Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Coastal Aquaculture in the Indian Sundarban: Evaluating Production Economics and Farmers Perception of Mangrove Ecosystem Benefits
Authors: Milon Sinha, Nimai Bera, R. C. Bhatta, Thies Geertz, Sabyasachi Chakraborty, Ajanta Dey, Prasanna Surathkal, Natasha Majumder
DOI Link: https://doi.org/10.22214/ijraset.2024.58535
Certificate: View Certificate
Abstract
Expansion of shrimp-based aquaculture in Sundarban is one of the major causative factors for the degradation of mangroves though has proven positive impact on aquaculture. In order to understand the perception of the local coastal community on the contribution of Mangrove Ecosystem Service in brackish water aquaculture in Indian Sundarban, a situational analysis was carried out in 2019-2020 with 30 farmers of Kultali block under South 24 Parganas district of West Bengal. Based on the descriptive and inferential statistical analysis of the data collected from aquaculture pond owners, it was observed that the smaller farms, located in the vicinity of the mangroves (<50 mts from the mangrove creek) showed significant benefits through higher yield and lower operational costs. The results also showed that the farmers were not adequately aware of ecosystem benefits and the need to conserve mangroves for sustainable pond-based aquaculture.
Introduction
I. INTRODUCTION
The ‘mangrove ecosystem services’ (MES) contributes directly or indirectly to the well-being, livelihoods, and betterment of the socio-cultural and socio-economic condition of the coastal community (Uddin et al., 2013; Bandaranayake, 1998; Sathirathai & Barbier, 2001; Richman, 2002). Mangroves provide four types of ecosystem services, such as (1) provisioning services such as fish and shellfish, woods and honey (Gilbert & Jansen, 1997) (2) cultural services like mangrove tourism, religious value etc. (Uddin et al., 2013) (3) regulatory services such as carbon sequestration (Twilley et al., 1992; Bouillon et al., 2008) and (4) other supporting services such as trapping of mangrove sediments, soil formation and regeneration, uptake and transformation of nutrients (Hussain & Badola, 2008). Although there are well-known benefits of the mangrove ecosystem services (MES), mangroves have been indiscriminately cut down globally and transformed into aquaculture ponds in order to utilize untapped brackish water aquaculture resources to meet the demand for seafood (FAO, 2008; Smith et al., 2010). Unsustainable aquaculture and overexploitation of natural resources have created an imbalance in the mangrove-centric ecosystem (Martinez-Porchas & Martinez-Cordova, 2012). Ecosystem services and livelihoods are often interrelated to each other, and only a few such services have been truly understood (IIED, 1995; Narendran et al., 2001; Delang, 2006). Thus, it is very important that the local community who rely on natural resources for their livelihood have a better understanding of the ecosystem services provided by mangroves (Sinare et al., 2016; Owour et al., 2017). However, the perceptions of such ecosystem services are mostly context-specific and are found to differ between individuals and social groups, varying with the geographical location and socioeconomic characteristics at the community level (Fedele et al., 2017; Costanza et al., 2017) and the local management institutions (Costanza et al., 2017; Casado-Arzuaga et al., 2013).
The transboundary Sundarban mangrove ecosystem shared between India and Bangladesh constitutes the largest contiguous tropical mangrove ecosystem in the world and includes a series of innumerable islands and crisscross distributaries to form a bio-diverse estuarine complex of flora and fauna (Gopal & Chauhan, 2016). The Indian Sundarban forms an uninterrupted delta patch in the Ganga-Brahmaputra estuary (Rogers & Goodbred, 2014) and comprises a highly productive mangrove-centric ecosystem that serves as a potential nursery and breeding ground for the variety of shellfish, finfish and crabs etc. (Miller et al., 1983; Little et al., 1988) and also plays a very crucial role in blue carbon sequestration (Twilley et al., 1992; Bouillon et al., 2008).
They act as the natural barriers against tropical cyclones and tidal surges forming a coastal bio-shield and protecting the coastal community (Moberg & Ronnback, 2003). The local people living in this environment face several challenges like climate extremities, illiteracy, poverty and others and have to largely depend on the bio-resources in and around the mangroves (Ekka & Pandit, 2012; Hussain & Badola, 2010).
In the Indian Sundarbans, nearly60 % of the total working population depend on agriculture as a primary occupation, either as cultivators (23.6 %) or as agricultural labourers (36.1 %). (World Bank, 2014). However, studies show that the available agricultural land has reduced from 2149 km2 to 1691 km2 during the period 2001-2008 mostly due to the increase in population (Hazra et al., 2010) and uncontrolled expansion of profitable intensive shrimp aquaculture in the last few decades (Giri et al., 2021). While there has been unprecedented growth in the aquaculture sector it has also contributed largely to the degradation of the mangrove-centric ecosystem (Primavera, 2006; Simental & Martinez-Urtaza, 2008). Indiscriminate and unsustainable shrimp farming practices have also led to the salinization of paddy fields and freshwater sources in these areas (Chopra et al., 2009). In the Sundarban areas of West Bengal, many state policies are driving development in search of new opportunities that can balance the tradeoffs between mangrove conservation and aquaculture (Chopra et al., 2009; Sanchez-Triana et al., 2014; Dasgupta et al., 2019). However, knowledge about the exact relationship between the mangroves and pond aquaculture among the stakeholders would enhance their participation in promoting mangrove restoration and conservation measures. Through better understanding and awareness of ecosystem services, one can explain the relationship between humans and their environment (He et al.,2018) which also leads to a better understanding of an individual’s appreciation of the ecosystem services and their role in sustaining peoples’ livelihoods and well-being (Su et al., 2020).
