Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Extraction of Phytochemicals from Pomegranate Peels: A Sustainable Approach
Authors: Sunita Patil, Shreya Joshi, Prapti Sutar
DOI Link: https://doi.org/10.22214/ijraset.2024.63244
Certificate: View Certificate
Abstract
Pomegranate (Punica granatum), often referred to as the ‘Tree of Life,’ is known for its extensive pharmacological properties. The peels of this fruit, typically considered waste, are rich in phytochemicals that offer numerous health benefits, including antioxidant, anticancer, antibacterial, and anti-aging properties. Despite the known benefits, pomegranate peels are often discarded due to their bitterness and high fibre content. This represents a missed opportunity to harness their valuable phenolic compounds. This study aimed to evaluate the total phenolic content (TPC) of pomegranate peel extract and to investigate effect of parameters like solvent type, particle size, and temperature on TPC yield. Methanol was found to be the most effective solvent, with total phenolic content of 27.11 mg GAE /g plant material due to its polar nature. The optimum particle size for extraction was determined to be 400 mm and temperature was identified as 50 °C, yielding a higher phenolic concentration. This study demonstrates the significant potential of pomegranate peels as a source of valuable phenolic compounds. By optimizing the extraction parameters, we can maximize the yield of these compounds, contributing to sustainable practices and reducing waste.
Introduction
I. INTRODUCTION
The pomegranate, scientifically called as Punica granatum, is a fruit-bearing juicy shrub that belongs to the Lythraceae family. Originating in the Middle Eastern region of Afghanistan and Iran the shrubs grow 5 to 10 m tall. They are widely cultivated for their culinary and pharmacological uses. The fruit has three main parts, the outer shell, the inner membrane, and the arils. A white spongy membrane covers the sweet, juicy deep red seeds called the pericarp which in turn is covered by a dark purple-red skin called the rind or the peel. The fruit has a thick-skinned, crown-like structure at the top known as the calyx which is attached to the other components of the shrub.[1]
Pomegranates are popularly known for their pharmacological properties. The phytochemicals present in the fruit are known to exhibit a multitude of health benefits including antioxidant, antibacterial, anticancer, and anti-aging properties.[2]
This research focuses on the fruit peels, which are often discarded as waste due to their bitterness and excessive fibre content. They are a rich source of nutrients and phenolic compounds and contain significant amounts of vitamin C, potassium, and magnesium. There are almost 500 phytochemicals identified among which phenols, flavonoids, phenolics, steroids, ellagitannins, gallotannins, anthocyanins, tannins, carbohydrates, and saponins are the major ones. The presence of these compounds makes the peels an underutilized yet undervalued resource [3]. Phenolic compounds are the most vital part of a human diet. They are secondary metabolites found in all plant species. The redox properties of phenolic compounds are responsible for necessary antioxidant activity and the hydroxyl groups are responsible for free radical scavenging
Given the potential of these peels, this study aims to sustainably explore their use in extracting their phytochemicals. This adds a sustainable aspect to the research while also utilizing a commonly wasted part of the fruit. The experiment focuses on determining the phytochemicals present in pomegranate peels and the effect of parameters like solvent, particle size and temperature on extraction of phenolic content. Hence batch experiments were conducted using orbital shaker to study effect of parameters on total phenolic content (TPC) concentration and phenolic content was analysed by Folin-Ciocalteu Reagent (FCR) method by using UV double beam spectrophotometer. The FCR method is widely used in phytochemical studies due to its sensitivity and ability to provide reliable quantitative measurements of phenolic compounds [4]. This study seeks to harness the untapped potential of pomegranate peels by for the extraction TPC.
II. MATERIAL AND METHODS
A. Materials
- Collection of Raw material
Pomegranate fruits were collected from a local farm in Pune, Maharashtra, India. The peels of the fruit were separated, washed using distilled water, and sun-dried for almost two weeks. The dried peels were then powdered using a mechanical grinder. Using a sieve shaker, the powder was separated into three sizes as 400 mm, 600 mm, and 850 mm. All the dried and ground material of different size was packed in close container and used as required.

