (Channa punctatus) were collect-ed and analysed in an ICP-MS for six HTEs (Cu, Pb, Zn, As, CD and Cr). Then, the health risk to human through the con-sumption of L. stagnalis and C. Punctatus was calculated using the Target Hazard Quotient (THQ) and Hazard Index (HI). It is observed that the average level of Pb (12.96 µg/L) and Cd (3.83µg/L) in water is higher than the limit prescribed by WHO, 2004. In sediment, the average level of Zn (118.14 µg/g) and Cd (0.43 µg/g) is higher than the average shale value of the earth\'s crust. The higher value of Pb, Cd and Zn in the canal ecosystem may be due to the discharge of chemical, primer etc. from industries as well bricks industry. In mollusc (L. stagnalis), the average levels of HTEs are the highest in compar-ison to limits prescribed by FAO (1983) and account for 82.74 µg of Cu per g, 20.09 µg of Pb per g, 530.49 µg of Zn per g, and 0.86 µg of Cd per g. In edible fish (C.punctatus) parts, level of As (2.41 µg/g) and Cd (0.66 µg/g) are higher than the FAO limits. Mollusc, being the primary consumer of the aquatic food chain, accumulates more HTE than that of fish; hence mollusc can be used as a potential bio-indicator. THQ values of metals due to consumption of mollusc and fish show that As contributes almost 50 % in HI. Also, among the population of two age groups (adult and children); children are found more susceptible to HTE exposure. Hence, regular monitoring and assessment are required for aquatic ecosystems for the safeguard of human health.
Introduction
I. INTRODUCTION
Freshwater ecosystems are the inevitable source of water for various domestic and industrial applications. Any alteration in the freshwater ecosystem can affect human health. Heavy and Trace Elements (HTEs) derived from human-made sources viz. run-off from farming land, domestic and sewage discharge and industrial effluent discharge are the primary cause of these alterations (Giri and Singh 2015). Moreover, some natural sources such as atmospheric fallout, forest fire and weathering of rocks can also alter the ecosystem. The freshwater ecosystems mainly comprise of lentic and lotic ecosystems.
In any ecosystem, there is a linear flow of energy and nutrients from one organism to others due to their feeding habit. This linear sequential flow from one trophic level to another is regarded as the food chain. It is well known that HTEs can cause a hostile effect on the different trophic level through its longevity, bio-accumulative behavior and biomagnifying characteristics (Li et al. 2015). An elevated level of HTEs deposits in either water or sediment of freshwater ecosystems in the soluble, colloidal or suspended form which different trophic levels readily absorb. Fish and mollusc absorb HTEs either through their gills, food or through their body surface. Mollusc, the primary consumers, belongs to gastropod can tolerate continuous exposure of HTEs in its body (Goldberg 1975). In human, HTEs enter into the digestive tract through consumption of fish and mollusc and impart in health problems by damaging vital organs like kidney, liver, and cause cardiovascular disease (Rahman et al. 2012). The potential health risk posed by consuming aquatic organisms by the human is studied by estimating Target Hazard Quotient (THQ) and Hazard Index (HI). Health risk assessment is a useful tool to delineate the composite picture of HTEs exposure caused by specific HTE; hence it has been extensively used in various literatures (Tao et al. 2012; Yi et al. 2011).
The present study deals with the Dimna Canal, which is an interlinking between Dimna Lake and Subarnarekha River. The canal receives water from Dimna Lake, which is an artificial reservoir. During rain, when the water levels of Dime Lake rise, the sluice gate opens, and the canal is enriched with lake water. This result in an outflow of water from the canal under gravity to travel about 10 km and mingles with Subarnarekha River. During its movement, it crosses a densely populated region of Jamshedpur. Jamshedpur is more than 150 years old, first planned industrial city of India. Now, it houses > 1200 industries. The rapid industrial development in the city promoted from the agglomeration of people from the rural area to the main city. The urbanization resulted in the emergence of pollution problems.
