Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Review on Enhancing Biogas Production and Process Stability in Anaerobic Digestion through Biochar Integration
Authors: Jumbom Ruti, Mudo Puming
DOI Link: https://doi.org/10.22214/ijraset.2025.66892
Certificate: View Certificate
Abstract
Biochar produced from different biomass sources like softwoods (pine, spruce, fir) and hardwoods (oak, maple, birch, teak), is an excellent additive for anaerobic digestion. The pine (Pinus spp.) biochar with its porous structure is ideal for microorganisms to attach and improve methane production. Spruce (Picea spp.) biochar, recognized for its large surface area, enhances microbe interactions and accelerates gas generation. The Oak (Quercus spp.) biochar has a significant effect on stability and prevents extreme fluctuations in pH that may adversely affect digestion. The Maple (Acer spp.) biochar aids in facilitating electron transfer for optimal AD operation. The Fir (Abies spp.) biochar enhances the retention of nutrients while supporting the growth of microorganisms, resulting in a relatively stable digestion environment. More recently, the effects of biochar on ammonia build-up reduction and stabilization of biogas yield in addition to general improvement of system performance with teak-based biochar for AD have also been found.
Introduction
I. INTRODUCTION
The digestion of organic matters is a naturally occurring biological activity that produces digestate rich in nutrients and produces biogas, which serves as a form of renewable energy [1,2]. It is, however, plagued with problems such as pH fluctuation, ammonia inhibition, and lower methane production levels [3]. To overcome some of these bottlenecks in the process, researchers have applied biochar-which is pyrolytically produced material from biomass that is carbon-intensive-to improve performance in Anaerobic Digestion (AD) [4].
Biochar may be obtained from a range of biomass feedstocks, such as industrial wood wastes, forestry residues, and agricultural byproducts [5]. The major feedstocks that are typically used for the production of biochar include softwoods, such as pine (Pinus spp.), spruce (Picea spp.), and fir (Abies spp.), and hardwoods used include oak (Quercus spp.), maple (Acer spp.), and birch (Betula spp.) [6]. Each type of biochar is unique with its own set of properties that will impact AD performance. For instance, oak biochar is very alkaline, which promotes stable pH in the digester [7]. Pine biochar has high porosity, giving a favourable surface upon which microorganisms can grow for their enhancement in retention and activity in the system. Spruce biochar has a large surface area, thus facilitating interaction among the microorganisms and accelerating gas production. Fir biochar supports nutrient retention. Its use enhances microbial growth, which further stabilizes digestion.
A recent study suggests that Tectona grandis biochar may provide additional AD-specific benefits, including ammonia inhibition and steady biogas production [8]. Biochar presents a promising additive in enhancing the stability of the process and advancing microbial activity in AD. The applications of biochar in AD are described within this paper and are disseminated in terms of its success in countering major operational challenges in the process and, thus, optimising system efficiency.
II. OBJECTIVE OF THE STUDY
- To review the physiochemical properties of biochar derives from various biomass sources.
- To evaluate the role of biochar in addressing key challenges in anaerobic digestion
- To analyse the mechanism through which biochar enhances methane production and overall AD efficiency
- To highlight future research directions and potential industrial applications of anaerobic digestion systems.
III. RESEARCH METHODOLOGY
This study follows a descriptive approach which relies on information gathered from existing literature, reports and case studies. The analysis synthesizes findings from sources to present a comprehensive review of biochar’s effectiveness, mechanisms of action and potential for scaling up anaerobic digestion systems. The data is collected from:
- Peer-reviewed journal articles and documented case studies focusing on anaerobic digestion and biochar.
- Publications from government agencies and organizations in the renewable energy and waste energy management sectors.
IV. BIOCHAR PRODUCTION AND OPTIMIZATION PROCESS
Biochar is a carbon-rich material obtained from pyrolysis, a thermochemical process under oxygen-limited conditions from biomass. Recent interest has focused on the stabilizing of pH, improving microbial activity, and reducing ammonia inhibition of AD with the help of biochar. The effectiveness of biochar in AD relies upon feedstock selection, pyrolysis parameters, and post-treatment techniques.
Fig. 1 Flowchart for Biochar Production and Optimization Process
A. Feedstock Selection
Biochar is derived from agricultural residues, forestry byproducts, and industrial wood waste. Feedstock selection has an impact on porosity, alkalinity, and surface area of biochar that are considered as performance enhancing agents in AD [8,9].
