Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Encapsulation of Camphor in Nanocellulose based Pickering Emulsion and Study of its Antibacterial Activity
Authors: Sakshi Dudhani, Guruprasad R. Mavlankar, Deepa N. Rangadal, Minakshi N. Bhatu, Shubhangi P. Patil
DOI Link: https://doi.org/10.22214/ijraset.2023.56335
Certificate: View Certificate
Abstract
Camphor is a natural and renewable antimicrobial agent. However, it is highly sublime, which limits its practical application as an effective antibacterial agent. To improve camphor\'s stability and retention properties, we have microencapsulated camphor into an almond essential oil-based Pickering emulsion (AO-PE), stabilized by cellulose nanocrystals (CNC). The CNC was synthesized from corn husk using an eco-friendly homogenization approach to stabilize the AO-PE. Various factors affecting the formation and stability of Pickering emulsions (PE) were studied, such as the source of cellulose (corn husk and mung hull), oil type (sesame oil and almond oil), oil in water concentration, and stability with time and shear. Camphor-loaded CNC-stabilized AO-PE exhibited a small droplet size and had long-term stability at 6.67% by volume oil concentration, owing to the presence of 0.2 g of CNC at droplet interfaces. The results showed that the PE exhibited better antibacterial performance against Staphylococcus aureus than the equivalent concentrations of camphor, almond oil, CNC, and camphor dissolved in almond oil, individually. This research shall provide fresh insights into the significant enhancement of the antimicrobial properties of medicinal natural products by incorporating them into PEs.
Introduction
I. INTRODUCTION
Thanks to their relative ease of formulation for poorly soluble drugs and other substances, emulsion-based systems have been applied for several decades in a wide variety of fields ranging from drug delivery and pharmaceuticals to cosmetics and the food industry [1]. In early 1900s, was introduced the fascinating world of Pickering emulsions, when the ability of particles to stabilize the wax and water mixture and create emulsions was first identified by Ramsden [2] in 1903. Pickering [3] later proposed employing nanoparticles and microparticles to stabilize emulsions, which advanced emulsion research. The term, "Pickering emulsion," which can be oil-in-water (O/W), water-in-oil (W/O), water-in-water (W/W), or even mixed, was thus recognized and described. Pickering emulsions (PE) offer a world where oil and water can peacefully coexist without constantly separating [4]. They provide a stable, long-lasting mixture of immiscible liquids that do not coalesce to form aggregates. These are uniquely stabilized by particles, rather than traditional surfactants.
Their mesmerizing structure and patterns have the potential to revolutionize diverse industries [5]. Being a truly remarkable and intriguing phenomenon, PEs have recently drawn significant research interest as templates due to their several advantages over conventional emulsions [6].
Among all the natural bio-macromolecules used as emulsifiers in PEs, cellulosic resources have attracted extensive attention for their techno-functional, economic, renewable, and non-toxic characteristics [7]. Being one of the most abundant polymeric bio-based resources in the world with an annual worldwide production of 1.5×1012 tons, cellulose is isolated from the cell walls of plants, certain microorganisms like bacteria and fungi, and tunicates [8]. Nanocellulose satisfies all the increasing demands for a sustainable and eco-friendly stabilizer [9]. Its amphiphilic surface nature, originating from the hydrophobic face and hydrophilic edge of cellulose chains, leads to efficient stabilization of oil/water interfaces [10]–[12]. Its excellent mechanical properties, when coupled with surface modification, tailor the wettability at the interfaces effortlessly [1], [13]. Nanocellulose is therefore a versatile material with multifunctional properties for a variety of applications [14]–[16].
Known to have pain-relieving, anti-inflammatory, decongesting, antispasmodic, antimicrobial, analgesic, relaxing, healing, and nerve stimulation properties, Cinnamomum camphora is a versatile and valuable natural remedy for a variety of health issues [17]–[21]. Camphor has been used for centuries for its medicinal properties [22]. Its effectiveness in preventing and treating infections caused by bacteria and fungi has been acknowledged for years [23]. However, the sublimation property of camphor can make it less effective in certain applications. Due to sublimation, some of its active components are released into the air, resulting in the weaker concentration of the compound in the product being used. This can make the product less efficient than required and intended. Additionally, sublimation also makes it less stable and more prone to evaporation or degradation, which can reduce its shelf life and overall efficacy. Therefore, in order to ensure maximum potency, camphor is used in products that are specifically formulated to stabilize its properties, such as ointments or creams, rather than in its pure, solid form. Incorporating Camphor into Pickering formulations can pave the way for a widely increased range of medicinal and antimicrobial applications.
