Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Extraction of Bacterial Pigments and Evaluation of its Antioxidant Activity
Authors: Dr. Anupriya Chudiwal, Nandana Nair , Mahesh Salunke
DOI Link: https://doi.org/10.22214/ijraset.2024.64351
Certificate: View Certificate
Abstract
Carotenoid are pigment that exist in a wide variety of plant and microorganism they are characterized by red, yellow or orange coloration .These important pigment play significant role in animal and human health .In contrast to plant production, the advantages of microbial fermentation of carotenoids were lower media cost, fast growth rate of microorganism and the ease of culture control . When the microbial cells are used to produce the color the term refers to ‘microbial pigments’. Different types of pigments extracted from different microbes. In this study, soil and water samples were collected from different areas of MGM University, Chh. Sambhajinagar .Different pigmented colonies were observed on nutrient agar plate .Out of these, the orange and yellow colonies were selected and sub-cultured further to obtain their pure cultures. These samples were inoculated onto nutrient agar plates, and after an incubation period of 24 hours, different colonies were observed on the plates. Out of these, the orange and yellow isolated were selected and sub-cultured further to obtain their pure cultures. After production of the pigment in liquid medium, it was further processed for the extraction of these pigments. Further, antioxidant assay by radical scavenging activity of DPPH was carried out, which revealed that the pigment had an radical scavenging activity of 60% as compared to that of the standard ascorbic acid, which showed radical scavenging activity of 33 %. Thus, the current study can be a useful step for large-scale production of pigments, their purification and application in cosmetic industries
Introduction
I. INTRODUCTION
Plants, animals, and microbes are the sources of natural pigments. Different types of pigments extracted from different microbes. Microbial pigment production is a recent phenomenon. They display all the colors from rainbow including light or dark tinges and unusual colors like black, white, brown, golden, silver and fluorescent green, yellow or blue (Chandran et al., 2014).
Among microorganisms, pigments displaying a wide diversity of colour, structure, and function have been reported. Though much has been published regarding microbial pigments, much more remains to be investigated, particularly regarding their biological role in the producing organisms. Many of the pigments produced by pathogenic bacteria have been shown to act as a virulence factor (Kothari et al., 2016). Dyes and pigments have become an indispensable part of our daily life. Since they colour and enhance the visual appeal of various products, they have different applications in many fields ranging from agriculture to textiles, cosmetics to pharmaceuticals. To meet the demands in these fields, they are produced on a large scale. Synthetic dyes have the advantage of offering a wide range of colour alternatives and consistent colour quality, as well as being suitable for large-scale production at an economical price. However, they can have unfavourable consequences on both human health and environment (Pizzicato et al., 2023). Most of the synthetic dyes are not readily biodegradable, persisting in the environment for a long time, leading to long-term environmental impacts. The production of synthetic dyes often requires the use of non-renewable resources or even petroleum-based chemicals (Pizzicato et al., 2023).
Production of all the pigments shown in this figure is believed to be associated with quorum-sensing (QS) in bacteria. QS refers to a population-density dependent regulation of the behaviour of the given microbial population. Since pigment production in many of the pathogenic bacteria (e.g. Pseudomonas aeruginosa, Staphylococcus aureus, etc.) is under control of QS, the genetics of pigment production assumes high importance in clinical context. Many of the screening assays for identifying novel QS-inhibitors also employ pigment production as the test parameter. Some of these bacterial pigments (e.g. prodigiosin from Serratia marcescens) have the potential of being developed as a commercial product, and hence the metabolic pathways and the genes involved in biosynthesis of these pigments attract attention from an industrial perspective too (Siddharthan et al., 2020). The textile dyeing industry has long been environmentally challenging in several respects. First, there is very high water consumption during all stages of dyeing, which consequently generates a significant amount of contaminated water waste.
Second, the use of very harsh chemicals is also a significant threat to the environment, especially given that residue from these chemicals can come into contact with consumers during their use of textile products. Third, the energy consumption during dyeing through the heating and drying stages is also very high. Finally, the dyes that are currently used are mostly of synthetic origin. One of the ways to reduce these issues is to use natural dyes. Natural dyes were in use before synthetic ones; however, their extraction and use are technologically time-consuming and very expensive. In the past decade, research has focused on emerging, novel and exciting sources of natural dyes: bacteria .