In this context, a socio-ecological-economic survey was conducted to assess the present scenario of the mode of aquaculture practice and also to understand the socio-economic status of 28 aquaculture farmers residing in the villages of Madhabpur, Madhusudanpur, Kaikhali, Gopalganj, Shyamnagar of Gopalganj and Deulbari-Debipur Gram Panchayat of South 24 Parganas district under Kultali block of West Bengal who benefit directly and/or indirectly from mangroves. The main objectives of the survey study were: 1. To study the socio-economic status of the farmers in the region 2. To study the relationship between farm size and economic efficiency 3. To delineate the influence of mangrove ecosystem services on aqua farms, and 4. To understand the level of awareness of the benefits and costs of aquaculture in relation to mangrove ecosystems.
II. MATERIAL AND METHODS
A survey was conducted under the Kultali Community Development (CD) block of Indian Sundarban, South 24 Parganas, West Bengal, India (Figure. 1) where aquaculture is practiced predominantly. Geographically, the Kultali CD block is located at 22°05′12″N; 88°35′37″Eand bounded by the Jaynagar-I and Canning- I CD blocks in the north, a part of the Basanti CD block in the east, Sundarbans National Park in parts of the east and south, and the Jaynagar- II CD block in the west. The Kultali CD block has an area of 306.18 km2. A total of 28 aquaculture farmers were surveyed in 2019-2020residing in the villages of Madhabpur, Madhusudanpur, Kaikhali, Gopalganj, Shyamnagar of Gopalganj and Deulbari-Debipur Gram Panchayat in the Kultali block.
A structured questionnaire was developed to investigate the socio-economic status, farm management practices, and the perceptions of the aquaculture farmers about the impact of mangrove-aquaculture interactions. The questionnaire was divided into two sections: Section (A) dealt with general information including basic profile about the farmers (such as their age, experience, education, livelihoods, stocking density, harvesting cost and return, and farm management practices) and Section (B) focused on the perception of the farmers on the impact of the interaction between mangrove environment and aquaculture. In section B, most of the questions were normal scientific statements that are commonly believed to cause adverse and/or beneficial impacts. A mixed method approach was used to extract a quantitative and qualitative response from selected stakeholders such as aquaculture farmers, cultivators, agricultural labourers etc.
After the survey during the analysis three types of post-stratification were done from the sampled farmers. Firstly, the farmers were post-stratified into four quartiles based on their farm size in order to assess the influence of economies of scale on techno-economic differences. The second post-stratification was based on the size of the farm into small and marginal in order to comply with the standard method of classification followed in the literature. The third post-stratification of the samples was very important from the point of the present study namely assessment of the benefits of ecosystem services received from the mangroves. Thus, it was hypothesized that the farms located very close to mangroves have distinct benefits compared to farms away from the mangroves. Accordingly, farms were stratified into two categories namely those farms located within 50 meters from the mangrove creeks and greater than 50 meters from the mangrove creeks to test the assumption that the benefits of nutrient supply and water quality uniformly flow to all the farms and it does not influence the pond productivity.
If the creek is well maintained and equitably utilized, there should not be any difference between the yields between the two categories. The primary data was used to understand the influence of farm size, distance from the creek, and operational costs on the yield of the individual species. A linear mixed model of the following form was fit to the data[1]:
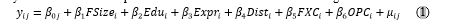
where y represents the yield, subscript i represents individual farms, subscript j represents the species groups farmed, FSize is the farm size (ha), Edu is the education (number of years) received by the farmer, Expr is the number of years of experience, Dist is the distance of the farm in meters from the mangrove creek, FXC is the total fixed costs of the farm (in rupees), OPC is the total operational costs of the farm (in rupees), and μi is the stochastic disturbance term that is assumed to be identically and independently distributed.The βk are coefficients of the regression model to be estimated where β0j are group-specific intercepts (fixed effects) that capture the heterogeneity in yield attributable to the species groups. Five groups of species were created in the data: crab; mullet; seabass; shrimp; and an “other” category. Interpretation of the fixed effects is the same as that of any dummy/indicator variable in a linear regression model with log-transformed dependent variable. The crab category was used as the base/reference category, and the fixed effects coefficients of the other categories can be used for deriving the percentage change in yield comparatively with the crab category. The variables yield, Dist , FXC, and OPC in the regression model were in logarithms to smoothen their distribution. Data processing and descriptive analyses of the data were done using Microsoft Excel 2016. The regression model was fit using the open-source statistical software R (R Core Team, 2023) and the fixest package contributed by Berge (2023).
A survey was conducted under the Kultali Community Development (CD) block of Indian Sundarban, South 24 Parganas, West Bengal, India (Figure. 1) where aquaculture is practiced predominantly. Geographically, the Kultali CD block is located at 22°05′12″N; 88°35′37″Eand bounded by the Jaynagar-I and Canning- I CD blocks in the north, a part of the Basanti CD block in the east, Sundarbans National Park in parts of the east and south, and the Jaynagar- II CD block in the west. The Kultali CD block has an area of 306.18 km2. A total of 28 aquaculture farmers were surveyed in 2019-2020residing in the villages of Madhabpur, Madhusudanpur, Kaikhali, Gopalganj, Shyamnagar of Gopalganj and Deulbari-Debipur Gram Panchayat in the Kultali block.