- Softwoods (Pine, Spruce, Fir): These types are rich in porosity and possess large surface areas, ideal for microbial colonization.
- Hardwoods (Oak, Maple, Birch): Oak biochar stabilizes the pH level while maple biochar facilitates electron transfer, increasing the activity of microorganisms.
- Teak (Tectona grandis): Recent research shows that teak biochar has the potential to mitigate ammonia inhibition and stabilize biogas production, hence being a promising material for AD [8].
B. Pyrolysis Process
The efficacy of biochar in AD depends on pyrolysis conditions:
- Temperature (300–700°C)
- Lower temperatures (300–500°C) retain more nutrients.
- Higher temperatures (500–700°C) enhance surface area and porosity, hence increasing microbial adhesion [11,17].
- Residence Time: Longer residence times produce more carbonized biochar, which increases stability in AD [16].
- Heating Rate: Higher porosity is promoted by slower heating rates, thus enhancing microbial attachment and gas production [18].
C. Optimization and Post-Treatment
The properties of biochar can be optimized and enhanced through optimization and post-treatment techniques [18,19].
- Adjustment of pyrolysis conditions to optimize porosity, alkalinity, and adsorption capacity.
- Post-treatment techniques include washing (removing inhibitors) and activation (enhancing surface properties) to enhance the effectiveness of biochar in AD.
Selection of appropriate feedstocks, refinement of conditions for pyrolysis, and optimization with post-treatment techniques would evolve and optimize biochar to improve production of methane, stability, and reduce ammonia toxicity in the end, thereby adding value to AD systems.
V. APPLICATION OF BIOCHAR IN ANAEROBIC DIGESTION
Biochar is an input used to improve the performance of anaerobic digestion systems through the promotion of microbial activity, stabilization of pH, and increases in methane production and the removal of inhibitions to process development. Its effectiveness in anaerobic digestion is highly variable, depending on its feedstock source.
A. Biochar for Microbial Growth and Stability
- Pine (Pinus spp.) Biochar: Such high porosity of pine biochar creates an excellent surface for microbial attachment, thereby showing higher microbial density and increased methane production in AD [1,2].
- Spruce (Picea spp.) Biochar: Spruce biochar supports microbial colonization because it has a relatively large surface area and shows minimal reactor-fluctuation along the long-term stabilization operation [2,3].
- Fir (Abies spp.) Biochar: Such a feedstock is known for nutrient retention to ensure continuous microbial growth and higher biogas yields, making them applicable for nutrient-limited digesters [4].
B. Biochar for pH Regulation and Ammonia Reduction
- Oak (Quercus spp.) Biochar: Oak biochar is alkaline and useful for buffering pH fluctuations and reducing ammonia toxicity. Methanogenic bacteria perform better in such conditions of AD [1,7].
- Teak (Tectona grandis) Biochar: Recent studies have shown that the biochar of teak lowers ammonia concentration, which thus avoids inhibition and guarantees a constant biogas production. The environment-friendly characteristic of this plant makes it an ideal additive for the sustainable AD application [8].
C. Biochar for Electron Transfer and Methane Enhancement
- Maple (Acer spp.) Biochar: As it has electrical conductivity, maple biochar stimulates direct interspecies electron transfer (DIET), thereby increasing organic matter degradation rate and methane production [3,4].
- Birch (Betula spp.) Biochar: It enhances nutrient release and stabilizes anaerobic digestion, and thus is a good addition for digesters using nutrient-poor feedstocks [5,6]
D. Biochar for toxin adsorption and inhibition reduction
- Activated Biochar: Some biochars, especially post-treated with activation, enhance the adsorption capability of these biochars, thereby removing toxic sulfides and heavy metals. Thus, it prevents microbial inhibition and ensures a more stable AD process, thereby making biochar a useful biogas optimization tool [9].
VI. MECHANISM OF BIOCHAR IN ANAEROBIC DIGESTION SYSTEM
Biochar plays a crucial role in enhancing anaerobic digestion by supporting microbial growth, stabilizing pH, mitigating ammonia inhibition, and facilitating electron transfer. The mechanisms by which biochar improves AD are as follows:
A. Microbial Support
- Biochar's porous structure offers an ideal surface for microbial colonization, allowing higher densities and activities of methanogenic communities [2].