In any country, there are several lentic and lotic ecosystems for fulfilling the water requirement. Also, there are many canals sources of which may be lakes, rivers or any industrial body. Although research has been carried out in HTE pollution in the lentic and lotic ecosystem (Gupta et al. 2014; Chen et al. 2016) but hardly any articles are available that delineate the pollution scenario of such interlinking canals. Such types of canals, when located in any industrial city, exhibit various pollution characteristics. Dimna canal also flows through a densely populated region of Jamshedpur city; hence it can reflect the pollution status of the whole city.
A few decades ago, the canal was a pellucid water body with its salubrious flora and fauna (based on personal observations). But now, the canal has turned into a drainage canal which resulted in the ecological deterioration and species loss. People residing in the canal also claim that polluted canal has a detrimental effect on their health and crops. Therefore, to determine the status of pollution in Dimna canal, the concentration of HTEs and their probable impact on human through consumption of aquatic organisms are studied. The major objective of the present study is to: (a) assess the level of HTEs (Cu, Pb, Zn, As, CD and Cr) in water and sediment; (b) determine the HTE content in mollusc (Limnaea stagnalis) and fish (Channa punctatus); and (c) calculate probable health risk in an adult and child population due to ingestion of contaminated fish and mollusc.
II. MATERIALS AND METHODS
The maximum and mean depths of the canal were approx. 1.2 m and 0.5 m, respectively, especially in the dry season, and its catchment area comprises a large population of about 70000 people. Seven Sampling sites were selected to assess the HTE pollution in Dimna Canal (Fig. 1). Five replicates of composite grab sample of water were collected from seven sites in the dry season (July-Aug 2017) (Fig. 1). Also, sediment samples (in five replicates) were collected using a PVC corer of diameter 5 cm and length 30 cm. Ten replicates of fish samples were collected using the fishing net, and twenty replicates of mollusc samples were collected by hand picking method. The selected fish and mollusc species was Channa punctatus and Limnaea stagnalis. Fish and mollusc samples were stored in plastic bags. All sediment, water and biological samples were stored in an icebox and transported to the laboratory at an earliest. In Laboratory, 1 L of water samples was treated with 5 mL of conc. HNO3 (65%, Merck, Germany) and heated for 15 (APHA 2012). Sediment samples were placed in a hot air oven (REMI 24, India) oven (temperature = 105°C, time = 12 h); then ground with pestle and mortar and sieved through 63 µ sieve. 0.2 gm of sediment samples was digested in microwave digester with a mixture of HNO3 (4.5 ml), HF (40%, Merck, Germany, 2 ml), HCl (35%, Merck, Germany, 1 ml) and H2O2 (30%, Merck, Germany, 0.5 ml) (USEPA 1996). The Fish samples were washed with the water of their respective sites, and then muscle samples were taken out with the help of knives, scissors and forceps. Similarly, the soft part of molluscs were removed with a spatula. All the biological samples were ashes in a muffle furnace at 50°C for 10 minutes, 150°C for 1 h and 450°C for 8 h. The ashed samples were digested on the hottest block with a mixture of HNO3 (10 ml), HClO4 (4 ml) and H2SO4 (1 ml) (AOAC 1990). All the digested samples were diluted (if required) with double distilled water and filtered through Whatman #42 filter paper. The samples were then analysed in ICP-MS (Inductively Coupled Plasma-Mass Spectrometry, Perkin Elmer Elan DRC-e) for HTES (As, Cd, Cr, Cu, Pb and Zn). For maintaining the calibration status of ICP-MS, a calibration blank and calibration standard solution was run after every ten samples. The calibration standards used were DOLT-5 (dogfish liver) and NIST 1640(a) (natural water) supplied by National Research Council, Canada and National Institute of Standards and Technology, USA, respectively. The recovery % varied from 96.8 to 104.3 for DOLT-5 and 89.6-107.2 for NIST 1640(a). Map representing study area was prepared in ARC-GIS software.