A structured questionnaire was developed to investigate the socio-economic status, farm management practices, and the perceptions of the aquaculture farmers about the impact of mangrove-aquaculture interactions. The questionnaire was divided into two sections: Section (A) dealt with general information including basic profile about the farmers (such as their age, experience, education, livelihoods, stocking density, harvesting cost and return, and farm management practices) and Section (B) focused on the perception of the farmers on the impact of the interaction between mangrove environment and aquaculture. In section B, most of the questions were normal scientific statements that are commonly believed to cause adverse and/or beneficial impacts. A mixed method approach was used to extract a quantitative and qualitative response from selected stakeholders such as aquaculture farmers, cultivators, agricultural labourers etc.
After the survey during the analysis three types of post-stratification were done from the sampled farmers. Firstly, the farmers were post-stratified into four quartiles based on their farm size in order to assess the influence of economies of scale on techno-economic differences. The second post-stratification was based on the size of the farm into small and marginal in order to comply with the standard method of classification followed in the literature. The third post-stratification of the samples was very important from the point of the present study namely assessment of the benefits of ecosystem services received from the mangroves. Thus, it was hypothesized that the farms located very close to mangroves have distinct benefits compared to farms away from the mangroves. Accordingly, farms were stratified into two categories namely those farms located within 50 meters from the mangrove creeks and greater than 50 meters from the mangrove creeks to test the assumption that the benefits of nutrient supply and water quality uniformly flow to all the farms and it does not influence the pond productivity. If the creek is well maintained and equitably utilized, there should not be any difference between the yields between the two categories. The primary data was used to understand the influence of farm size, distance from the creek, and operational costs on the yield of the individual species. A linear mixed model of the following form was fit to the data[2]:

where y represents the yield, subscript i represents individual farms, subscript j represents the species groups farmed, FSize is the farm size (ha), Edu is the education (number of years) received by the farmer, Expr is the number of years of experience, Dist is the distance of the farm in meters from the mangrove creek, FXC is the total fixed costs of the farm (in rupees), OPC is the total operational costs of the farm (in rupees), and μi is the stochastic disturbance term that is assumed to be identically and independently distributed.The βk are coefficients of the regression model to be estimated where β0j are group-specific intercepts (fixed effects) that capture the heterogeneity in yield attributable to the species groups. Five groups of species were created in the data: crab; mullet; seabass; shrimp; and an “other” category. Interpretation of the fixed effects is the same as that of any dummy/indicator variable in a linear regression model with log-transformed dependent variable. The crab category was used as the base/reference category, and the fixed effects coefficients of the other categories can be used for deriving the percentage change in yield comparatively with the crab category. The variables yield, Dist , FXC, and OPC in the regression model were in logarithms to smoothen their distribution. Data processing and descriptive analyses of the data were done using Microsoft Excel 2016. The regression model was fit using the open-source statistical software R (R Core Team, 2023) and the fixest package contributed by Berge (2023).
III. RESULT AND DISCUSSION
The socio-economic characteristics, technical factors, cost and returns were collected from the sampled farmers through personal interviews.
A. Post-Stratification of Sampled Farmers
As explained in the methodology section the sampled farmers (28 samples) were post-stratified and the results are presented in Table-1.
B. Socioeconomic attributes, farm management, and techno-economics of the sampled farmers with respect to aquaculture
General information on the socio-economic & techno-economic variables of the surveyed farmers revealed that all the farmers were male and their ages ranged from 32 to 75 years. Of the total sample, half of the farmers had joint families whereas others had nuclear families. From the study, it was observed that on average, the aquaculture farmers possessed more than 20 years of experience in brackish water aquaculture. The present findings of the study also corroborate the findings reported by Badola and Hussain (2003). The average age, percentage of joint families, the number of years of establishment, and experience increased with the increase in the farm size. It was also found that most of the older farms were jointly owned and the elderly owned larger ponds with similar education levels. It was also interesting to note that as the farm size increased, many socioeconomic factors which had an influence on the yield and farm management practices showed significant differences. The larger farms were also older and set up by experienced farmers. The farmers took up extensive aquaculture as livelihood considering their skills and inherited experience and traditional knowledge system. Kunstadter et al. (1986) also reported that the coastal community is significantly dependent on the harvest of marine and coastal resources as well as the adoption of extensive aquaculture as a livelihood option. The aquaculture farmers mentioned that most of the aquaculture ponds had been inherited. However, during the interaction, most of the farmers were unaware of any subsidy/scheme from the state/central government for aquaculture. There were also a few cases of conversion of paddy land into shrimp farms. The nature of land ownership of most of the farms was by virtue of “Patta” (User’s right) and the farmers operated their business either jointly or individually. Mostly, extensive or semi-intensive mode of aquaculture is being practised in the farms. The primary source of water supply in the farms is tidal exchange from mangrove creek. Most of the farms are located adjacent to the mangrove creek. During the survey, the farmers informed that as a part of their farm management, they had to invest in various capital assets for the establishment of the farm and operational expenses for their aquaculture practice. The costs are classified into a) fixed costs which include pond preparation, watch and ward, and total maintenance cost, b) capital costs which include land value or lease rent, pond construction or shaping, inlet-outlet, farmhouse cum store room, nets and crafts, pump set, generator, harvesting devices etc. and c) operational costs which include seed, feed, fertilizers, pond health management, labour and transportation respectively.