- The increased microbial retention leads to greater metabolic activity, the organic matter being broken down faster and better biogas production [2,3].
B. Ammonia Adsorption
- Biochar has adsorption functional groups that would subsequently reduce ammonia toxicity on methanogenic microbes [1].
- A further mitigation against microbial inhibition is enacted through the regulation of ammonia accumulation that biochar exerts to ensure continued methane production and process stability [1,7].
C. pH Buffering
- Some of the alkaline hardwood biochars like oak biochar buffers pH variation and inhibits acidification of the AD reactors [3].
- Stable pH ensures optimal performance of methanogenic activity to avoid failures associated with excessive acid buildup [3,6].
D. Electron Transfer Enhancement
- The maple biochar shows high electrical conductivity. It facilitates direct interspecies electron transfer [4].
- Accelerated decomposition of recalcitrant organic substances leads to quicker methane production, improving system efficiency [4,5].
Table 1. Comparison of Biochar Types and Their roles in AD systems
|
Biochar Type |
Key Properties |
Role in Anaerobic Digestion |
|
Pine (Pinus spp.) |
High porosity |
Promotes microbial attachment, enhancing methane yields. |
|
Spruce (Picea spp.) |
High surface area |
Stabilizes AD processes by supporting microbial growth. |
|
Fir (Abies spp.) |
Moderate ash content, nutrient retention |
Improves microbial activity and methane production |
|
Oak (Quercus spp.) |
High alkalinity |
Buffers pH fluctuation and mitigates ammonia inhibition |
|
Maple (Acer spp.) |
High electrical conductivity |
Facilities DIET, improving methane production efficiency. |
|
Birch (Betula spp.) |
Nutrient release |
Enhances microbial activity and Stabilizes AD performance |
|
Teak (Tectona grandis) |
Ammonia reduction potential |
Stabilizes biogas production and reduces ammonia levels. |
VII. ENVIRONMENTAL AND ECONOMICAL BENEFITS OF BIOCHAR IN AD SYSTEM
Biochar significantly improves the environmental and economic viability of anaerobic digestion systems. The most significant environmental benefit it has is carbon sequestration; it locks up carbon in the digestate, preventing it from being released into the atmosphere. This diminishes the impact of climate change since carbon stays in a stable form rather than adding to the emission of greenhouse gases. Biochar also reduces methane and nitrous oxide emissions, which otherwise would have escaped from digesters if not managed properly. Biochar improves the efficiency of organic waste breakdown, thus minimizing process failures and supporting sustainable waste management. Another important environmental benefit of the biochar is enhanced biogas production, reduction in dependence on fossil fuels, and promotion of clean energy transitions. Such an upgrade in renewable energy production upholds the circular bioeconomy by providing a more sustainable source of energy. Additionally, improving digestate quality, biochar makes it safer for use in agriculture as organic fertilizer, avoiding pollution risks, and advancing sustainable farming practice.
Beyond sustainability, biochar has economic advantages in that it uses low-cost feedstocks, such as agricultural and forestry residues, to reduce raw material costs. Technological advancements in pyrolysis have made the production of biochar more efficient and accessible for large-scale AD facilities. Furthermore, biochar increases methane output by enhancing microbial activity and stability in digestion, which results in higher biogas yields. In this case, it would mean higher revenues for industries and farm businesses that seek biogas as renewable energy. Furthermore, biochar solidifies the digestion process, thus reducing system failures and maintenance costs, which means ongoing production of energy. Its inclusion in AD systems will provide two benefits: lower emissions as well as enhanced biogas production. This is a win-win investment in sustainable AD technologies.
VIII. CHALLENGES IN BIOCHAR APPLICATION
Biochar provides several benefits for AD systems, but its application is confronted with several challenges such as scalability, feedstock variability, and long-term effects. One of the major drawbacks is that the scalability is difficult, as large-scale biochar production requires significant capital investment and specialized infrastructure. High costs related to pyrolysis technology, feedstock collection, and processing make its widespread use economically challenging for many AD facilities. Furthermore, logistical issues associated with sourcing, transporting, and integrating biochar into AD systems can also add to further complications. Feedstock variability is another vital issue. This means that a biochar has very much different quality and compositions depending on feedstock and condition of pyrolysis used. The microbe interactions would be affected at different levels; the surface areas, porosities, alkalinity, or adsorption will all affect processes and thus present inconsistent results within AD performance, making it more challenging to lay down universal recommendations for the applications of biochars.