In this study, PE stabilized by cellulose nanocrystals (CNC) obtained from corn husk was chosen as a captivating medium to trap and retain the excellent medicinal properties of the naturally revitalizing compound, camphor. Synergistically, after formation, these PEs exhibited a significant retention in the antibacterial activity of camphor, which sustained for the long term. The novel aspect of the current work exhibited that the CNC-stabilised Camphor containing AO-PE has had an enhanced effect on antimicrobial activity against S. aureus, attributing to the encapsulated Camphor. Therefore, the objectives of this study were as follows: (1) CNC were synthesized from bio-waste like corn husk and mung hull. (2) The efficiency of synthesized CNC in stabilizing the PEs with two different organic phases, almond oil and sesame oil, was investigated comparatively. (3) Camphor was encapsulated in AO-PE stabilized by CNC obtained from corn husk and its morphological and stability parameters were studied for potential applications. (4) The antibacterial activity of camphor-loaded AO-PE was evaluated against S. aureus (gram positive), in comparison with that of free Camphor and Camphor added O/W emulsions.
II. MATERIALS AND METHODS
A. Chemicals and Materials
Corn husk, mung hull, camphor, almond oil, sesame oil, sodium hydroxide, hydrogen peroxide, sulfuric acid, peptone, agar, beef broth, sodium chloride, Gram-positive bacteria Staphylococcus aureus (S. aureus). Throughout the process, distilled water was used as a solvent and for formation of aqueous phase of the emulsions.
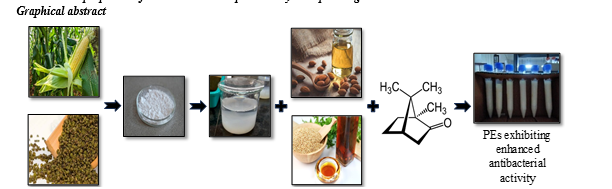
B. Preparation of CNC from corn Husk and mung hull
1) Alkali Pretreatment and Bleaching: 20 gm of corn husk and mung hull was given alkali treatment using 5% w/v sodium hydroxide with constant stirring for 180 min at 60?C for delignification. This pretreated material was then bleached with 6% of hydrogen peroxide for 120 min at 40?C, to remove residual lignin, hemicellulose and other impurities. It was followed by a controlled chemical treatment given through acid hydrolysis to remove local interfibrillar contacts, split the amorphous domains and release crystalline nanocellulose after subsequent ultrasonication.
2) Acid Hydrolysis: 1 g of delignified cellulose was dispersed in 64 wt % sulfuric acid solutions and magnetically stirred with a range of reaction time (15-60 min) at temperature 45?C. The mixture was then diluted 10 times the amount with cold distilled water to terminate the hydrolysis. The suspension formed was centrifuged at 5000 rpm for 10 min and washed with distilled water for many consecutive cycles to reach neutral pH. Also, the suspension was deduced to the quantity of 50 ml while washing. The neutralized suspension was then homogenized via ultrasonication.
3) Ultrasonication: The acid hydrolyzed and later neutralized cellulose suspension was defibrillated into nanocrystals through ultrasonication for 1 min in ice bath to avoid over-heating and crystalline nanocellulose was obtained. Figure 1 is the schematic representation of the overall process of cellulose extraction.
III. CHARACTERIZATION
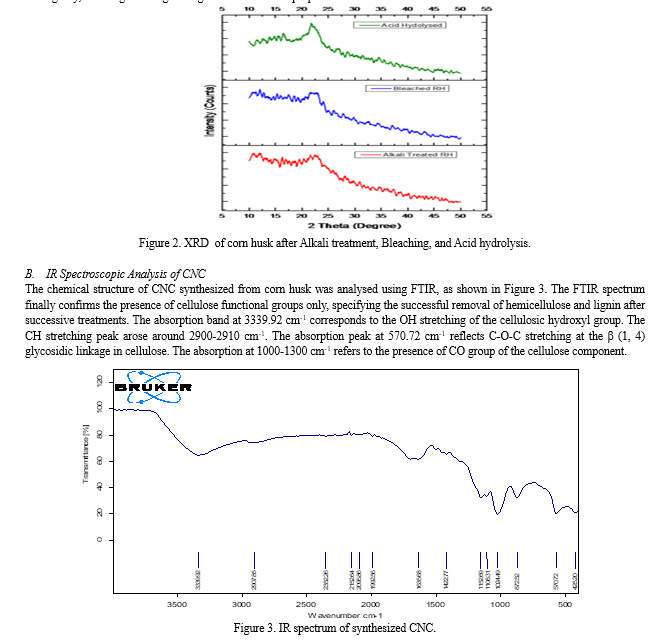
A. XRD Analysis of CNC
The crystallinity of the corn husk after different treatments was determined using an X-ray diffractometer (Bruker D-8 Discover) with CuKa radiation (l = 0.1542 nm). The scanning range and the scanning speed were 5-40° and 5 deg/s, respectively.