C. Analysis of Costs and Returns based on the farm size
Table-2 represents the results obtained from the survey of the four classes of farm size. It was observed that as the farm size increases, the capital costs and fixed costs per hectare decrease indicating the economies of scale. It was also found that the total capital investment cost of Group IV farmers was higher by 4 times compared to Group II farmers per hectare.
The Group I farmers invested almost 4 times higher cost per hectare compared to Group III farmers. The results showed that the farm size of Group III was most efficient in terms of the total cost of production per hectare. However, the highest yield of black tiger shrimp (Penaeus monodon) is shown among Group I farms (233 kg/ha) compared to only 99 kg/ha of the Group III farms. Interestingly, the per hectare production of all the stocked fish and shrimp such as sea-bass, crabs and other fishes were higher for the smaller farm size families except for mullets. The total production of crabs per family was higher for smallest farm group compared to larger farm family. Small ponds had more production of mullets than marginal ponds. According to Uddin et al. (2013), due to the bigger size, the availability of natural feed (plankton) was more in the bigger ponds compared to the smaller ponds. Moreover, increased water area in the bigger ponds enabled the free movement of the fishes which was more conducive for their growth. The cumulative effect of these two factors results in better growth which in turn, gives better yield. It was also evident that the size of the aquaculture farm is an important factor that influences the yield in crop productivity. The net profit per hectare earned by the different farm size classes showed that the net profit of Group I farmers was much higher compared to the other groups (Table-3).
D. Analysis of Costs and Returns of Small and Marginal Farmers
Through the second method of stratification adopted in the present study, the sampled farmers were classified into the standard small and marginal farmers. The results presented in Table 3 reveal the farm management systems which indicate the intensity of stocking and its impact on yield. The yield of all species such as shrimp, sea-bass and crabs were higher among the small farms except mullets and other fishes indicating that small farms are better managed compared to marginal farms. Access to the infrastructure, quality inputs and better farm management practices could have helped the small farmers to enhance their gross income. The total capital cost of small and marginal farms was ?68,635 and ?30,078 respectively which indicates the modern methods of scientific farming followed by small farmers. The operational costs of marginal farmers are almost double the small farmers indicating the economic inefficiency of marginal farmers in procuring materials. The gross profit per hectare of marginal farms was lower compared to small farms. Thus, the small farms earn higher net profit because of the higher gross revenue and lower operational costs.
E. Analysis of Costs and Returns based on the location of farms from the mangrove creeks
The results of the analysis of the stratification of sampled farmers based on the location of farms from the mangrove creeks are presented in Table 4. There are more farms within 50 meters from the mangrove creek. The operational cost of farms located 50 meters away was much higher compared to the farms located within 50 meters from the mangrove creek. Except for crabs, the yield of all other fish and shrimp species was much higher in the closer farms compared to farms located away from 50 meters. This indicated that the due to small farm size, and absence of strong embankments and crab nets, the crabs from these ponds would have easily escaped resulting in less yield. Some of the major varieties of fish species, shrimps and crabs harvested from the surveyed farms in the area are Black Tiger Shrimp (Penaeus monodon), Brown shrimp (Metapenaeus monoceros), Indian white prawn (Penaeus indicus), Asian Sea Bass (Lates calcarifer), Mullets (Liza parsia, Mugil cephalus&Liza tade), Long whisks catfish (Mystusgulio), White legged Shrimp (Penaeus vannamei) and Orange Mud Crab (Scylla olivacea). It was observed that the farm within 50 meters distance from the creek realized a higher net profit compared to the farm located 50 meters away from the creek.
Thus, there are mutual benefits between aquaculture farms and mangrove creeks with the lower cost of production. The results indicate that many direct and indirect ecosystem goods and services generated from mangroves benefit the aquaculture farms which are closer to the mangrove creeks (Iqbal, 2020). Thus, the integration of mangrove aquaculture is defined in this context as the integration of mutual benefits and tradeoffs of the two ecosystems in such a way that both ecosystems get benefits (Eddy et al., 2016).
F. Impact of Socio-ecological Factors on Yield: Regression Analysis
The results of the multiple regression analysis for all the selected species are presented in Table 5. The dependent variable is the logarithm of species-wise yield in individual farms. Table 5 shows that (logarithm of) farm size has a negative and statistically significant effect on farm yield, suggesting that larger farmers obtain lower yields than smaller farmers. This probably indicates the existence of significant diseconomies of scale in aquaculture practiced in the area of the farmer characteristics, the experience of the farmer has positive and significant impact on aquaculture yields. Distance of the farm from the creek has a negative and significant impact on farm yields. Given that farms closer to the creek are also more profitable as seen previously, distance appears to play an important role in determining the yields and economic sustainability of the farms in the area. Among the cost variables, only variable costs have a significant impact on the yield.
In Table 5, the crab category is the base category for the fixed effects. Since the dependent variable - yield - is in logarithms, the coefficient for mullet represents the percentage change in average yield for mullet farms compared to the farms growing crab. Mullet yield is lower by 42.12 percent compared to the yield of the base category, i.e., the crab category[3]. Along the same lines, shrimp yield is higher by 121.37 percent compared to the yield of the crab category. Technology for shrimp farming has been well-established and there is a structured value chain from hatcheries to final consumer, whereas for other species such as mullets and seabass there is a dearth of such markets especially for the supply of inputs like fingerlings.