III. RESULT AND DISCUSSION
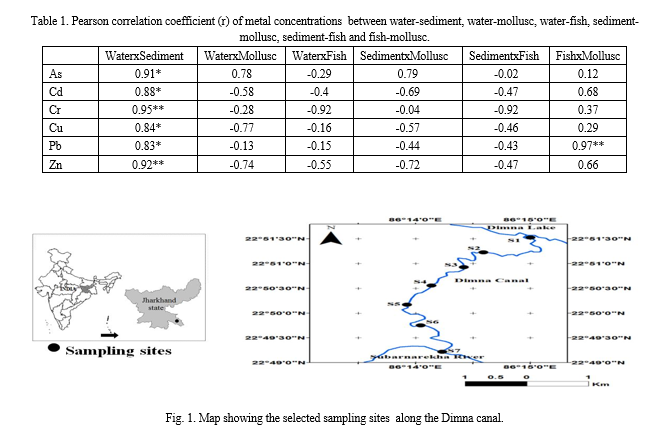
The concentration of different HTEs (µg/l, Fig. 2) in water were Cu (21.62), Pb (13.00), Zn (303.48), As (3.85), Cd (3.83) and Cr (34.76). While comparing with drinking water guidelines by WHO (2004) (Cu=2000 µg/l, Pb=10 µg/l, Zn=3000 µg/l, As=10 µg/l, Cd=3 µg/l, Cr=50 µg/l), there were two metals (Pb and Cd) for which the concentration was found higher. The higher level of Pb and Cd may be due to elevated industrial and sewage disposal into the canal (Banerjee et al. 2015). An elevated level of Pb also indicated that these HTEs came from traffic load of road and industrial discharge from chemical, paint, primer, coal tar and petroleum product manufacturing industry (Bai et al. 2011; Giri and Singh 2014).
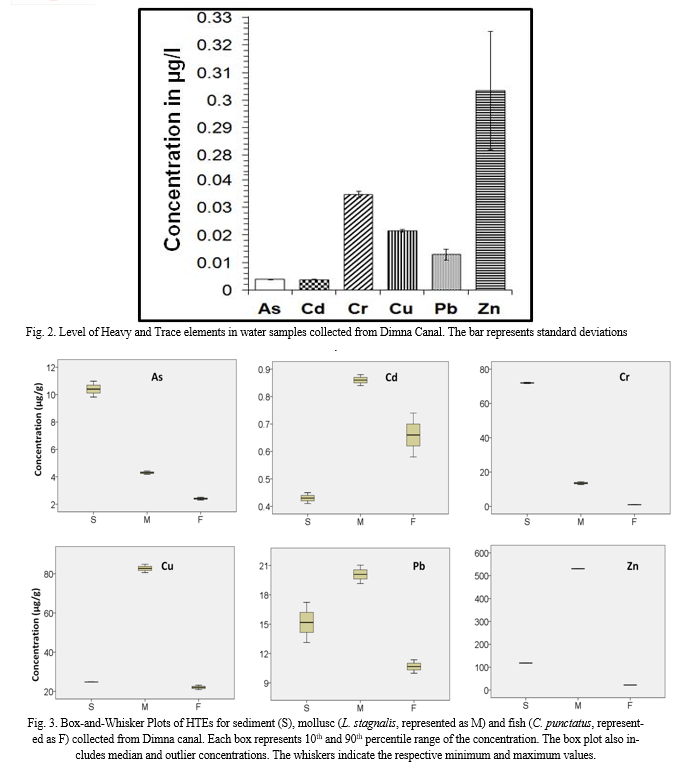
In sediments, the HTE levels (µg/g dry weight, Fig.3) were as follows: Cu (24.77), Pb (15.18), Zn (118.14), As (10.40), Cd (0.43) and Cr (71.97). The average level of HTEs in earth’s crust is 45 µg Cu/g, 20 µg Pb/g, 95 µg Zn/g, 13 µg As/g, 0.3 µg Cd/g, and 90 µg Cr/g (Turekian and Wedepohl 1961). Comparison of the concentration with that in shale/earth’s crust showed that the levels of Zn and Cd were higher in Dimna canal may be due to the discharge from bricks (coal consumer), rubber, paints and auto-lubricant etc. industry (Giri and Singh 2014). Furthermore, Zn was also known to be released from the mixing of agricultural runoff in the sediment during the dry season (Banerjee et al. 2015). Sediment and water analysis revealed that the HTE content in sediment was much higher than those determined in the water in this study as had been observed by Giri and Singh (2014) and Banerjee et al. (2015).