The long-term impact of biochar in AD systems is not well understood. Very little research has been conducted on its prolonged interaction with microbial communities, possible accumulation in digesters, and impact on system efficiency over time. Concerns include microbial adaptation, possible clogging of reactor components, and changes in biochar's effectiveness after repeated use. Further research, standardized production methods, and cost-effective implementation strategies are needed to maximize the potential of biochar as a sustainable and efficient additive in AD systems.
IX. CASE STUDIES ON BIOCHAR IN AD
Application of biochar in AD has been evaluated in many practical environments. In all of these cases, improvements in methane yield, process stability, and inhibition control were noted. An industrial-scale digester showed that using pine biochar increased the methane yield by 20%. The reason is the high porous structure of this kind of biochar, enhancing microbial attachment and activity, hence improving biogas efficiency [1,6]. In a municipal AD plant, oak (Quercus spp.) biochar was applied there to neutralize the pH fluctuation and decrease ammonia inhibition. It was quite very effective in providing a buffer because of its alkaline nature in the reactor environment and steady biogas production at high organic loading rates [5,7].
A mixture of birch and spruce (Betula spp. and Picea spp.) biochars was tested within a European biogas facility to improve retention of microbes along with the better quality of the gas by having higher methane content. The stabilization of the digester process upon mixing these two biochars presented multi-biochar utilization opportunities for performance improvements in AD processes [3,4]. Further, an agricultural waste-based AD system showed that adding biochar from teak (Tectona grandis) reduced ammonia toxicity and maintained a stable output of biogas. Its strong capacity to adsorb neutralized the imbalances of nitrogen and was very suitable as a type of biochar application in AD for managing agricultural waste [8].
X. COMPARATIVE ANALYSIS OF BIOCHAR
Biochars from various feedstock types exhibit differing properties, which significantly influence anaerobic digestion (AD). Softwood biochars from species such as pine (Pinus spp.), spruce (Picea spp.) shows high porosity and large surface area that proves to enhance the microbial attachment to the biochar, increasing the microbial retention, resulting in higher methane yields and stability of the process [1,2]. Hardwood biochars from oak (Quercus spp.) and maple (Acer spp.) enhance better buffering capacity that prevents pH fluctuation and acidification leading to continued optimum methanogenic activity. Additionally, maple biochar enhances direct interspecies electron transfer (DIET), accelerates organic matter degradation, and improves methane production efficiency [3,4].
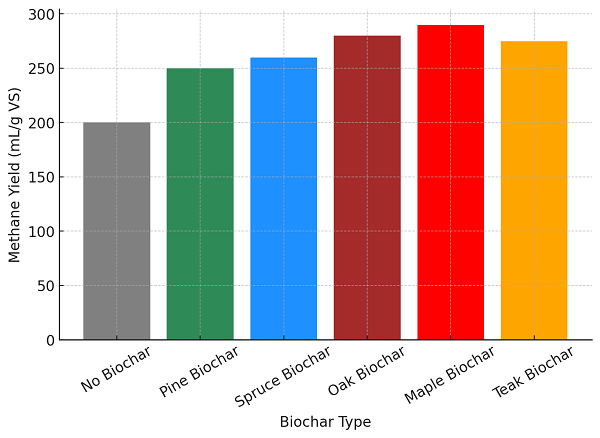
Fig. 2 Impact of Different Biochar Types on Methane Yield in AD System
Figure shows that various types of biochar improve methane yield in AD systems as biochar helps support microbes, acts as a pH buffer, and inhibits ammonia. Control with no addition of biochar showed the least amount of methane produced, that is, 200 mL/g VS, showing issues such as unstable pH and microbial inhibition [1,3]. Softwood biochars, such as pine (250 mL/g VS) and spruce (260 mL/g VS), enhance the attachment of microbes and interspecies electron transfer to increase methane yield [4,6,7]. Oak biochar (280 mL/g VS) offers a better buffering capacity and ammonia production reduction to produce stable methane yield [5,9]. Biochar derived from maple wood (290 mL/g VS) achieves the highest electrical conductivity and DIET, thereby producing the highest methane yield [3,11]. Teak biochar (275 mL/g VS) reduces ammonia toxicity, stabilizing biogas production and holds promise for large-scale AD applications [12,13].