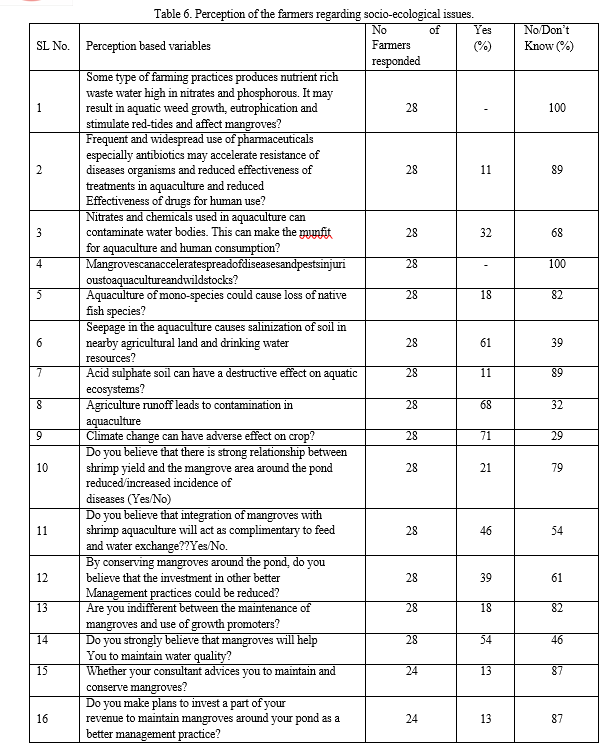
G. Socio-ecological Perceptions on Mangrove-aquaculture Linkages
The socio-economic survey also included a section to understand the perceptions of the beneficiaries regarding the relationship between aquaculture and water quality and mangroves. The selected questions have been presented in Table 6 to outline the critical socio-ecological relationships. A small number of farmers were aware of the negative impact of the use of antibiotics which may result in the development of disease resistance. Interestingly, none of the farmers agreed with the statement that mangroves could spread diseases through water contamination. One of the negative aspects of monoculture is the loss of aquatic biodiversity, was supported by 18% of the farmers and the rest were not aware. The presence of rice farms around shrimp ponds made farmers believe that the runoffs of rice farms could contaminate the water received by the pond aquaculture. Hence, the majority of the farmers agreed with the statement. Similarly, the majority of the surveyed farmers also agreed seepage of saline water would be affecting the rice fields. The majority of the farmers observed that there is a negative relationship between climate change (water temperature, salinity and variability of rainfall) and shrimp yield. Most of the farmers have answered affirmatively regarding the benefits of mangrove conservation on aquaculture such as maintenance of water quality, nutrition cycle, integration of mangroves with shrimp farming etc. In general, it was observed that the farmers strongly believe in the benefits of integration of mangroves with aquaculture considering their responses to social and ecological questions (Iqbal, 2020).
IV. ACKNOWLEDGEMENT
The authors are grateful to Global Nature Fund (GNF), Germany, for the financial cooperation through the project titled “Multi-Stakeholder Partnership (MSP) To Strengthen Transformative Processes in Shrimp Trade As A Basis For The Protection Of Mangrove Ecosystems In South Asia” or “Sustainable Aquaculture in Mangrove Ecosystem (SAIME)” The authors are also grateful to the field supervisor of Nature Environment & Wildlife Society (NEWS) who helped during the field survey. The authors also express their sincere gratitude to the local communities of the Kultali block for providing valuable information required for this study.
V. FUNDING
Funding cooperation for the study was provided by the Global Nature Fund in consortium with Naturland e.V., Germany under the project “Multi- stakeholder partnership (MSP) to strengthen transformative processes in shrimp trade as a basis for the protection of mangrove ecosystems in South Asia” (Project No-4286).
VI. CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Table 1. Post-stratification of surveyed farmers based on the data collected through key informant interviews with the help of a structured questionnaire
|
Group |
No. of observations (N) |
Minimum farm area (Hectare) |
Maximum farm area (Hectare) |
Mean ±SD |
|
I Quartile |
7 |
0.20 |
0.53 |
0.38±0.11 |
|
II Quartile |
7 |
0.54 |
1.20 |
0.89±0.25 |
|
III Quartile |
7 |
1.30 |
1.61 |
1.44±0.15 |
|
IV Quartile |
7 |
1.74 |
2.80 |
2.14±0.36 |
|
Overall |
28 |
0.20 |
2.80 |
1.21±0.70 |
|
Marginal farms |
18 |
0.20 |
1.34 |
0.79±0.41 |
|
Small farms |
10 |
1.60 |
2.80 |
1.98±0.39 |
|
< 50-meter distance |
24 |
0.20 |
2.80 |
1.25±0.73 |
|
>50-meter distance |
4 |
0.53 |
1.74 |
0.95±0.55 |
Table 2. Costs and returns of different farm size
|
Category/Farm Size quartile |
Group-I (N=7) ( 0.20-0.53 ha) |
Group-II (N=7) ( 0.54-1.20 ha) |
Group-III (N=7) (1.30-1.61 ha) |
Group-IV (N=7) (1.74-2.80 ha) |
|
Total Capital cost (?/Ha) |
55,332 |
20,760 |
35,771 |
76,549 |
|
Total Fixed Cost/Ha (?/Ha) |
9,260 |
5,162 |
3,363 |
21,592 |
|
Total operational cost (?/Ha) |
1,41,681 |
92,720 |
30,733 |
43,780 |
|
Total cost (?/Ha) |
1,50,941 |
97,881 |
34,096 |
65,372 |
|
Yield-Shrimp (P. monodon) (Kg/Ha) |
233 |
153 |
99 |
127 |
|
Yield-Sea Bass (Kg/Ha) |
53 |
32 |
15 |
27 |
|
Yield-Mullets (Kg/Ha) |
51 |
31 |
8 |
137 |
|
Yield-Other fish (Kg/Ha) |
147 |
77 |
52 |
135 |
|
Yield-Crab (Kg/Ha) |
184 |
50 |
16 |
55 |
|
Sale-Shrimp (P. monodon) (?/Ha) |
1,14,806 |
76,621 |
48,572 |
51,808 |
|
Sale-Sea Bass (?/Ha) |
15,809 |
10,485 |
4,452 |
10,896 |
|
Sale-Mullets (?/Ha) |
20,326 |
12,582 |
15,927 |
77,545 |
|
Sale-Other fish (?/Ha) |
34,065 |
71,217 |
22,258 |
48,265 |
|
Sale-Crab (?/Ha) |
1,30,615 |
35,609 |
12,366 |
34,427 |
|
Gross Revenue (?Ha) |
3,15,621 |
2,06,514 |
1,03,574 |
2,22,943 |
|
Gross Profit (?Ha) |
1,73,940 |
1,13,794 |
72,841 |
1,79,163 |
|
Net Profit (Total Revenue-Total Cost) ?/Ha |
1,64,680 |
1,08,633 |
69,478 |
1,57,571 |
Table 3. Costs and returns of small and marginal farmers.