II. METHODOLOGY
A. Sample Collection and Isolation of Pigmented Bacteria
- Soil Sample: Soil samples were collected from MGM University campus Chh.Sambhainagar .Samples of collected soil were serially diluted up to a concentration of 10-6. On sterile Nutrient agar (NA) Petri plates, dilutions ranging from 10-5 to 10-6 were plated and incubated for 72 hours at 37°C.
- Water Samples: A water sample was placed in a clean container at several points on the JNEC campus of MGM University. After that, it was hurriedly brought to the lab to be prepared for bacteriological examination. Water samples were serially diluted to a ratio of 10–6. After sterilizing the plates, dilutions ranging from 10-5 to 10-6 were added to the nutrient agar (NA). After that, the plates were incubated at 37°C. The isolated colonies of pigment-producing organisms were streaked over extra sterile NA plates in order to separate pure cultures.
B. Selection and Purification of Pigment Producing Isolates
The isolated colonies were purified on Nutrient agar medium using three different streaking procedures. To identify potential isolates, a color difference in the colony was used. To do this, isolates were spread out on nutrient agar medium, incubated at 25°C for 48 hours, and the development of pigment was observed. Color was used to select the colonies, and in order to get pure cultures, streaking was done repeatedly. After purification, isolates were stored at 02°C.
C. Characterization
Morphological Characterization : The morphological characterization was done on the basis of their colour, size, shape, margin, opacity, consistency, elevation, Gram staining and motility..
Biochemical characterization: For characterization other biochemical tests were carried out.
- Indole test: The orange and yellow isolates were inoculated to the test tubes containing tryptone water and incubated at 37°C for 24 hrs. 1ml of Kovac’s reagent was added along the side of the test tube. The development of a bright red colour ring at the inter phase of reagent and broth constitutes a positive test.
- MR-VP Test: For this, MRVP broth was inoculated with the orange and yellow isolates, and uninoculated broth was used as a control. Cultures were incubated at 37°C for 48 hours. For MR test, 5 drops of methyl red was added in the test solution. Change of colour to yellow indicates a negative result, while change of colour to bright red indicates a positive result. For VP test, Barrit’s reagent A and Barrit’s reagent B was added to the test solution. Change of colour to brownish red would indicate a positive result, and if the test solution remains colourless, it would indicate a negative result.
- Catalase Test: A smear of the isolated bacterial colony was prepared on the glass slide, and 2-3 drops of H2O2 were added to the smear. Appearance of bubbles within 1 minute after the addition of H2O2 indicates positive result.
D. Production and Extraction of Microbial Pigments
Bacterial isolates (yellow and Orange) were grown in LB medium. An equivalent number of cells were inoculated in 100 milliliters of freshly prepared LB medium for large-scale pigment production. The cells were then cultured in an incubator shaker at 28°C and 130 rpm for seven days, during which time the production of pigment was visually monitored. A variety of solvents, including ethanol, chloroform, petroleum ether, methanol, and acetone, were employed to test the pigments' maximum solubility. Additionally, the solvent was chosen by looking at the pigment's maximal solubility in it. The greatest solubility of pigment in methanol is observed.
100 ml of freshly prepared LB medium was cultivated in an incubator shaker at 130 rpm and 28°C to produce microbial pigments from yellow and orange isolates for 7 days .The bacterial culture was centrifuged at 6000 rpm for 20 minutes after 7 days. The supernatant was disposed away after centrifugation, and bacterial cell pellets were prepared for pigment extraction. One millilitre of methanol was added to the cell pellets, and they were centrifuged until they lost colour.
After that, the cell debris was disposed of, and the supernatant was moved to a glass petri plate to dry in an incubator for the entire night at 37°C. Shahitha and Poornima (2012) state that dried pigment was removed and dissolved in methanol at a concentration of 1 mg/ml. The antioxidant activity of the crude pigments was also checked .
3.5: Antioxidant property by DPPH method
The antioxidant property of the crude pigments was determined by it’s ability to scavenge 2,2-diphenyl-1- picrylhydrazyl (DPPH). The absorbance of each supernatant sample was measured at λ=517 nm and 1ml of 95% methanol was used as a control. Ascorbic acid was used as a standard. The antioxidant activity was given as percent (%) DPPH scavenging, calculated by the following formula:
% of antioxidant activity(RSA%) = [(Ac−As) ÷Ac] × 100
Where Ac is the absorbance of control, and As is the absorbance of test solution.
