Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Exploring Esterification and Pyrolysis Pathways for Sustainable Biofuel and Biomass Production
Authors: Walunj Ankita A, Wagh Snehal A, Varhadi Shritej M, Shinde Abhishek N, Pate Shubham
DOI Link: https://doi.org/10.22214/ijraset.2023.55950
Certificate: View Certificate
Abstract
The recent Years Increase the demand of the biofuels along with need of reduce the production cost of Improving the environmental sustainability of the biomass production or the biofuels. Biomass is on area of importance to reduce the overall uses of fossil fuels. In which the focusing the kinetic properties or the optical property of the production of the Biofuel or Biomass production. Process optimization and reaction kinetic model development were carried out for two stage esterification transesterifications... reactions of waste biomass. This study focused on these traditional processes due to their techno- economic feasibility- Which is an important factor before deciding on a type of Feedstock for Industrilisation.Since esterification reactions are acid catalyzed the synthesis of an efficient acidic catalyst will be very helpful for pretreatment. esterification of acetic acid which was used model reaction in Stabilization of biomass. The esterification is a potential route to remove the organic acid in biomass reacting them with alcohol Present in biomass with added alcohol. Water is a product of the esterification reaction. The concentration of acetic acid used to acidification; acetic acid is a common chemical species in biomass & was used as the model compound. The research compared the quality of slow pyrolysis produced. biomass collected. by condensing pyrolysis vapor using atomized ethanol (EtOH).Were also carried out for the analysis of biomass produced.
Introduction
I. INTRODUCTION
Alternative fuels developed form renewable feedstocks are gaining market share recently. Biofuel is recognized as a green fuel is the one of the alternatives of the vegetable fuel. The demand for renewable energy sources has made biofuels on attractive alternative that can reduce the consumption of the traditional fossils fuel.
Development of second-generation bioethanol processes will not enable replacement of diesel or jet fuels. For these applications new processes, or third generation biofuels must be developed to which we will here refer to as advanced biofuels. Presently the most widely used biofuel is ethanol produced from sugarcane, corn, wheat but the use of feedstock that can be used for food production prevents this process form expanding further. Bioenergy represents the utilization of biomass as starting material to produce sustainable fuels.
The comprehensive idea on the developing technologies for bioenergy production which includes cell surface engineering of yeast to produce bioethanol from various biomass resources and the enzymatic and whole cell catalyzed transesterification of plant oils to biodiesel fuel.
Biofuels have a closed loop for the CO2 that is the main green house gas and beside that they can contribute to the reduction in the emission of toxic gases such as SO2, SO3 and carbon. Biomass has considered as a green fuel however is production process as resulting in high environmental impact.
The yeast saccharomyces cerevisiae is workhorse in the current biofuel industry as it is used for production of esterified.
A. Types Lignocellulosic Biomass
Examples- Maize, straw, rice, wheat straw, corn stalks, sugarcane, corn stover
For biodiesel production, lignocellulosic biomass constitute is largest renewable resources.
Ethanol and esterified production through bioconversion of lignocellulosic biomass, have been already successfully achieved by many research worldwide laboratory scale, industry scale. The amount of residue is obtained from agricultural, industrial, forest sources.
Table 1: List of materials
|
Material’s |
Quantity |
|
Potato |
200gm |
|
Agar |
4gm |
|
soil |
0.5gm |
|
Sacchromyces Cerevisiae broth |
19ml |
|
Corn |
10gm |
|
Honey |
19ml |
|
Sugarcane Juice |
6ml
|
|
Molasses |
40ml |
|
Gram Straw |
42gm |
|
Jaggery |
160gm |
|
Distilled Water |
250ml |
B. Equipment
Water Bath11, Incubator12, PH meter13, Autoclave14, Calorimeter15, Weighing Balance16.
II. METHOD
A. Culture Media
Preparation of culture media
Potato Dextrose Agar it is a frequently used microbial growth media for cultivation of malfeasants and other fungi. Take 200gm of potato for 1litre of PDA media preparation. wash the potato to remove dirt. Peel of the skin and dice them. Add the pieces to 1Litre of distilled water. Boil for 20-25min on hot plate. Filter / collect the extract through the muslin cloth. Then add 3-4 agar. Then autoclave 121°C for 15 PCI for 15 min. Then Cool It. Pour into 2 Petri plate then Solidify. Add yeast in one Petri dish and another add pinch of soil. Incubate, The Petri dish at 37°C for 3-4 days.
