Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Review on Effective Usage of Recycling of Lubricating Engine Oil
Authors: Dr. Purusothaman M, Harshith M, Ramana Nanda M
DOI Link: https://doi.org/10.22214/ijraset.2025.66754
Certificate: View Certificate
Abstract
This paper focuses on the treatment of waste engine oils that have gone through an acetic acid recycling process. A recycling process was invented, and the findings eventually became similar to some of the more conventional approaches. Recycled oil can be put back into automobile engines. Using acetic acid has the advantage that it reacts very little, if at all, with base oils. Recycling happens in a room with ambient temperature. According to studies on its properties, acetic acid doesn\'t really affect base oils or oil additives. After being treated with 0.8 percent acetic acid, the used oil was separated into two layers, a dark, black sludge at the container\'s bottom and a transparent, dark red oil on top of it. The findings of this paper were compared to base oils provided by other recycling methods. They revealed that the recycled engine oil obtained by acetic acid treatment is equal to that obtained through other traditional approaches.
Introduction
I. INTRODUCTION
Engine oil Waste from engines is a very harmful material that must be handled carefully. The ecosystem may suffer if spent motor oil is dumped in the ground or water. sewers and streams alike. This could lead to groundwater and pollution of the soil. Reusing such tainted materials will help down the price of engine oil. It will also significantly improve the situation for the surroundings. The traditional techniques for recycling waste engine oil either call for expensive technology such as vacuum distillation or the use of dangerous materials like acid that contains sulfur. (Tiwari et al., 2024).
Additionally, these methods lead to contamination from products that have high levels of sulfur. The primary purposes of grease oils are to minimize material waste and friction between moving parts of different machines or equipment wear, increase machinery and equipment efficiency, and for energy and fuel conservation Having access to lubricants is necessary for any contemporary civilization, because lubrication not only lessens friction
The wear caused by a thin liquid layer positioned between moving not only does it keep surfaces clean, but it also eliminates heat and stops rusting.
Lubricating oil waste pertains to the hydraulic, cutting, engine, and transmission oils after use. It also describes the deterioration of newly added lubricating materials tainted by carbon, ash, metals, gums, varnish, water, residue, and other contaminated materials, as well as the asphaltic chemicals that lead from the engines' surface. (Nowak et al., 2019).
These oils need to be altered and taken out of the car after a few thousand miles driven due to anxiety from a severe decline in service quality. The quantity of lubricant that is gathered yearly in the USA and Europe is enormous, roughly 1.7. This enormous quantity of waste oils significantly affects the economy and environmental factors. Whether released into the air, water or even burned as a poor-quality fuel, it could result in major pollution issues when they discharge dangerous metals and other contaminants into the surroundings. The viscosity, viscosity-temperature characteristics, and fluidity at low temperatures are the primary service features of engine oil. temperatures, stability of chemicals, and defensive qualities. At 100°F, the viscosity of lubricating oils ranges from 10 to 1000 centistokes. One suggested fix for this issue is the retrieval of the waste oil to lubricating oil. procedures for recycling that use Materials that are affordable and harmless may be the best option. (Pinheiro et al., 2021).
A. Experimental Work
The used engine oil from light automobiles gathered in a container from nearby Tripoli garages and repair shops. Previous to vacuum distillation as a method of treating oil after analysis lubricating oil was extracted using a solvent and stored for days to give substantial suspended particles time to settle in the presence of gravity to recycle used oil. (Pinheiro et al., 2021).
II. MATERIALS AND METHODS
Acetic acid, sodium hydroxide, waste engine oil, and activated carbon are the ingredients needed for our research project. A beaker, funnel, and viscometer are the tools and equipment that are employed. The following are the primary parts that were created and manufactured for the refining process: Base frame, oil immersion heater, heating container, mixing container, and thermometer (Eldeen F. Hegazi, 2017).
A. Treatment with Acetic Acid
Corrosive Intervention An eight-liter container was estimated to have held five liters of used motor oil. Similarly, a different 1.2 Liter measuring glass was used to determine 1 Liter of acids (acidic corrosive). After turning on the controller hot plate, the purposeful base oil was poured on top. The used motor oil, or base oil, was maintained at a temperature between 40 and 45?C. The acidic corrosive was added to the used motor oil at this temperature and the mixture was blended for ten minutes. Acidic destructive is mixed with used engine oil in an equal ratio of 45 milliliters for every five liters of used engine oil. The blended liquid is then spun at 100 degrees Celsius for ten minutes in a closed chamber (Rawal et al., 2015).