The concentrations of different HTEs (µg/g, dry weight) in L. stagnalis and C. punctatus were represented in Fig. 3. It was observed that HTE level was higher in L. stagnalis in comparison to fish may be due to the reason that mollusc can produce non-mineralized granules in its digestive glands that can trap an excess of HTEs (Goldberg 1975). While comparing with the Food and Agricultural Organization (FAO 1983), levels of all HTE elements in L. stagnalis were beyond the recommended level for human consumption. The levels of Cu, Pb, Zn, As, Cd and Cr in L. stagnalis were 82.73 µg/g, 20.09 µg/g, 530.49 µg/g, 4.30 µg/g, 0.86 µg/g and 13.52 µg/g, respectively. While comparing with the previous literature, it was observed that the level of Cd and Cr in L. stagnalis corroborates with the work of Gupta et al. (2014). However, the levels of Cu, Pb and Zn in L. stagnalis of the present study were quite higher than that of Gupta et al. (2014) and Abdallah (2013).
The fish, C. punctatus also accumulated a significant amount of HTEs. The levels in C. punctatus were 22.02 µg Cu/g, 10.67 µg Pb/g, 22.02 µg Zn/g, 2.41 µg As/g, 0.66 µg Cd/g, and 0.94 µg Cr/g. While comparing with FAO (1983), the levels of As, Cd and Pb were found higher than the acceptable limits. The HTE levels in C. punctatus were compared with previous literature. It was observed that the levels of Cu, Pb, Cd and As were higher than those observed by Batvari et al. (2008); Malik et al. (2010); Begum et al. (2005); Bhupander and Mukherjee (2011); Giri and Singh (2015) and Sekhar et al. (2004). However the level of Cr in C. punctatus was much higher than those reported in C. punctatus from Pulicat lake (Batvari et al. 2008), Bhopal lake (Malik et al. 2010) and Subarnarekha River (Giri and Singh 2015), but twenty times lower than that of Kolleru lake (Sekhar et al. 2004). The level of Zn in C. punctatus was quite higher than those reported by Batvari et al. (2008) and Malik et al. (2010), but lower than those reported by Begum et al. (2005); Bhupander and Mukherjee (2011); Giri and Singh (2015) and Sekhar et al. (2004). The variation in levels in fish and mollusc may be due to their feeding habits, floating characteristics, genetic tendency, organ structure and age etc. (Zhao et al. 2012).
Table 1 showed the pearson correlation between HTEs in water-sediment, water-mollusc, water-fish, sediment-mollusc, sediment-fish and fish-mollusc. The result showed that there were strong positive correlation (p>0.05) between HTEs in water and sediment showing that there are common source of HTEs in both medium. However, Cr between sediment and fish was revealed to have a strong negative association represented that Cr was absorbed through contaminated sediment in the fish species.
USEPA (2000) has recommended the calculation of Target Hazard Quotient (THQ) and Hazard Index (HI) to assess the probable health risk to the human population due to consumption of aquatic organisms (fish and mollusc). The formula for calculating THQ is mentioned below.