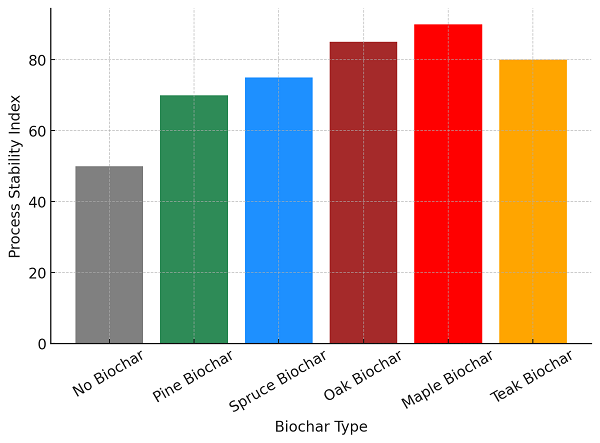
Fig. 3 Effect of Different Types of Biochar on Process Stability in AD System.
In the figure, it is shown that by applying different biochar types, process stability in AD systems is significantly enhanced. Thus, reactor efficiency and consistency during operation are improved. If there is no addition of biochar, the stability index is again the lowest, which is 50, reflecting the problems of pH fluctuation, microbial inhibition, and failure in the conventional AD system [1,3]. Softwood biochars, for example, pine (70) and spruce (75), increase microbial retention and long-term process stability by reducing reactor fluctuations and improving consistency in biogas production [4,6]. Hardwood biochars, such as oak (85) and maple (90), provide superior pH buffering, ammonia reduction, and electron transfer, leading to higher digestion stability [5,7,9]. The emerging feedstock teak biochar (80) stabilizes biogas production by preventing ammonia toxicity and is promising for large-scale AD applications [8,12].
The most recent study concludes that the latest biochar candidate for AD is teak, Tectona grandis. This one is capable of stabilizing biogas production because it eliminates ammonia inhibition that occurs during digestion on high-nitrogen feedstock. The elimination of this common problem happens due to excess ammonia adsorption and balanced microbial environment without causing toxicity, and continued biogas yields are guaranteed [5]. The comparative benefits of different biochars suggest that the right feedstock-based biochar can improve the efficiency of AD systems to a great extent. As the research advances, tailored biochar applications will further optimize biogas production, process stability, and waste-to-energy conversion in AD systems.
XI. FUTURE RESEARCH DIRECTION
The AD application of biochar is promising but requires further optimization and research before scaling up. Optimization of the pyrolysis process to further modify characteristics such as biochar porosity, alkalinity, and surface functionality enhances the biological activity of microbes, retention of nutrients, and pH stability in AD systems [1,3]. Making biochars tailored toward various digestion processes and feedstocks through optimized pyrolysis would be a better approach [5].
Further research is needed on the longer-term interaction of biochar with microbial communities. Although studies have indicated biochar to enhance microbial colonization and DIET, its role in supporting novel methanogenic strains as well as the stabilization of microbial dynamics is not identified [7,8]. The biochar compositional optimization would establish the highest biogas yield with process stability [4]. Long-term environmental and economic evaluations are important to assess the effects of biochar on greenhouse gas emissions, waste valorization, and digestate quality [6,9]. These findings will inform policy and industrial take-up, so that biochar is kept affordable and sustainable for large-scale AD applications [10,11]. Scaling up low-cost, sustainable feedstocks for biochar will increase the efficiency and viability of AD systems [12].
Conclusion
Biochar has shown potential to enhance the systems of anaerobic digestion with a capability of addressing significant issues like ammonia inhibition, pH instability, and low methane yields. Improving the stability of the process and the production of biogas is critical for the optimization of AD for both industrial and small-scale applications. Various types of biochar have different benefits, with softwood biochars (such as pine and spruce) offering high porosity that encourages microbial attachment and retention, thus providing higher yields of methane. Hardwood biochars (such as oak and maple) contribute to pH buffering and electron transfer, ensuring optimal methanogenic activity and enhanced organic matter breakdown. Emerging biochars, teak, amongst others, offer good prospects with reducing ammonia toxicity to levels, implying higher efficiencies and sustainability when used in the applications of AD. Despite many merits, future studies are to optimize the biochar production parameters by tailoring physicochemical properties for various conditions of digestions. Studies at longer scales to understand long-term effects on the microbial community and process efficiencies or biogas production also should be required for the ultimate comprehension of this phenomenon in stabilization processes of AD. Such interactions with different feedstocks and populations of microbes will expand its applicability in sustainable waste management. As advancements continue, the applications of biochar-enhanced AD systems will significantly contribute towards energy recovery, waste reduction, and circular bioeconomy, thus emerging as a key innovation for renewable energy and environmental sustainability.