B. Detection of mold
Preparation of potato dextrose Broth PDB Take 200 gm of potato for 1 Liter of PDB media preparation wash the potato remove dirt. Peel off the skin. and dice them Add the pieces to 1 Liter of water. Boil for 20-25 min on Hot plate. Filter the extract through the muslin cloth. Then autoclave 121 °C for 15 pci for 15 min. Then cool it. Then, add 250 mL of PDB into conical Flask. Then Inoculate yeast into PDB by using sterile inoculating loop and another flask inoculate yeast which are present
in soil. Incubate the conical flask at 37°C for 3-4 days. Then detection of mold.
C. Acid Fast staining
The most Common staining technique used to identify acid fast bacteria is the Ziehl Neelsen Stain in which the acid-fast species are stained bright red. Firstly Air-dry Heat fix a thin film of microorganism on slide. Flood the slide with carbolfuchsin dye. Dry Heat for 2 min. cool and rinse with water wash the top and bottom of slide with water and clean the slide bottom well. Counterstain with methylene Blue for 30 sec to 1 min. Then observe under the microscope, Then High content of my colic acid in their cell walls. So Acid fast bacteria will be red in color. Acid fast bacteria was found.
D. Fermentation Tank
A fermentation tank is the vessel used to house work and yeast in the production of beer fermentation is. based on the principle of Anaerobic respiration for deriving energy from the breakdown of carbohydrates Such as glucose. In this process. glucose is first broken to pyruvate by glycolysis. The pyruvate is then converted to alcohol or lactic acid along with the regeneration of NAD. Fermentation of sugar by saccharomyces cerevisiae for production of ester fire in a batch experiment was Conducted to Improve the performance. of the Fermentation process. The thermotolerant ability of saccharomyces cerevisiae to grow and ferment glucose at elevated temperature, like the optima for Saccharification was Investigated.
The two essential raw material required for ferment carbon sources for ex. starch, cane molasses, beet molasses etc. Nitrogen sources ex. corn, Steep liquor, urea These are raw material extracted from venous Sources.
fermentation of biomass by Saccharomyces cerevisiae for production OF biofuel to improve the performance of the fermentation process the thermotolerant ability of saccharomyces cervices to grow and ferment glucose at elevated temperature Base of Fermentation tank is compose with wheat grain, corn, sugarcane Juice, yeast, molasses, Honey, Jaggery and water respectively.
Wheat is composed of 70% Starch, 10% crude protein and remaining part composed with water, fat, ash and fiber, soaking and fermenting grain improve their nutritional benefits corn is a primary biomass source being used for producing cellulose ethanol. Sugar cane juice used for production of biofuel like ethanol molasses is mainly used as a Source of sugar for the fermentation. It is a used as a microbiological energy source. Honey can work to. Feed yeast. It helping in to produce the neutral by product of carbon. dioxide and alcohol Jaggery contain. carbon sources as compared with Sucrose It provide feed to yeast. Saccharomyses cerevisiae & seethe production of acetyl co A derived form n-butane, fermentation were performed in 250 mL borosilicate glass bottle, Containing wheat grain, sugar cane Juice, Jaggery and water. yeast, Honey
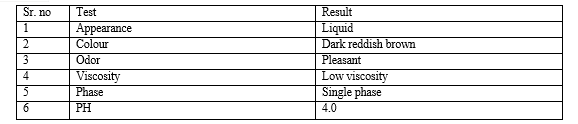
The maximum biofuel production rate were observe between 30 to 45? with difference. Initial glucose concentration increase Substrate supply did not. improve the specific ethanol production rate when pH was not controlled. PH 4.0-5.0 was optimal. range for biofuel production process a changes in the main fermentation pathway was observe with various PH range. The Highest specific biofuel production rate at pH 4.0 formation of acetic acid increase when PH was below 4. When temp was increase to us of the System still show High cell growth and production of Biofuel.