III. RESULT AND DISCUSSION
A. Isolation Of Pigment Producing Bacteria And Sub-Culturing
Fountain Water sample was plated on nutrient Agar plates. Most of the colonies were white, except one light orange coloured colony was observed on third day of incubation at 37°C (Fig.1 A). The soil sample was also plated on Nutrient agar plate and incubated at 37°C for one week. On third day of incubation, orange colonies were observed along with yellow and deep orange-coloured bacterial colonies. The two (yellow and Orange) coloured colonies were picked for further study. Out of these, the orange and yellow colonies were sub-cultured onto nutrient agar plates to obtain pure cultures
B. Characterization
Characterization were done by gram staining, indole production test, methyl red test, Voges-proskeaur test, catalase and oxidase test. Characterization of both the yellow and orange bacterial isolates were done .results were mentioned in table no .1 and 2
Colony morphology: The colony morphology of both the yellow and orange isolates were carried out on the basis of criteria as mentioned below:
|
Criteria |
Orange isolate |
Yellow isolate |
|
Colour |
Orange |
Yellow |
|
Opacity |
Translucent |
Opaque |
|
Elevation |
Convex |
Convex |
|
Texture |
Mucoid |
Brittle |
|
Shape |
Circular |
Irregular |
Table 1: Colony morphology
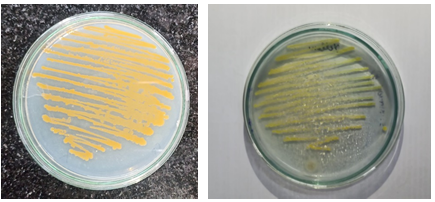
Plate 1: Orange isolate Plate 2: Yellow isolate
Fig 1: Pure cultures of orange and yellow obtained by successive sub-culturing
- Gram’s Staining: Upon microscopic examination, it was observed that the orange isolate retained the violet colour of crystal violet, while the yellow isolate did not retain the stain crystal violet, and was counter stained by saffranin. It was observed that the orange isolate was gram positive, cocc; while the yellow isolate was gram negative, cocci.
- Indole Test: Since no development of bright red coloured ring was observed at the interphase of reagent and test sample, it can be concluded that the test was negative for both the orange and yellow isolates.
- Methyl Red Test: Upon addition of methyl red indicator, it was observed that the test solution containing orange isolate showed change in colour to red, while the test solution containing yellow isolate showed change in colour to yellow. This indicates a positive and negative result, respectively.
- Voges-Proskaeur’s Test: Upon addition of Barrit’s reagent A and Barrit’s reagent B to the test solutions both orange and yellow isolates, no change in colour was observed which indicated a negative result.
|
Sr no. |
Test |
Orange isolate |
Yellow isolate |
|
1. |
Indole |
Negative |
Negative |
|
2. |
Methyl red |
Positive |
Negative |
|
3. |
Voges Proskaeur’s |
Negative |
Negative |
|
4. |
Oxidase |
Negative |
Negative |
|
6. |
Gram Staining |
Gram positive, cocci |
Gram negative, rod |
|
7. |
Motility |
Non-Motile |
Non-Motile |
Table 2: Biochemical characterization
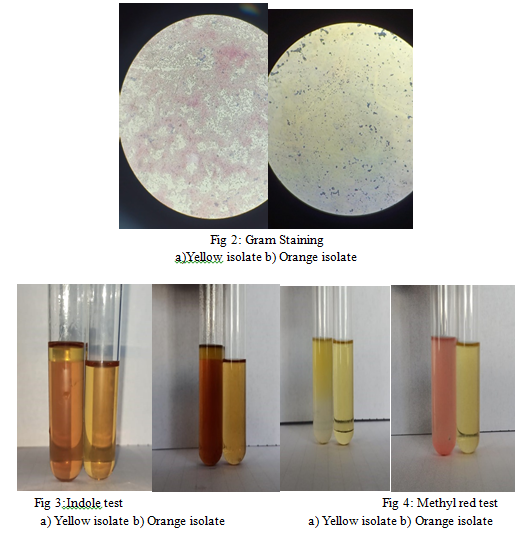
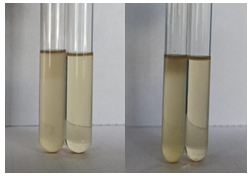
Fig 5: Voges-Proskeaur’s test
a) Yellow isolate b) Orange isolate
C. Extraction of Pigment
Production of microbial pigments: Bacterial isolates (orange and yellow) were inoculated in 150 ml freshly prepared nutrient broth and incubated in shaking incubator for a period of 7 days, and were visually observed for pigment production.