References
[1] P. Devi and C. Eskicioglu, \"Effects of biochar on anaerobic digestion: A review,\" Environmental Chemistry Letters, vol. 2024. [2] S. Tang, Z. Wang, Z. Liu, Y. Zhang, and B. Si, \"The role of biochar to enhance anaerobic digestion: A review,\" Journal of Renewable Materials, vol. 2020. [3] A. I. Osman et al., \"Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review,\" Environmental Chemistry Letters, vol. 2022. [4] S. Singhal et al., \"Review of performance enhancement of anaerobic digestion with the aid of biochar and future perspectives,\" Journal of Renewable and Sustainable Energy, vol. 2023. [5] M. Manga et al., \"Biochar and Its Potential Application for the Improvement of the Anaerobic Digestion Process: A Critical Review,\" Energies, vol. 2023. [6] J. Pan, J. Ma, L. Zhai, T. Luo, Z. Mei, and H. Liu, \"Achievements of biochar application for enhanced anaerobic digestion: A review,\" Bioresource Technology, vol. 292, pp. 122058, 2019. [7] W. Zhao, H. Yang, S. He, Q. Zhao, and L. Wei, \"A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms, and economic assessments,\" Journal of Cleaner Production, vol. 255, pp. 120287, 2020. [8] H. Fathima, S. A. CK, A. D., and N. George, \"Exploring Tectona grandis: Phytochemicals, medicinal value, and green innovations: A review article,\" Journal of Pharmacognosy and Phytochemistry, vol. 13, no. 6, pp. 102–111, 2024. Available: https://doi.org/10.22271/pHyto.2024.v13.i6b.15170. [9] D. Mohan, A. Sarswat, Y. S. Ok, and C. U. Pittman, \"Organic and inorganic contaminants removal from water with biochar, a renewable, low-cost, and sustainable adsorbent – A critical review,\" Bioresource Technology, vol. 160, pp. 191-202, 2014. [10] J. Lehmann and S. Joseph, Biochar for environmental management: Science, technology and implementation, Earthscan, 2015. [11] N. A. Qambrani et al., \"Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review,\" Renewable and Sustainable Energy Reviews, vol. 79, pp. 255-273, 2017. [12] P. Dhull, R. K. Lohchab, S. Kumar, M. Kumari, Shaloo, and A. K. Bhankhar, \"Anaerobic Digestion: Advance Techniques for Enhanced Biomethane/Biogas Production as a Source of Renewable Energy,\" BioEnergy Research, vol. 17, pp. 1228–1249, 2024. Available: https://link.springer.com/article/10.1007/s12155-023-10621-7. [13] K. Stoknes, F. Scholwin, W. Krzesi?ski, E. Wojciechowska, and A. Jasi?ska, \"Efficiency of a Novel \'Food to Waste to Food\' System Including Anaerobic Digestion of Food Waste and Cultivation of Vegetables on Digestate in a Bubble-Insulated Greenhouse,\" Waste Management, vol. 56, pp. 466–476, 2016. Available: https://en.wikipedia.org/wiki/Digeponics. [14] L. Appels, J. Baeyens, J. Degrève, and R. Dewil, \"Principles and Potential of the Anaerobic Digestion of Waste-Activated Sludge,\" Progress in Energy and Combustion Science, vol. 34, no. 6, pp. 755–781, 2008. [15] R. E. F. Lindeboom, \"Autogenerative High Pressure Digestion: Anaerobic Digestion and Biogas Upgrading in a Single Step Reactor System,\" Water Science and Technology, vol. 64, no. 3, pp. 647–653, 2011. Available: https://en.wikipedia.org/wiki/Autogenerative_high-pressure_digestion. [16] \"Cradle-to-gate analyses of biochar produced from agricultural crop residues by slow pyrolysis,\" Clean Energy, Oct. 2023. Available: https://academic.oup.com/ce/article/8/6/1/7826174. [17] S. Chakrabarti and S. Shinde, \"A Three-Phase Analysis of Synergistic Effects During Co-pyrolysis of Algae and Wood for Biochar Yield Using Machine