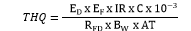
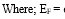 exposure frequency (for adult and children 208 days/year); ED = the exposure duration which is 68.35 years for adults and eight years for children (So et al. 2009). IR = the food ingestion rate. C = the HTE level in the food (µg/g). RFD = the reference dose (As: 0.0003 µg/g/day; Cd: 0.001 µg/g/day; Cr: 0.003 µg/g/day Cu: 0.04 µg/g/day; Hg: 0.0003 µg/g/day; Pb: 0.0035 µg/g/day; Zn: 0.3 µg/g/day). Bw = the body mass (adult-57.7 kg, children-15 kg) (USEPA 2000). AT = the average exposure time (days/year) and is calculated as 208 days/year ×68.35 years for adults and 208 days/year ×8 years for children (USEPA 2000). A field questionnaire of >40 people of adult group (18-35 year old) was performed at each of the seven locations, and it was concluded that average consumption of fish for the adult was 16 gm/day (d.w., assuming 79 % moisture). Since there was lack of adequate data on the investigation of consumption rate of mollusc (L. stagnalis), we assumed it to be 4 gm/day (d.w., assuming 82 % moisture as computed by author) based on local knowledge and questionnaire. In the questionnaire, one adult person represented a family of 2 adults + 2 children, thus, a total of 160 persons were effectively surveyed. Questionnaire revealed that people habitually do not consume fish and mollusc on two days in a week due to their religious reverences, hence for this study EF for adult and children was estimated as 208 days/year.
exposure frequency (for adult and children 208 days/year); ED = the exposure duration which is 68.35 years for adults and eight years for children (So et al. 2009). IR = the food ingestion rate. C = the HTE level in the food (µg/g). RFD = the reference dose (As: 0.0003 µg/g/day; Cd: 0.001 µg/g/day; Cr: 0.003 µg/g/day Cu: 0.04 µg/g/day; Hg: 0.0003 µg/g/day; Pb: 0.0035 µg/g/day; Zn: 0.3 µg/g/day). Bw = the body mass (adult-57.7 kg, children-15 kg) (USEPA 2000). AT = the average exposure time (days/year) and is calculated as 208 days/year ×68.35 years for adults and 208 days/year ×8 years for children (USEPA 2000). A field questionnaire of >40 people of adult group (18-35 year old) was performed at each of the seven locations, and it was concluded that average consumption of fish for the adult was 16 gm/day (d.w., assuming 79 % moisture). Since there was lack of adequate data on the investigation of consumption rate of mollusc (L. stagnalis), we assumed it to be 4 gm/day (d.w., assuming 82 % moisture as computed by author) based on local knowledge and questionnaire. In the questionnaire, one adult person represented a family of 2 adults + 2 children, thus, a total of 160 persons were effectively surveyed. Questionnaire revealed that people habitually do not consume fish and mollusc on two days in a week due to their religious reverences, hence for this study EF for adult and children was estimated as 208 days/year.
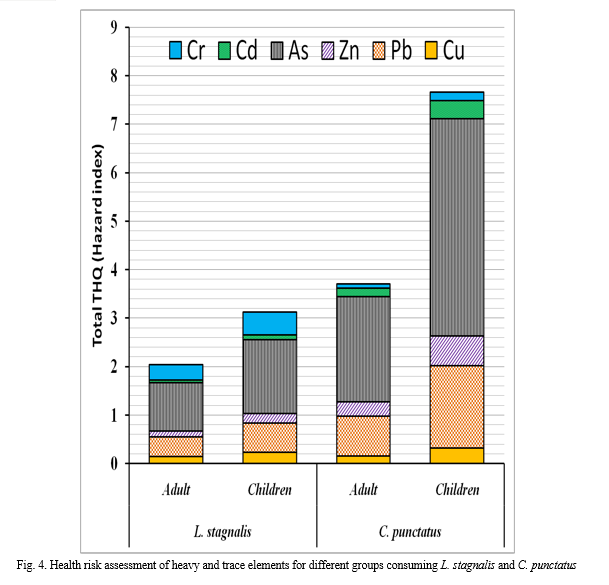
The THQ values of HTEs due to dietary intake of mollusc (L. stagnalis) and fish (C. punctatus) for adult and children populations are shown in Fig. 4.