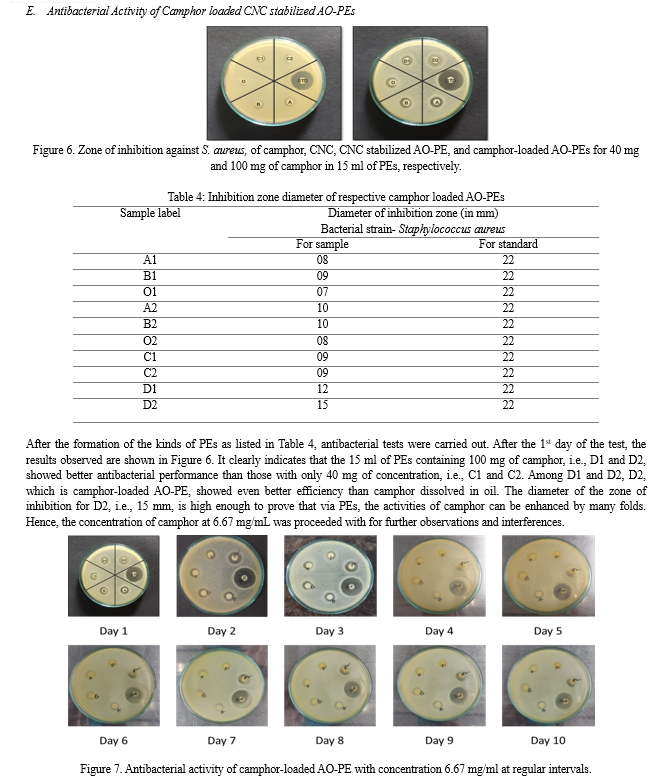
B. Fourier transfer-infrared (FT-IR) Spectroscopic Analysis of CNC
The IR spectra of CNC were determined by means of a Perkin Elmer FT-IR spectrometer to confirm the functional groups present in the synthesized material. The CNC synthesized from corn husk was analyzed within the range of 400–4000 cm-1 using the KBr palette method.
C. SEM Micrographs of CNC
Scanning electron microscopy (SEM) analysis was performed using a Jeol JSM-6400 scanning electron microscope to observe the surface morphology of CNC. The samples were air-dried and coated with gold to avoid charging. The images were taken with an accelerating voltage of 15 kV.
D. Morphological Analysis of Pickering emulsions
The storage stability of all kinds of PEs was checked by analyzing the optical images of the emulsions at regular intervals. The morphology of the oil-water interface between the droplets was observed, giving a clear idea of comparatively stable emulsions for the selection of oil and O/W concentration.
E. Shear stability of Pickering Emulsions
Firstly, all the primary emulsions were subjected to centrifugation at 1000 rpm for 2 min, and later at 5000 rpm for 2 min, so as to check for the effect of shear stress on stability. Freshly prepared AO-PEs containing 0.2 g of CNC and 6.67% by volume almond oil encapsulating 40 mg and 100 mg camphor were then subjected to centrifugation at 3000 rpm for 2 min. Also, emulsions were viewed under an optical microscope at regular intervals before and after applying shear stress to keep a check on morphological changes.
IV. INHIBITION ACTIVITY OF CAMPHOR-LOADED AO-PES
The Gram-positive bacteria S. aureus were chosen for antibacterial activity test. The antibiotic Chloramphenicol was used as the standard. The in vitro antibacterial activity of camphor-loaded AO-PEs was compared with that of free camphor, plain CNC suspension, and CNC-based AO-PEs.