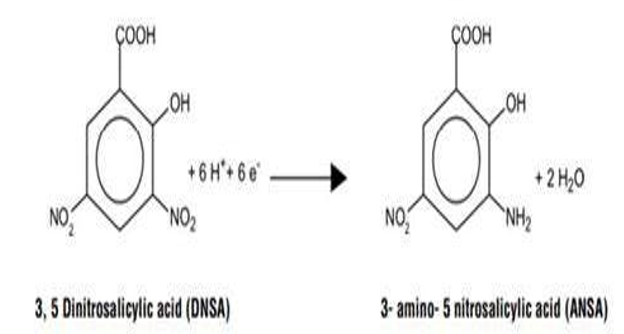
E. DNSA Test
3,5-dinitrosaalicylic acid is used extensive In biochemistry for the estimation. of reducing sugar, it detect the presence of free carbonyl group of reducing sugars. This involves the oxidation of aldehyde functional group and the ketone functional group.
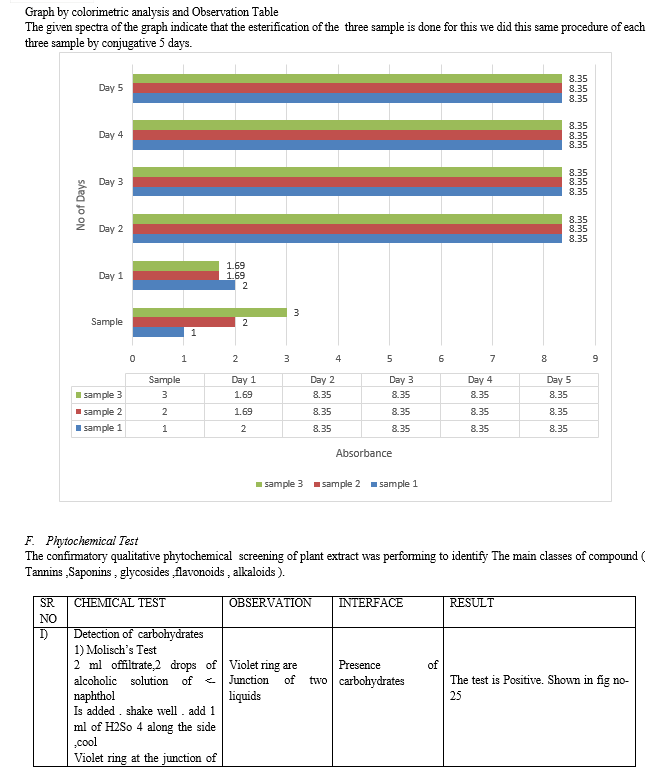
In amber colour bottle 1 gm of 3,5-dinitro Salicylic acid slowly add is gm of Sodium potassium tartarate phenol, Then add 10 gm of NAOH. Slowly added and continuous shaking in 100 ml of Water store in Dark place Prepare test tube for test sample and one control. Take 1 mL of dinitro solution and odd 4 mL of reducing sugar Sample in test tube. Boil in water both for 15 min at 70? temp. Get reading for 5 days to cheek the Stability of reducing sugar of it.







III. DISCUSSION
Certainly, let's discuss the various aspects of the subject you've presented:
- *Importance of Biofuels*: The increasing demand for biofuels is driven by the need to reduce our reliance on fossil fuels and their associated environmental impact. Biofuels, derived from renewable feedstocks, offer a more sustainable energy source.
- *Cost Reduction and Environmental Sustainability*: Reducing production costs is essential for the widespread adoption of biofuels. Simultaneously, improving the environmental sustainability of biomass production and biofuel processing is crucial to mitigate their environmental impact.
- *Kinetic vs. Optical Properties*: Your text mentions focusing on the kinetic properties or optical properties of biofuel and biomass production. It's important to clarify the specific properties you're referring to, as this can influence the efficiency and quality of production processes.
- *Process Optimization*: Process optimization plays a pivotal role in enhancing the efficiency of biofuel production. Optimization includes factors such as reaction kinetics, temperature, pressure, and catalysts.
- *Esterification and Transesterification*: These are key reactions in biofuel production, particularly in the synthesis of biodiesel. Esterification reactions, often acid-catalyzed, are used to pretreat biomass and remove organic acids, making the feedstock more suitable for biofuel production.
- *Pyrolysis and Condensation*: Slow pyrolysis and condensation of pyrolysis vapor using atomized ethanol are techniques used to produce biomass. The quality and composition of the resulting biomass can vary based on these processes.
- *Types of Biofuels*: Biofuels can be categorized into different generations. Second-generation bioethanol processes aim to complement traditional fuels. Third-generation biofuels, often referred to as advanced biofuels, are needed for applications like aviation and are less reliant on food crops.