Learning,\" arXiv, May 2024. Available: https://arxiv.org/abs/2405.11821. [18] J. Hyväluoma, M. Hannula, K. Arstila, H. Wang, S. Kulju, and K. Rasa, \"Effects of pyrolysis temperature on the hydrologically relevant porosity of willow biochar,\" arXiv, Oct. 2018. Available: https://arxiv.org/abs/1810.07417. [19] R. Wood, O. Masek, and V. Erastova, \"Biochars at the molecular level. Part 1 -- Insights into the molecular structures within biochars,\" arXiv, Mar. 2023. Available: https://arxiv.org/abs/2303.09661. [20] Wikipedia, \"Biochar,\" Dec. 2024. Available: https://en.wikipedia.org/wiki/Biochar. [21] Wikipedia, \"Pyrolysis,\" Dec. 2024. Available: https://en.wikipedia.org/wiki/Pyrolysis. [22] Md M. Uddin and M. M. Wright, \"Anaerobic digestion fundamentals, challenges, and technological advances,\" Physical Sciences Reviews, vol. 7, no. 1, 2022. Available: https://www.degruyter.com/document/doi/10.1515/psr-2021-0068/html. [23] O. Yenigün and B. Demirel, \"Ammonia inhibition in anaerobic digestion: a review,\" Process Biochemistry, vol. 48, no. 5–6, pp. 901–911, 2013. [24] Y. Chen, J. J. Cheng, and K. S. Creamer, \"Inhibition of anaerobic digestion process: a review,\" Bioresource Technology, vol. 99, no. 9, pp. 4044–4064, 2008. [25] N. Eldem, Ö. Akgiray, ?. Öztürk, E. Soyer, and B. Çall?, \"Ammonia and pH inhibition in anaerobic treatment of wastewaters, Part II: Model development,\" Journal of Environmental Science and Health, Part A, vol. 39, no. 9, pp. 2407–2421, 2004. [26] Q. Niu, W. Qiao, H. Qiang, T. Hojo, and Y. Y. Li, \"Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: stability, inhibition and recovery,\" Bioresource Technology, vol. 137, pp. 358–367, 2013. [27] A. M. Shabbirahmed et al., \"Challenges and strategies for waste food anaerobic digestion: insights and future directions,\" Environment, Development and Sustainability, 2024. Available: https://link.springer.com/article/10.1007/s10668-024-04820-1. [28] J. H. Long, T. N. Aziz, F. L. de los Reyes III, and J. J. Ducoste, \"Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations,\" Process Safety and Environmental Protection, vol. 90, no. 3, pp. 231–245, 2012. [29] X. He, F. L. De Los Reyes, and J. J. Ducoste, \"A critical review of fat, oil, and grease (FOG) in sewer collection systems: Challenges and control,\" Critical Reviews in Environmental Science and Technology, vol. 47, no. 13, pp. 1191–1217, 2017. [30] R. E. F. Lindeboom, \"Integrating microbial electrochemical technologies with anaerobic digestion to accelerate propionate degradation,\" arXiv, Oct. 2020. Available: https://arxiv.org/abs/2010.10829. [31] M. A. Shabbirahmed et al., \"Current challenges of high-solid anaerobic digestion and possible solutions,\" Biotechnology for Biofuels and Bioproducts, vol. 15, Article 52, 2022. Available: https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-022-02151-9. [32] P. B. Patil and D. V. Pinjari, \"Plastic Waste to Wealth: Sustainable Approach Toward a Circular Economy,\" in Springer Science and Business Media LLC, 2024. [33] T. G. Ambaye, E. R. Rene, A.-S. Nizami, C. Dupont, M. Vaccari, and E. D. van Hullebusch, \"Beneficial role of biochar addition on the anaerobic digestion of food waste: A systematic and critical review of the operational parameters and mechanisms,\" Journal of Environmental Management, vol. 2021
Copyright
Copyright © 2025 Jumbom Ruti, Mudo Puming. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66892
Publish Date : 2025-02-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