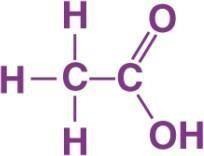
Fig: 2.1 Chemical formula of Acetic Acid
B. Decantation and Sedimentation
After the acid treatment stage, the acidic oil was allowed to settle for a whole day, resulting in the formation of sediment at the bottom of the beaker. Following this period, the acidic oil was carefully sedimented and decanted using a piece of cloth into another 8-liter beaker, while the residue (acidic sludge) at the beaker's bottom was disposed away. (Rawal et al., 2015).
C. Activation of Clay
After the removal of soil, sand, and stone, clay was mixed with distilled water to create a slurry. The slurry was transferred into an aluminum pan and allowed to sit at a temperature of 90–1000?C for an hour. Following the allotted time, distilled water was used to rinse the mixture to get rid of any extra acid. Washing water's pH was measured until it was determined to be neutral. After being cleaned, the clay mixture was ground into a powder and dried for an hour in an oven. (Rawal et al., 2015).
D. Activated Carbon is used for Bleaching
15% of activated charcoal is added to the oil after it has been heated to 40°C and moved to a mixing chamber. The mixture is stirred for approximately ten minutes and allowed to settle for twenty-four hours. A dark red, transparent base oil remains when the residue sinks to the bottom and is disposed of, and this oil is then placed in a base oil bottle. As the dying system ends, the fading oil is eliminated (Sharad Shanbhag et al., 2020).
E. Neutralization
After the oil has been moved to the blending chamber, sodium hydroxide is added and mixed for around ten minutes. The combination is then permitted to agree to 24 hours, during which the buildup settles at the base. It is then positioned inside the compartment of base oil. (Sharad Shanbhag et al., 2020).
F. Filtration
At long last, the sedimented oil was shifted through a channel fabric; the filtrate was then gathered in a filtration jar and viewed as clear, while the buildup — otherwise called the channel cake was discarded. (Raabe et al., 2022).
III. ANALYSIS AND SPECIFICATIONS OF RECYCLING OIL
As of right now, the lubricating oil that was produced appears to be comparable to lubricating oil that is sold commercially and may be utilized in automobile engines if the proper additives have been added. But to be sure that its attributes meet the ones offered by the Automotive Society, further analyses were carried out. (Raabe et al., 2022).
A. Kinematic Viscosity
Kinematic Viscosity is a measurement of flow resistance and can be thought of as either the internal or the lubricating oil's fluid friction. It is the most crucial lubricant characteristic that needs to be understood when assembling lubricants. It is an essential metric for identifying several used oil circumstances, such as fuel dilution, oxidation, and pollution. Products of oxidation and impurities including water, glycol, soot, and dirt lead to viscosity rise as fuel is diluted, and cutting off of Viscosity in multi-grade oils is caused by the viscosity index improver (S. & M., 2013).
The viscosity was evaluated using equation n2 = ρ2t2/ρ1t1 ∗ n1
B. Viscosity Index
The viscosity-temperature relationship is represented by the viscosity index (VI), an arbitrary number that indicates the degree to which oil viscosity varies with temperature. Because internal combustion engines must operate in a wide temperature range, this is one of the most important factors to take into account when selecting lubricating oil. The influence of temperature on an oil's kinematic viscosity decreases with increasing viscosity index for oils with comparable kinematic viscosities. (Stanciu, 2020)
C. Specific Gravity
The Specific gravity of engine oil is an estimation of the oil's weight comparable to water. As a rule, this number ranges from 0.85 to 0.95 for motor oils. This demonstrates that engine oil has a lower thickness than water. For example, an oil with a particular gravity of 0.9 is 90% as thick as water. Contingent upon the sort of oil and any added substances it incorporates, the exact sum might change. The proportion of the mass of a given volume of the item to the mass of an identical volume of water at a similar temperature is known as a relative entity for oil-based commodities. ASTM D1298 is utilized to quantify explicit gravity; the higher the particular gravity, the heavier the substance. (Khalafvandi et al., 2022).
D. Density and Specific Gravity
A material's thickness can be calculated by dividing its mass by its volume. Specific gravity is the ratio of a substance's density to that of water at the same temperature. The specific gravity and quantity of wasted oil can be measured to determine the number of impurities present. As the oil's fragrant fixation increments, so does its particular gravity. Spend engine oil has a higher specific gravity and density as a result of the contaminants. (Hamawand et al., 2013).