- *Environmental Impact*: While biofuels are considered greener alternatives, their production processes can have a significant environmental impact. Finding ways to reduce this impact is crucial for their sustainability.
- *Lignocellulosic Biomass*: This type of biomass, derived from sources like maize, straw, and sugarcane, is abundant and suitable for biofuel production, particularly biodiesel.
- *Yeast Saccharomyces cerevisiae*: This yeast is widely used in the biofuel industry, especially for esterification processes. It's a workhorse for converting feedstock into biofuels.
- *CO2 Reduction*: Biofuels offer a closed-loop system for CO2 emissions, as they absorb CO2 during growth and release it when burned. This makes them a valuable tool in reducing greenhouse gas emissions.
- *Challenges and Future Research*: Discuss the challenges in scaling up biofuel production and the need for ongoing research to improve efficiency, reduce costs, and minimize environmental impacts.
In conclusion, the development and adoption of biofuels are critical steps toward a more sustainable energy future. Achieving these goals requires a multidisciplinary approach that combines chemistry, biology, engineering, and environmental science to optimize processes and reduce their environmental footprint.
Conclusion
In conclusion, the recent increase in demand for biofuels, coupled with the necessity to reduce production costs and improve environmental sustainability in biomass production, highlights the significance of optimizing the processes involved. This study specifically focused on the kinetic and optical properties of biofuel and biomass production, with a particular emphasis on esterification reactions as a means to efficiently convert waste biomass. The development of an efficient acidic catalyst for pretreatment was identified as a key step in this process. Esterification of acetic acid, a common component in biomass, was investigated as a model reaction. This method offers the potential to remove organic acids from biomass by reacting them with alcohol present in biomass, producing water as a byproduct. Additionally, the quality of slow pyrolysis-produced biomass was compared with biomass collected by condensing pyrolysis vapor using atomized ethanol (EtOH). The findings of this study underscore the importance of carefully considering the techno-economic feasibility of various processes when selecting feedstock for industrialization. Furthermore, the development of efficient catalysts and optimization of esterification reactions hold promise for enhancing the overall efficiency and sustainability of biofuel and biomass production. These efforts contribute positively to the reduction of fossil fuel dependency and the promotion of a more environmentally friendly energy future.
References
[1] Aarnio TH, Suihko ML, Kauppinen VS (1991) Isolation of acetic acid-tolerant bakers-yeast variants in a turbidostat. Appl Biochem Biotechnol 27:55–63 [2] Albers E, Larsson C, Lide´n G, Niklasson C, Gustafsson L (1996) Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195 [3] Khandekar ML, Murty TS, Chittibabu P (2005) The global warming debate: a review of the state of science. Pure Appl Geophys 162:1557–1586 [4] Verho R, Putkonen M, Londesborough J, Penttila¨ M, Richard P (2004) A novel NADH-linked L-xylulose reductase in the L-arabinose catabolic pathway of yeast. J Biol Chem 279:14746–14751 [5] Ballerini D, Desmarquest JP, Pourquie J, Nativel F, Rebeller H (1994) Ethanol production from lignocellulosics: large scale experimentation and economics. Bioresour Technol 50:17–23 [6] Gervais P, Sarrette M (1990) Influence of age of mycelia and water activity on aroma production by Trichoderma viride. J Ferment Bioeng 69:46–50 [7] Green M, Kimchie S, Malester I, Shelef G (1989) Ethanol production from municipal solid waste via acid hydrolysis. In: Klas DL (ed) Energy from biomass and wastes XIII. Institute of Gas Technology, Chicago, pp 1281–1293 [8] Hari KS, Janardhan RT, Chowdary GV (2001) Simultaneous saccharification and fermentation of lignocellulosic wastes to ethanol using thermotolerant yeast. Bioresour Technol 77:193 196 [9] Zhuang XY, Zhang HX, Yang JZ, Qi HY (2001) Preparation of levoglucosan by pyrolysis of cellulose and its citric acid fermentation. Bioresour Technol 79:63–66 [10] Ziegler MT, Thomas SR, Danna KJ (2000) Accumulation of a thermostable endo-1,4-b-D-glucanase in the apoplast of Arabidopsis thaliana leaves. Mol Breed 6:37–46
Copyright
Copyright © 2023 Walunj Ankita A, Wagh Snehal A, Varhadi Shritej M, Shinde Abhishek N, Pate Shubham. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55950
Publish Date : 2023-09-30
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