The result showed that the HI values of HTEs are higher in C. punctatus in comparison to L. stagnalis. It should be noted that due to unavailability of RFD value of the total Cr, the value of Cr (VI) is taken in place of total Cr considering that the Cr s present in Cr (VI) form in the sample. Generally, the THQ value of Cu, Pb, Zn, Cd and Cr were <1; however the maximum THQ value accounted for C. punctatus for children, i.e. 0.31, 1.71, 0.61, 4.49, 0.37 and 0.18, respectively, for Cu, Pb, Zn, As, Cd and Cr. Moreover, maximum THQ value was observed for As, i.e. 0.99 and 1.53, respectively for adult and children consuming L. stagnalis; and 2.17, 4.49, respectively, for adult and children consuming C. punctatus. For the assessment of the overall human health risk from consuming aquatic food contaminated by HTEs, THQ of each HTE was added to calculate Hazard Index (HI) (Giri and Singh 2015; USEPA 2000). The HI value in all cases exceeded one suggesting that dietary intake of L. stagnalis and C. punctatus can impart health risk on adult and children population. However, the maximum HI value of 7.66 for the children consuming .C. punctatus indicated that these groups are more vulnerable to HTEs exposure. Furthermore, high THQ values for As in mollusc and fish may be due to industrial discharge in the form of copper arsenate, arsenic sulphide from As based pesticides and fertilizer manufacturing industries and agricultural runoff from the cultivated lands (Alloway 2013). Moreover, the source of Pb in C. punctatus may be the vehicular emission as the canal runs parallel to the roads and highways (Yi et al. 2011). It was observed that As was a significant risk contributor, for both local adult and children individual depending on the Dimna canal for aquatic food and contributed about 49% and 59% of the HI. The risk contributions of the rest of the HTE were relatively low and ranged between 3-20%.
Conclusion
The present study concludes that 1) The Dimna Canal is heavily contaminated with Pb, Cd and Zn may be due to the industrial discharge from bricks manu-facturing plants, chemical, paint, primer, coal tar and petroleum product manufacturing industry, vehicular emission, ag-ricultural run-off and domestic discharge. 2) Health risk assessment shows that the local population is exposed to HTE contamination due to consumption of C. punc-tatus and L. stagnalis. THQ and HI values indicate that the children are more susceptible to HTE exposure primarily due to exposure from As. Hence it can be stated that HTE pollution is a severe concern for the human population. Health risk assessment result showed an alarming situation for population consuming aquatic organisms like C. punctatus and L. stagnalis. It should be noticed that the dietary intake of the human population is not restricted to the selected L. stagnalis and C. punctatus species. The ac-tual consumption of HTEs by the local people is quite higher than the present study. Also, only a few HTEs were considered for health risk assessment. If all these factors would be considered, the combined risk from all HTE will be much higher. Hence it is necessary to regularly monitor the pollution status of the freshwater ecosystem and work on the remedial actions to eliminate the pollutant source.
References
[1] Alloway, B. J. (2013). Heavy metals in soils. Trace metals and metalloids in soils and their bioavailability. Environmental Pollution, 22, 465–496. [2] Abdallah, M. A. M. (2013). Bioaccumulation of heavy metals in mollusca species and assessment of potential risks to human health. Bulletin of Environmen-tal Contamination and Toxicology, 90(5), 552-557. [3] AOAC. (1990). Official methods of analysis (15th ed., p. 858). Association of Official Analytical Chemists Washington, DC: [4] APHA. (2012). Standard methods for the examination of water and wastewater (22nd ed.) American Public Health Association, Washington, DC: [5] Bai, J., Cui, B., Chen, B., Zhang, K., Deng, W., Gao, H., & Xiao, R. (2011). Spatial distribution and ecological risk assessment of heavy metals in surface sedi-ments from a typical plateau lake wetland, China. Ecological Modelling, 222(2), 301-306. [6] Banerjee, S., Maiti, S. K., & Kumar, A. (2015). Metal contamination in water and bioaccumulation of metals in the