|
Category/Farm Size quartile |
Marginal farmers < 1.5 Ha (N=19) |
Small farmer > 1.5 Ha (N=11) |
All farmers (N=28) |
|
Total Capital cost (?/Ha) |
30,078 |
68,635 |
52,541 |
|
Total Fixed Cost/Ha (?/Ha) |
5,551 |
16,950 |
12,192 |
|
Total operational cost (?/Ha) |
80,466 |
39,334 |
56,502 |
|
Total cost (?/Ha) |
86,017 |
56,284 |
68,695 |
|
Yield-Shrimp (P. monodon) (Kg/Ha) |
160 |
112 |
132 |
|
Yield-Seabass (Kg/Ha) |
32 |
22 |
26 |
|
Yield-Mullets (Kg/Ha) |
27 |
105 |
73 |
|
Yield-Other fish (Kg/Ha) |
76 |
118 |
101 |
|
Yield-Crab (Kg/Ha) |
65 |
44 |
53 |
|
Sale-Shrimp (P. monodon) (?/Ha) |
78,392 |
47,359 |
60,312 |
|
Sale-Sea Bass (?/Ha) |
10,099 |
8,703 |
9,286 |
|
Sale-Mullets (?/Ha) |
10,664 |
65,877 |
42,831 |
|
Sale-Other fish (?/Ha) |
45,340 |
42,350 |
43,598 |
|
Sale-Crab (?/Ha) |
45,393 |
28,587 |
35,602 |
|
Gross Revenue (?Ha) |
1,89,888 |
1,92,876 |
1,91,629 |
|
Gross Profit (?Ha) |
1,09,422 |
1,53,542 |
1,35,126 |
|
Net Profit (Total Revenue-Total Cost) ?/Ha |
1,03,871 |
1,36,592 |
1,22,934 |
Table 4. Costs and Returns of the farms located within and outside 50 meters of the creek.
|
Category/Farm distance quartile |
Farms< 50meters (N=24) |
Farms>50metres (N=6) |
|
Total Capital cost(?/Ha) |
54,418 |
56,610 |
|
Total Fixed Cost/Ha(?/Ha) |
12,637 |
8,673 |
|
Total operational cost (?/Ha) |
54,417 |
73,009 |
|
Total cost(?/Ha) |
67,054 |
81,682 |
|
Yield-Shrimp(P.monodon)(Kg/Ha) |
134 |
118 |
|
Yield-Sea Bass (Kg/Ha) |
26 |
26 |
|
Yield-Mullets (Kg/Ha) |
79 |
20 |
|
Yield-Other fish (Kg/Ha) |
107 |
55 |
|
Yield-Crab (Kg/Ha) |
50 |
78 |
|
Sale-Shrimp(P.monodon)(?/Ha) |
59,299 |
68,331 |
|
Sale-Sea Bass (?/Ha) |
9,463 |
7,884 |
|
Sale-Mullets (?/Ha) |
47,280 |
7,622 |
|
Sale-Other fish (?/Ha) |
47,479 |
12,878 |
|
Sale-Crab (?/Ha) |
33,136 |
55,125 |
|
Gross Revenue (?Ha) |
1,96,655 |
1,51,840 |
|
Gross Profit (?Ha) |
1,42,238 |
78,830 |
|
Net Profit (Total revenue-Total Cost) ?/Ha |
1,29,602 |
70,158 |
Table 5. Multiple regression analysis of the farm yields of shellfish and finfish species.