E. Flash Point
The term "flash point" refers to the lowest temperature at which engine oil needs to be heated under specified conditions to generate enough vapor to mix with air and ignite on its own when exposed to a given flame. Engine oil's flash point serves as a marker for oil pollution. An engine oil's significantly low flash point is a good sign that it has gotten tainted with volatile substances like gasoline and is a tool for determining the flammability of a sampling mixture of air and vapor. Calculating the temperature at which a sample becomes combustible is another application for it. (Ljubas et al., n.d.).
F. Absolute Corrosive Number
Used engine oils treated with acetic acid are among the recycled or re-refined lubricants whose corrosive qualities are evaluated using the absolute corrosive number (ACN), a crucial metric. This statistic aids in assessing the oil's propensity to corrode engines and other devices. Sludge, metal particles, and other pollutants are frequently eliminated during the recycling process by using acetic acid.
The remaining acid from this treatment, although its effectiveness, may affect the end product's corrosive qualities. (Josiah & Ikiensikimama, 2010).
G. Absolute Base Number
The amount of base in an oil is measured by the Absolute Base Number (TBN). To destroy the acidic item arrangement, gasoline motor oils comprise a package of very alkaline base additions. An extension of this group, the TBN, can be used to determine when the motor's oil needs to be changed. This is because TBN eventually runs out of power. Oil with higher TBN values is better at killing acids over longer periods. The oil's anticipated assistance life is shown by the rate at which the additional substances are utilized. (Josiah & Ikiensikimama, 2010)
H. Color Index
Color is the most crucial aspect to take into account when choosing lubricating oils. Glacial acetic acid and formic acid reacted with base oils virtually as little as sulfuric acid, which reacted severely with the utilized oil. This demonstrates unequivocally that the original composition of the oil is unaffected by acetic or formic acids. Moreover, recycling used oil can benefit greatly from the application of formic and acetic acids. Sulfur dioxide and other dangerous pollutants were not released into the atmosphere by this innovative technique of recovering spent fuel oil. Moreover, glacial acetic and formic acids are less detrimental to manufacturing machinery than sulfuric acid. Because of its minimal reactivity with the spent oil, the foundation can only require a modest amount of additive regenerated using the acetic acid-clay technique. To move this method to the commercial stage, more study is needed. While several variables have been examined in this research, many more, including temperature, pressure, mixing, settling time, centrifugation speed, and adsorbent type, still require further research (Jock et al., 2023).
Table 1: Physical properties of the used engine oil, fresh engine oil, and recycled oil: (M. U. Dubai and N. Bello 2019).
|
S/N O |
Sample |
Flash Point (oC) |
Pour Point (oC) |
Kinemati c Viscosit y @ 40oC |
Kinemati c Viscosit y @ 100oC |
Vis cos ity Ind ex |
Refrac tive index |
Specif i c Gravit y |
|
1 |
Virgin engine oil |
264 |
-19 |
149. 91 |
16.30 |
115 |
1.493 |
0.69 7 |
|
2 |
Used engine oil |
232 |
-18 |
169. 00 |
15.80 |
94 |
1.591 |
0.83 1 |
Table 2: Chemical properties of the used engine oil, fresh engine oil, and recycled oil: (M. U. Dubai and N. Bello 2019).
|
Property |
Fresh Engine Oil |
Used Engine Oil |
|
|
Total Acid Number (TAN) |
1-2 |
3-5 or higher |
|
|
Total Base Number (TBN) |
8-12 |
2-4 or lower |
|
|
Viscosity Index |
100-120 |
80-100 or lower |
|
|
Sulfated Ash Content (%) |
0.5-1.5 |
0.5-2.0 |
|
|
Sulfated Ash Content (%) |
<0.1 |
0.1-0.5 |
|
A key criterion used to assess the risk of fire and explosion during motor oil capacity and treatment is flashpoint. It illustrates how the oil contains erratic mixes. The treated motor oil had glimmer points of 208 and 214°C, whereas the untreated motor oil had glimmer points of 121 and 131°C. The dissolvable extraction and mud permeation techniques used for the treatment may have caused the pollution in the untreated oil to be expelled, as seen by the increased glimmer point in the treated instances. In a greasing-up framework, the pour point is a marking that indicates the temperature at which oil becomes too slick to successfully stream due to gravity.
Conclusion
Compared to sulfuric acid and formic acids which interacted strongly with the utilized oil, acetic acid has essentially little interaction with base oils. This demonstrates unequivocally that the oil\'s natural structure is unaffected by acetic acid. Furthermore, recycling used oil is greatly benefited by the application of acetic acid. The innovative method of recycling spent motor oil did not release harmful gasses into the atmosphere, such as sulfur dioxide. Furthermore, compared to sulfuric acid and formic acid have less of an adverse effect on the processing machinery. Because of its low reactivity, the base oil regenerated via the acetic acid-clay technique may require fewer additions.