Extraction of the pigment: After 7 days, bacterial culture was centrifuged at 10,000 rpm for 15 min. After centrifugation, supernatant was discarded and to the cell pellets, 1 ml of solvent (methanol, acetone) was added and vortexed until the cell pellets turned colorless. The cell debris was discarded, while the supernatant was transferred to a glass petri-plate and dried overnight in an incubator at 37?. Dried pigment was scraped out and dissolved in the solvent, and 10 ml of the pigment extract was retrieved in the process. In this study, it was observed that the orange isolate showed better growth in less time as compared to the yellow isolate, which needed minimum 48 hours for its growth. The yellow isolate also showed better pigmentation with prolonged incubation period that is 3 days, as compared to the orange isolate. This observation was in accordance with the work conclusions drawn by Patil et al., 2023.
During the extraction of pigments, two different solvents were used for the extraction process, i.e. methanol and acetone. It was observed that the pellet turned colourless on addition of acetone, but when methanol was added, the pellet still retained some of the orange colour. Thus, it can be concluded that methanol served as a better solvent for the pigment than acetone, as it was also mentioned by Patil et al, 2023.

Fig 6: Production of microbial pigment Fig 7: Pigment extraction
D. Antioxidant Assay
The extracted pigment was then checked for antioxidant properties by using DPPH method. The antioxidant property was then calculated according to the mentioned above formula. By suppressing the DPPH radical, the pigmented extracts from this investigation demonstrated concentration-dependent antiradical action. Profound capacity DPPH scavenging potential of methanolic crude pigment extract of the microbial strain yellow and orange may be due to hydrogen groups present in the extracts .It was observed that the pigment extract showed a RSA of 60 %, as compared to the standard (ascorbic acid) which showed an RSA of 33%.

Fig 8: DPPH antioxidant assay
Conclusion
Natural dyes and pigments have emerged as an important alternative to potentially harmful synthetic dyes. The production of pigments from microorganisms is beneficial over other sources because microorganisms can grow rapidly which may lead to high productivity of product. In this study, bacterial strains showing orange and yellow pigments were isolated and characterized. Methanol served as a better solvent for the pigment as compared to acetone. The extracted pigment showed a considerable radical scavenging activity.
References
[1] Chandran M., Yuvaraj D., Vivek P., Narayanan P (2014). Production and extraction of pigments from novel strains and their applications. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 5(6): 584-593 [2] Patil M., Kalyani M., Ghodake B., Narwade B (2023). Production and characterization of bacterial pigment from soil and water bacterial isolates. Peer Reviewed & Indexed Quarterly Journal for Applied science 12(45): 545-550. [3] Siddharthan N., Sandhiya R., Hemalatha N (2020). Extraction and characterisation of antibacterial pigment from Roseomonas gilardi YP1 strain in Yercaud soil. Asian Journal of pharmaceutical and clinical research, 13(3): 116-120. [4] Pizzicato, B., Pacifico, S., Cayuela, D., Mijas, G., & Riba-Moliner, M. (2023). Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules, 28(16), 5954. [5] Kothari V , Joshi C and Patel P (2016) Colourful Side of Bacteriology: The Pigmented Bacteria Advancements in Genetic Engineering 5(1) 1-2. [6] Shahitha S, Poornima K. Enhanced production of prodigiosin in Serratia marcescens. Pharmaceutical Science . 2012, 13840. Journal of Applied 24. [7] Sharma D, Gupta C, Aggarwal S, Nagpal N. Pigment extraction from fungus for textile dyeing. Fibre and Textile Dyeing. 25.
Copyright
Copyright © 2024 Dr. Anupriya Chudiwal, Nandana Nair , Mahesh Salunke . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64351
Publish Date : 2024-09-26
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