This study was carried out by the Agar Disk Diffusion Method (DDM). The bacteria S. aureus, were cultivated overnight, followed by sterilization. The bacteria suspension was then diluted and spread onto an infusion agar plate. The circular samples were sterilized and then gently pasted on the inoculated plates. After incubation at 37 °C for 24 h, the diameters of inhibition zones around the circular discs were measured using a ruler. The experiments were performed in triplicate. The bacterial colonies growing in each section (as listed in Table 3) were recorded.
Table 3: Samples analyzed for comparative evaluation of enhanced antimicrobial activity exhibited by camphor-loaded CNC-based AO-PEs.
|
Label |
Description |
|
A |
Free camphor |
|
B |
Plain CNC suspension |
|
O |
CNC stabilized AO-PE |
|
C1 |
Camphor dissolved in almond oil (2.67 mg/ml of oil) |
|
C2 |
Camphor-loaded AO-PE (40 mg in 15 ml of CNC based AO-PE) |
|
D1 |
Camphor dissolved in almond oil (6.67 mg/ml of oil) |
|
D2 |
Camphor-loaded AO-PE (100 mg in 15 ml of CNC based AO-PE) |
V. RESULTS AND DISCUSSION
A. XRD Analysis of CNC
The X-ray diffraction pattern and the crystallinity index of CNCs were obtained after different stages of treatment of corn husk as shown in Figure 2. The crystalline peaks around 2? = 12°, 21°, and 22° were defined as cellulose I exhibiting crystallographic planes. Upon removing the non-cellulosic constituents of the corn husk by chemical treatment, the intensity of the peak becomes more defined. The cellulose nanocrystals show the highest crystallinity index value (68.33%), which displayed the strongest and sharpest peak at 2? = 22°. The increased crystallinity of treated corn husk compared to untreated one was attributed to the progressive removal of amorphous non-cellulosic materials. The increased crystallinity is also expected to increase their stiffness and rigidity, resulting in strengthening of the mechanical properties.

Reflected through Figure 7, is an observation that at some point of time, the bacterial growth started even within the standard zone, but it was considerably retarded and inhibited to a large extent in D2. The superior antibacterial activity is also attributed to the use of CNC as the stabilizer. However, it can be seen with successive days that the antibacterial performance of D2 is gradually decreasing. This gives scope for further studies to be carried out on different parameters to maximize the potential of camphor-loaded PEs. Moreover, the applications of this crucially retained medicinal property through AO-PEs can be extended in various fields like drug carriers, cosmetics, and agriculture.
VI. FUTURE PROSPECTS
With the rapid growth of scientific and technological interest in nanocellulose from academic and industry players for non-toxic-based applications, more studies have been focusing on the usage of nanocellulose as a Pickering emulsifier that meets the aspects of sustainability, stability, eco-friendliness, safety, edibility, and biocompatibility for food, beauty, personal care, and hygiene-based products. CNC-stabilized PEs have proven to be of great potential for various applications in the food, pharmaceutical, and cosmetic industries. The development of CNC-based emulsions may focus on improving their functional properties, such as rheology, texture, and sensory characteristics. This may involve optimizing CNC size, concentration, and surface modification, as well as exploring the use of other natural or synthetic particles for stabilization.
And to ensure safety, more studies on the toxic effects of nanocellulose and its derivatives are required to provide a firm conclusion regarding the human safety aspect before their implementation in consumer products. More edible particles like CNC rather than inorganic solid particles should be explored and applied to form PE systems to achieve higher and safer efficiency in food and other industries. These edible particles will provide more sustainable solutions. While the benefits of such precise release systems are clear, current research needs to push further to explore the full potential of these multi-faceted dynamic systems, in particular towards in vitro and in vivo behavior. It is clear, therefore, that the area of PEs for biomedical applications has enormous potential, with a lot still to be achieved. The efficient and improved antibacterial behavior of camphor, after encapsulation in CNC-based almond oil-Pickering emulsion, can further lead to investigations into the role of PEs as carriers of therapeutic natural products. The highly effective camphor-loaded AO-PEs can also be evaluated to accelerate the healing effect by reducing the microbial burden in burn wounds. This shall prove to be useful in sophisticated dressings. In agriculture, the use of camphor-loaded PEs shall be explored in animal husbandry to check the treatment of bacterial infections in livestock, which can in turn, improve animal health and productivity. The antibacterial PEs can also be used to preserve food and prevent bacterial growth in the production of perishable foods. Also, these could be helpful in disinfecting surfaces and preventing the spread of bacteria in homes, hospitals, and other public spaces. When incorporated into fabrics and textiles, due to their retention properties via PEs, camphor can reduce odours and prevent the spread of infection in clothing and other textiles. Edible essential oil-based PEs with healing properties can be added to soaps, lotions, serums, and other personal care products to reduce the risk of infection on applied areas.