References
[1] Armioni, M. D., Benea, M. L., & Ra?iu, S. A. (2020). Used engine oil recycling techniques: a comparison. https://www.researchgate.net/publication/342787432 [2] Eldeen F. Hegazi, S. (2017). Recycling of Waste Engine Oils Using Different Acids as Washing Agents. [3] International Journal of Oil, Gas and Coal Engineering, 5(5), 69. https://doi.org/10.11648/j.ogce.20170505.11 [4] Hamawand, I., Yusaf, T., & Rafat, S. (2013). Recycling of Waste Engine Oils Using a New Washing Agent. Energies, 6(2), 1023–1049. https://doi.org/10.3390/en6021023 [5] Jock, A. A., Magomya, A. M., & Essang, S. E. (2023). RECYCLING AND CHARACTERIZATION OF SPENT ENGINE OIL USING TWO STAGES: SOLVENT EXTRACTION AND CLAY PERCOLATION TECHNIQUES. FUDMA JOURNAL OF SCIENCES, 7(3), 182–185. https://doi.org/10.33003/fjs-2023-0703-1836 [6] Josiah, P. N., & Ikiensikimama, S. S. (2010). The Effect of Desludging and Adsorption Ratios on the Recovery of Low Pour Fuel Oil (LPFO) from Spent Engine Oil. Chemical Engineering Research Bulletin, 14(1). https://doi.org/10.3329/cerb.v14i1.4092 [7] Khalafvandi, S. A., Pazokian, M. A., & Fathollahi, E. (2022). The Investigation of Viscometric Properties of the Most Reputable Types of Viscosity Index Improvers in Different Lubricant Base Oils: API Groups I, II, and III. Lubricants, 10(1). https://doi.org/10.3390/lubricants10010006 [8] Ljubas, D., Krpan, H., & Matanoviae, I. (n.d.). Influence of engine oils dilution by fuels on their viscosity, flash point and fire point. [9] Nowak, P., Kucharska, K., & Kami?ski, M. (2019). Ecological and Health Effects of Lubricant Oils Emitted into the Environment. International Journal of Environmental Research and Public Health, 16(16), 3002. https://doi.org/10.3390/ijerph16163002 [10] Pinheiro, C. T., Quina, M. J., & Gando-Ferreira, L. M. (2021). Management of waste lubricant oil in Europe: A circular economy approach. Critical Reviews in Environmental Science and Technology, 51(18), 2015–2050. https://doi.org/10.1080/10643389.2020.1771887 [11] Raabe, D., Ponge, D., Uggowitzer, P. J., Roscher, M., Paolantonio, M., Liu, C., Antrekowitsch, H., Kozeschnik, E., Seidmann, D., Gault, B., De Geuser, F., Deschamps, A., Hutchinson, C., Liu, C., Li, Z., Prangnell, P., Robson, J., Shanthraj, P., Vakili, S., … Pogatscher, S. (2022). Making sustainable aluminum by recycling scrap: The science of “dirty” alloys. Progress in Materials Science, 128, 100947. https://doi.org/10.1016/j.pmatsci.2022.100947 [12] Rawal, D. G., Garg, D. N., Wani, D. U. R., & Yadav, D. S. (2015). Corrosive Poisoning- A Case report with Literature Review. International Journal of Medical Research and Review, 3(1), 132–135. https://doi.org/10.17511/ijmrr.2015.i1.23 [13] S., N., & M., A. (2013). Lubrication and Lubricants. In Tribology - Fundamentals and Advancements. InTech. https://doi.org/10.5772/56043 [14] Sharad Shanbhag, Swapnil Ramani, & Sanjeel Naik. (2020). Refining of used Engine Oil. International Journal of Engineering Research And, V9(05). https://doi.org/10.17577/IJERTV9IS050510 [15] Stanciu, I. (2020). Viscosity Index for Oil Used as Biodegradable Lubricant. Indian Journal of Science and Technology, 13(3), 352–359. https://doi.org/10.17485/ijst/2020/v13i03/147759 [16] Tiwari, R., Agrawal, P. S., Belkhode, P. N., Ruatpuia, J. V. L., & Rokhum, S. L. (2024). Hazardous effects of waste transformer oil and its prevention: A review. Next Sustainability, 3, 100026. https://doi.org/10.1016/j.nxsust.2024.100026
Copyright
Copyright © 2025 Dr. Purusothaman M, Harshith M, Ramana Nanda M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66754
Publish Date : 2025-01-30
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