|
Variable |
Coefficient |
p-value |
|
|
Farm size (logarithm) |
-0.4900*** |
0.0024 |
|
|
Education |
0.0007 |
0.9729 |
|
|
Experience |
0.0129* |
0.0855 |
|
|
Distance from the creek (logarithm) |
-0.1530* |
0.0890 |
|
|
Fixed costs (logarithm) |
0.0722 |
0.2040 |
|
|
Operating costs (logarithm) |
0.3843*** |
0.0024 |
|
|
Intercept |
-0.5328 |
0.7036 |
|
|
Fixed effect- Mullet |
-0.5468** |
0.0338 |
|
|
Fixed effect- Others |
0.0722 |
0.7534 |
|
|
Fixed effect- Seabass |
-0.3140 |
0.2196 |
|
|
Fixed effect- Shrimp |
0.7947*** |
0.0008 |
|
|
Model R2 : 43.39 Model F-statistic: 8.201 (p-value <0.001). Symbol *** denotes statistical significance at the 99 percent confidence level, ** denotes significance at the 95 percent confidence level, and * denotes significance at the 90 percent confidence level. |
|||

Conclusion
The present study was carried out to understand the baseline socio-economic status of the brackish water fish farmers residing in the villages of the Kultali block and also to investigate their perceptions of the ecological benefits of mangroves and their ecosystem services. This study also focused on the different livelihood activities practiced by these farmers by integrating the ecological benefits of mangroves into tidal-fed pond aquaculture in the Sundarban area. The results obtained during the study showed that there was a strong relationship between the farm location and mangrove creeks in maintaining the diversity of finfish and shellfish, their yield, and the cost of production. The present study showed that the farmers have traditional knowledge of the ecosystem benefits of mangroves and wetlands for maintaining water quality and nutrient cycle. However, proper scientific evidence on water quality parameters like salinity, temperature, pH and oxygen, water depth, and plankton density have to be researched upon to validate the local ecological knowledge of the farmers. The present findings support the view that mangroves offer an important fishing ground for the well-being of coastal communities through the enhancement of fisheries production (Seary, 2019). The perception of farmers on the mangrove-aquaculture linkages was found to be positive. Their traditional knowledge can be utilized to influence the community to participate in the promotion of mangrove restoration and conservation measures in Sundarban linking with livelihood. Mangrove ecosystem services can be integrated into aquaculture practices. Hence, it can be concluded that integrated mangrove aquaculture can be effectively used for conserving mangroves and promoting sustainable livelihoods.
References
[1] Badola R., & Hussain, S.A. (2003). Valuation of the Bhitarkanika mangrove ecosystem for ecological security and sustainable resource use. Study report. Wildlife Institute of India, Dehra Dun 101. https://irade.org/eerc/pdf/WB_FR_RuchiBadola.pdf. Accessed 10 July 2020
[2] Bandaranayake, W.M. (1998). Traditional and medicinal uses of mangroves, Mangroves Salt Marshes, 2(3),133–148.
[3] Berge, L. (2023). Efficient Maximum Likelihood Estimation with Multiple Fixed-Effects. https://cran.r-project.org/web/packages/FENmlm/vignettes/FENmlm.html
[4] Bouillon, S., Borges, A.V., Castan˜eda-Moya, E., Diele, K., Thorsten, D., Duke, N.C., Kristensen, E., Lee, S.Y., Marchand, C., Middelburg, J.J., Rivera-Monroy, V.H., Smith III, T.J., & Twilley, R.R. (2008). Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem Cycles, 22, GB2013. doi:10.1029/2007GB003052.
[5] Casado-Arzuaga, I., Madariaga, I., & Onaindia, M. (2013). Perception, demand and user contribution to ecosystem services in the Bilbao Metropolitan Greenbelt. Journal of Environmental Management, 28, 1–6.
[6] Chopra, K., Kapuria, P., & Kumar., P. (2009). Biodiversity, land use change and human wellbeing: A study of aquaculture in the Indian Sundarbans. OUP Catalogue, Oxford University Press
[7] Costanza, R., de Groot, R., Braat, L., Kubiszewski, I., Fioramonti, L., Sutton, P., Farber, S., & Grasso, M. (2017). Twenty years of ecosystem services: How far have we come and how far do we still need to go? Ecosystem Services, 28, 1–16.
[8] DasGupta., R., Hashimoto., S., Okuro., T. & Basu., M. (2019). Scenario-based land change modelling in the Indian Sundarban delta: An exploratory analysis of plausible alternative regional futures. Sustainability Science, 14, 221–240. https://doi.org/10.1007/s11625-018-0642-6.
[9] Delang, C.O. (2006). Not just minor forest products: the economic rationale for the consumption of wild food plants by subsistence farmers. Ecological Economics, 59 (1), 64–73.
[10] Eddy, S., Ridho, M. R., Iskandar, I., & Mulyana, A. (2016). Community-based mangrove forests conservation for sustainable fisheries. Journal Silvikultur Tropika, 7 (3), S42-S47.
[11] Ekka, A., & Pandit, A. (2012). Willingness to pay for Restoration of Natural Ecosystem: A study of Sundarbans Mangroves by Contingent Valuation Approach. Indian Journal of Agricultural Economics, 67 (3), 323-333.
[12] FAO. (2008). The State of the World Fisheries and Aquaculture. Rome, Italy: FAO; 2009.
[13] Fedele, G., Locatelli, B., & Djoudi, H.(2017). Mechanisms mediating the contribution of ecosystem services to human well-being and resilience. Ecosystem Services, 28, 54.
[14] Gopal, B., & Chauhan, M., (2016). The Transboundary Sundarbans Mangroves (India and Bangladesh) In book: The Wetland Book (pp.1-10) DOI:10.1007/978-94-007-6173-5_26-6.