While camphor has enormous potential medicinal benefits, it can also pose risks to human health, both directly and indirectly, if not used safely. Hence, the recommended guidelines should be carefully followed to avoid potential health risks while carrying out all the necessary investigations related to its applications.
VII. ACKNOWLEDGEMENT
The authors acknowledge the Institute of Science, Dr. Homi Bhabha State University, Mumbai, for providing assistance and support for the smooth conduct of the laboratory activities.
A. Declaration of Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. No conflict of interest exists.
Conclusion
CNC was synthesized using plant raw materials, corn husk, and mung hull. When formulated into PEs, CNC formed from corn husk showed better interfacial morphology, both immediately after the formation of PEs and after the application of shear stress through centrifugation. As per the XRD analysis, the CNC exhibited the strongest and sharpest peak at 2? = 22°, confirming the successful removal of non-cellulosic functional groups by successive treatments. The FTIR absorption spectrum of CNC confirmed the presence of cellulosic functional groups only. The absorption band at 3339.92 cm-1 corresponds to the OH stretching of the cellulosic hydroxyl group. The CH stretching peak arose at around 2900-2910 cm-1. The absorption peak at 570.72 cm-1 reflects C-O-C stretching at the ? (1, 4) glycosidic linkage. The SEM micrographs of the CNC confirmed the crystallinity, indicating the successful formation of cellulose nanocrystals. CNC from corn husk and almond oil were selected for further processing into PEs due to their excellent shear stability. All the crucial properties of camphor were locked into PEs so as to acquire their long-term retention for various future applications. Camphor-loaded AO-PE showed significantly higher antimicrobial activity than camphor, CNC, camphor in almond oil, and AO-PE, individually, proving the objective of this research. This research shall further prove to be useful to retain and enhance the properties of medicinal and antimicrobial drugs in a highly efficient and convenient manner through the applied concept of Pickering emulsions.
References
[1] S. Fujisawa, E. Togawa, and K. Kuroda, “Nanocellulose-stabilized Pickering emulsions and their applications,” Sci. Technol. Adv. Mater., vol. 18, no. 1, pp. 959–971, Dec. 2017, doi: 10.1080/14686996.2017.1401423. [2] “Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation).—Preliminary account,” Proc. R. Soc. Lond., vol. 72, no. 477–486, pp. 156–164, Jan. 1904, doi: 10.1098/rspl.1903.0034. [3] S. U. Pickering, “CXCVI.—Emulsions,” J Chem Soc Trans, vol. 91, no. 0, pp. 2001–2021, 1907, doi: 10.1039/CT9079102001. [4] E. Dickinson, “Use of nanoparticles and microparticles in the formation and stabilization of food emulsions,” Trends Food Sci. Technol., vol. 24, no. 1, pp. 4–12, Mar. 2012, doi: 10.1016/j.tifs.2011.09.006. [5] W. Li et al., “Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications,” Front. Nutr., vol. 9, p. 864943, May 2022, doi: 10.3389/fnut.2022.864943. [6] D. Gonzalez Ortiz, C. Pochat-Bohatier, J. Cambedouzou, M. Bechelany, and P. Miele, “Current Trends in Pickering Emulsions: Particle Morphology and Applications,” Engineering, vol. 6, no. 4, pp. 468–482, Apr. 2020, doi: 10.1016/j.eng.2019.08.017. [7] Q. Li et al., “Application of Nanocellulose as particle stabilizer in food Pickering emulsion: Scope, Merits and challenges,” Trends Food Sci. Technol., vol. 110, pp. 573–583, Apr. 2021, doi: 10.1016/j.tifs.2021.02.027. [8] D. Klemm, B. Heublein, H. Fink, and