planktons, molluscs and fishes in Jam-shedpur stretch of Subarnarekha River of Chotanagpur plateau, India. Water and Environment Journal, 29(2), 207-213. [7] Batvari, B. P. D., Kamala-Kannan, S., Shanthi, K., Krishnamoorthy, R., Lee, K. J., & Jayaprakash, M. (2008). Heavy metals in two fish species (Carangoidel malabaricus and Belone stronglurus) from Pulicat Lake, North of Chennai, Southeast Coast of India. Environmental monitoring and assessment, 145(1-3), 167-175. [8] Begum, A., Amin, M. N., Kaneco, S., & Ohta, K. (2005). Selected elemental composition of the muscle tissue of three species of fish, Tilapia nilotica, Cirrhina mrigala and Clarius batrachus, from the freshwater Dhanmondi Lake in Bangladesh. Food Chemistry, 93(3), 439-443. [9] Bhupander, K., & Mukherjee, D. P. (2011). Assessment of human health risk for arsenic, copper, nickel, mercury and zinc in fish collected from tropical wet-lands in India. Advances in Life Science and Technology, 2, 13-24. [10] Chen, H., Chen, R., Teng, Y., & Wu, J. (2016). Contamination characteristics, ecological risk and source identification of trace metals in sediments of the Le\'an River (China). Ecotoxicology and environmental safety, 125, 85-92. [11] FAO. (1983). Compilation of legal limits for hazardous substances in fish and fishery products. Food and Agricultural Organization (FAO) Fishery Circular No. 464, pp 5-100. [12] Giri, S., & Singh, A. K. (2015). Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environmental Monitoring and Assessment, 187(3),63. [13] Giri, S., & Singh, A. K. (2014). Assessment of surface water quality using heavy metal pollution index in Subarnarekha River, India. Water Quality Exposure and Health, 5(4), 173-182. [14] Goldberg, E. D. (1975). The mussel watch—a first step in global marine monitoring. Marine Pollution Bulletin, 6(7), 111. [15] Gupta, S. K., Chabukdhara, M., Kumar, P., Singh, J., & Bux, F. (2014). Evaluation of ecological risk of metal contamination in river Gomti, India: a biomoni-toring approach. Ecotoxicology and environmental safety, 110, 49-55. [16] Li, P., Lin, C., Cheng, H., Duan, X., & Lei, K. (2015). Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern Chi-na. Ecotoxicology and Environmental Safety, 113, 391-399. [17] Malik, N., Biswas, A. K., Qureshi, T. A., Borana, K., & Virha, R. (2010). Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bho-pal. Environmental Monitoring and Assessment, 160(1-4), 267. [18] Rahman, M. S., Molla, A. H., Saha, N., & Rahman, A. (2012). Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chemistry, 134(4), 1847-1854. [19] So, T. Y., Farrington, E., & Absher, R. K. (2009). Evaluation of the accuracy of different methods used to estimate weights in the pediatric population. Pediat-rics, 123(6), 1045-51. [20] Tao, Y., Yuan, Z., Xiaona, H., & Wei, M. (2012). Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and poten-tial health risk assessment from Taihu lake, China. Ecotoxicology and environmental safety, 81, 55-64. [21] Turekian KK and Wedepohl KH. 1961. Distribution of the elements in some major units of the earth’s crust. Geological Society of America Bulletin, 72(2), 175–92. [22] USEPA. (2000). Risk-based Concentration Table. United States Environmental Protection Agency, Philadelphia, PA; Washington, DC. [23] USEPA. (1996). EPA Method 3052: microwave assisted acid digestion of siliceous and organically based matrices. U.S. Environmental Protection Agency, Of-fice of Solid Waste and Emergency Response. U.S. Government Printing Office, Washington. [24] Sekhar, K. C., Chary, N. S., Kamala, C. T., Raj, D. S., & Rao, A. S. (2004). Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environment International, 29(7), 1001-1008. [25] WHO (2006) Guidelines for drinking water quality. World Health Organization, Geneva. [26] Yi, Y., Yang, Z., & Zhang, S. (2011). Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159(10), 2575-2585. [27] Zhao, S., Feng, C., Quan, W., Chen, X., Niu, J., & Shen, Z. (2012). Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Marine pollution bulletin, 64(6), 1163-1171.
Copyright
Copyright © 2023 Preeti Kumari, Puja Hansdah. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55935
Publish Date : 2023-09-29
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