[15] Gilbert, A.J., & Jansen, R. (1997). The use of environmental functions to evaluate management strategies for the Pagbilao mangrove forest. CREED Working Paper 15, pp 51
[16] Giles, D. E. (2011). Interpreting dummy variables in semi-logarithmic regression models: Exact distributional results. University of Victoria Department of Economics Working Paper EWP, 1101, 1-24. https://web.uvic.ca/~dgiles/downloads/working_papers/ewp1101.pdf
[17] Giri, S., Samanta, S., Mondal, P.P., Basu, O., Khorat, S., Chanda, A., & Hazra., S. (2021). A geospatial assessment of growth pattern of aquaculture in the Indian Sundarbans Biosphere Reserve. Environment, Development and Sustainability. https://doi.org/ 10.1007/s10668-021-01612-9
[18] Hazra, S., Samanta, K., Mukhopadhyay, A., & Akhand, A. (2010). Temporal change detection (2001-2008) study of Sundarban. Jadavpur, Kolkata, India: School of Oceanographic Studies, Jadavpur University. Retrieved December 7, 2015.
[19] He, S., Gallagher, L., Su, Y., Wang, L., & Cheng, H. (2018). Identification and assessment of ecosystem services for protected area planning: A case in rural communities of Wuyishan national park pilot. Ecosystem Services, 31, 169–180.
[20] Hussain, S.A., & Badola, R. (2008). Valuing mangrove ecosystem services: linking nutrient retention function of mangrove forests to enhanced agroecosystem production. Wetlands Ecol Manage, 16, 441–450
[21] Hussain, S.A., & Badola, R. (2010). Valuing mangrove benefits: Contribution of mangrove forests to local livelihoods in Bhitarkanika Conservation Area, East Coast of India. Wetlands Ecology and Management, 18(3), 321-331.
[22] IIED, (1995). The hidden harvest: The value of wild resources in agricultural systems. A summary. Sustainable Agriculture Program, IIED, London
[23] Kimberly, G. R. & Goodbred., S. (2014). The Sundarbans and Bengal Delta: The World’s Largest Tidal Mangrove and Delta System In book: Landscapes and Landforms of India (pp.181-187) DOI:10.1007/978-94-017-8029-2_18
[24] Kunstadter, P., Bird, E., & Sabhasri, S. (1986). Man in the mangroves. United Nations University, Tokyo.
[25] Little, M.C., Reay, P.J., & Grove, S.J. (1988). The fish community of an East African mangrove creek. Journal of Fish Biology, 32(5), 729–747.
[26] Martinez-Porchas, M., & Martinez-Cordova, L. R. (2012). World Aquaculture: Environmental Impacts and Troubleshooting Alternatives. The Scientific World Journal, 2012, 9. doi:10.1100/2012/389623.
[27] Owuor, M.A., Icely, J., Newton, A., Nyunja, J., Otieno,P., Tuda, A.O., & Oduor, N., (2017). Mapping of ecosystem services flow in MidaCreek, Kenya. Ocean and Coastal Management, 140, 11–21.
[28] Rogers, K.G., & Steven L. G. Jr., (2014). Landscapes and Landforms of India: The Sundarban and Bengal Delta: the World’s Largest Tidal Mangrove and Delta System (V.S., Kale, Ed) Springer, New York.
[29] Sánchez-Triana, E., T. Paul, & O. Leonard. (2014). Building resilience for sustainable development of the Sundarbans. Washington, DC: The International Bank for Reconstruction and Development, The World Bank.
[30] Smith, M. D., Roheim, C. A., Crowder, L. B., Halpern, B.S., Turnipseed, M., Anderson, J. L., Asche, F., Bourillón, L., Guttormsen, A. G., Khan, A., Liguori, L.A., McNevin, Aaron., O\'Connor, M.I., Squires, D., Tyedmers, P., Brownstein, C., Carden, K., Klinger, D.H., Sagarin, R., Selkoe, K. A. (2010). Economics. Sustainability and global seafood. Science, 327 (5967), 784-6. doi: 10.1126/science.1185345.
[31] Iqbal, H. (2020). Valuing ecosystem services of Sundarbans Mangrove Forest: Approach of choice experiment. Global Ecology and Conservation, 24, e01273
[32] Miller, J.M., Crowder, L.B., & Moser, M.L. (1983). Migration and utilization of estuarine nurseries by juvenile fishes: an evolutionary perspective. Contributions to Marine Sciences, 27: 338–352
[33] Moberg, F., & Ronnback, P. (2003). Ecosystem services of the tropical seascape: interactions, substitutions and restoration. Ocean & Coastal Management, 46 (1–2), 27–46.
[34] Narendran, K., Murthy, I.K., Suresh, H.S., Dattaraja, H.S.S., Ravindranath, N.H., & Sukumar, R. (2001). Non-timber forest product extraction, utilization and valuation: a case study from the Nilgiri Biosphere Reserve, Southern India. Economic Botany, 55(4), 528–538.
[35] Owuor, M.A., Icely, J., Newton, A., Nyunja, J., Otieno, P., Tuda, A.O., & Oduor, N. (2017). Mapping of ecosystem services flow in Mida Creek, Kenya. Ocean and Coastal Management,140, 11–21.
[36] Primavera, J. H. (2006). Overcoming the impacts of aquaculture on the coastal zone. Ocean & Coastal Management, 49, 531–545.
[37] R Core Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Copyright
Copyright © 2024 Milon Sinha, Nimai Bera, R. C. Bhatta, Thies Geertz, Sabyasachi Chakraborty, Ajanta Dey, Prasanna Surathkal, Natasha Majumder. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET58535
Publish Date : 2024-02-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