A. Bohn, “Cellulose: Fascinating Biopolymer and Sustainable Raw Material,” Angew. Chem. Int. Ed., vol. 44, no. 22, pp. 3358–3393, May 2005, doi: 10.1002/anie.200460587. [9] Y. Habibi, L. A. Lucia, and O. J. Rojas, “Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications,” Chem. Rev., vol. 110, no. 6, pp. 3479–3500, Jun. 2010, doi: 10.1021/cr900339w. [10] S. Tarimala and L. L. Dai, “Structure of Microparticles in Solid-Stabilized Emulsions,” Langmuir, vol. 20, no. 9, pp. 3492–3494, Apr. 2004, doi: 10.1021/la036129e. [11] I. Kalashnikova, H. Bizot, B. Cathala, and I. Capron, “Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface,” Biomacromolecules, vol. 13, no. 1, pp. 267–275, Jan. 2012, doi: 10.1021/bm201599j. [12] W. G. Glasser et al., “About the structure of cellulose: debating the Lindman hypothesis,” Cellulose, vol. 19, no. 3, pp. 589–598, Jun. 2012, doi: 10.1007/s10570-012-9691-7. [13] S. H. Teo, C. Y. Chee, M. Z. Fahmi, S. C. Wibawa Sakti, and H. V. Lee, “Review of Functional Aspects of Nanocellulose-Based Pickering Emulsifier for Non-Toxic Application and Its Colloid Stabilization Mechanism,” Molecules, vol. 27, no. 21, p. 7170, Oct. 2022, doi: 10.3390/molecules27217170. [14] S. Parajuli and E. E. Ureña-Benavides, “Fundamental aspects of nanocellulose stabilized Pickering emulsions and foams,” Adv. Colloid Interface Sci., vol. 299, p. 102530, Jan. 2022, doi: 10.1016/j.cis.2021.102530. [15] V. Mikulcová, R. Bordes, and V. Kašpárková, “On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles,” Food Hydrocoll., vol. 61, pp. 780–792, Dec. 2016, doi: 10.1016/j.foodhyd.2016.06.031. [16] H. Yu et al., “Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity,” Int. J. Biol. Macromol., vol. 170, pp. 24–32, Feb. 2021, doi: 10.1016/j.ijbiomac.2020.12.027. [17] H. Huo, Y. Gu, Y. Cao, N. Liu, P. Jia, and W. Kong, “Antifungal activity of camphor against four phytopathogens of Fusarium,” In Review, preprint, Mar. 2021. doi: 10.21203/rs.3.rs-274895/v1. [18] M. J. A. Fazmiya et al., “Current Insights on Bioactive Molecules, Antioxidant, Anti-Inflammatory, and Other Pharmacological Activities of Cinnamomum camphora Linn,” Oxid. Med. Cell. Longev., vol. 2022, p. 9354555, 2022, doi: 10.1155/2022/9354555. [19] S. Sobhy et al., “Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi,” Separations, vol. 10, no. 3, p. 189, Mar. 2023, doi: 10.3390/separations10030189. [20] D. Hoang, A. Wong, and R. P. Olympia, “Looking Back to Move Forward: The Current State of Research on the Clinical Applications of Camphor- and Menthol-Containing Agents,” Cureus, Jul. 2023, doi: 10.7759/cureus.41426. [21] Q. I. Usmani, N. Jahan, and Sofiya, “Kafur (C. camphora L.)–An Updated Review of its Ethnopharmacology, Phytochemistry and Pharmacology,” Int. J. Pharm. Pharm. Sci., pp. 10–17, Oct. 2022, doi: 10.22159/ijpps.2022v14i10.45766 [22] R. Hamidpour, S. Hamidpour, M. Hamidpour, and M. Shahlari, “Camphor (Cinnamomum camphora), a traditional remedy with the history of treating several diseases,” Int. J. Case Rep. Images, vol. 4, no. 2, p. 86, 2013, doi: 10.5348/ijcri-2013-02-267-RA-1. [23] R. B. Malabadi, K. P. Kolkar, N. T. Meti, and R. K. Chalannavar, “Camphor tree, Cinnamomum camphora (L.); Ethnobotany and pharmacological updates,” Biomedicine, vol. 41, no. 2, pp. 181–184, Jul. 2021, doi: 10.51248/.v41i2.779.
Copyright
Copyright © 2023 Sakshi Dudhani, Guruprasad R. Mavlankar, Deepa N. Rangadal, Minakshi N. Bhatu, Shubhangi P. Patil . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET56335
Publish Date : 2023-10-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