2. Chemical
Gallic acid, Folin–Ciocalteu reagent, methanol, ethanol, acetone and anhydrous sodium carbonate. All chemicals used were of analytical grade.
B. Methods
- Batch extraction
Batch experiments were performed by using an orbital shaker for solid to liquid ratio of 1:40 g/mL for solvents ethanol, methanol, acetone and distilled water; particle sizes (400 mm, 600 mm and 850 mm) and temperatures (40, 50 and 60 °C). Shaking avoids the settling of solid particles and improves mass transfer.
Effect of solvent on concentration of phenolic compounds was studied for 400 mm particle size, temperature 40 o C, solid to liquid ratio of 1: 40 g/mL and shaking time 60 min. After 60 min the conical flasks containing extract were removed and filtered using Whatman filter paper to obtain the clear extract. The effect of selected particle sizes on concentration of phenolic content was investigated for temperature 40 °C, solid to liquid ration of 1: 40 g/mL and shaking time 60 min and the effect of selected temperatures on concentration of phenolic compounds was investigated for particle size 400 mm, solid to liquid ration of 1: 40 g/mL and shaking time 60 min
2. Estimation of total phenolic content (TPC)
The Folin-Ciocalteau reagent method with slight modifications was used to determine the total phenolic compounds in each extract [5]. This method added 0.1 ml of extract and 0.5 ml of FCR to a 10 ml volumetric flask. Then 1.5 ml of 20 % (w/v) Na2CO3 was added and shaken well. The remaining flask was filled with distilled water up to the mark and shaken. The flasks were kept in a water bath for 30 minutes at 40 °C. The mixture was then cooled, and absorbance was measured using a UV double beam spectrophotometer (Model: Shimadzu UV-1800) at 765 nm. Total phenolic content was expressed in terms of gallic acid equivalent (mg/g of plant material). The unknown concentration of phenolic compounds was determined from the calibration plot of standard gallic acid solution with the regression equation y = 0.0017x+0.0635
3. Phytochemical analysis:
Methanolic extract of pomegranate peels was utilized for phytochemical analysis to identify various constituents using specific reagents and tests.
III. RESULTS AND DISCUSSIONS
A. Qualitative Analysis of Extract
The phytochemical analysis of pomegranate peel extract obtained using methanol as a solvent inferred the presence of flavonoids, phenolic compounds, tannins, saponins, steroids, and terpenoids as shown in Table 1. The following image 2 shows the observations during qualitative analysis. These heterogeneous groups are responsible for the antioxidant, anti-inflammatory, anti-bacterial, and anti-cancer properties of the pomegranate peels. [5]

Table 1: Qualitative Phytochemical analysis of pomegranate peel extract
|
Sr. No. |
Phytochemicals |
Test |
Method |
Observation |
Inference |
|
1 |
Flavonoids |
Alkaline reagent test |
Add four drops of 2% (w/v) NaOH in filtered, clear extract and shake well. |
Yellow colour appears |
Flavonoids are present |
|
2 |
Tannins |
Ferric chloride solution |
Add few drops of 5% lead acetate solution in clear extract and shake well |
White colour precipitate |
Tannins are present |
|
3 |
Saponins |
Foam test |
Add 3 ml of distilled water in extract. |
Foam appears |
Saponins are present |
|
4 |
Phenolic Compounds |
Ferric chloride solution |
Take clear filtered extract and add, a few drops of 5% (w/v) ferric chloride solution and shake well |
A dark violet colour appears |
Phenolic compounds are present |
|
5 |
Terpenoids |
Salkowski test |
Take filtered extract and add few drops of chloroform followed by concentrated H2SO4 |
A red ring on the surface was observed |
Terpenoids are present |
|
6 |
Steroids |
Liebermann-Burchard’s test |
Add 1ml acetic anhydride and a few drops of concentrated H2SO4 in the filtered and clear extract |
Various shades of dark colour were observed |
Steroids are present |
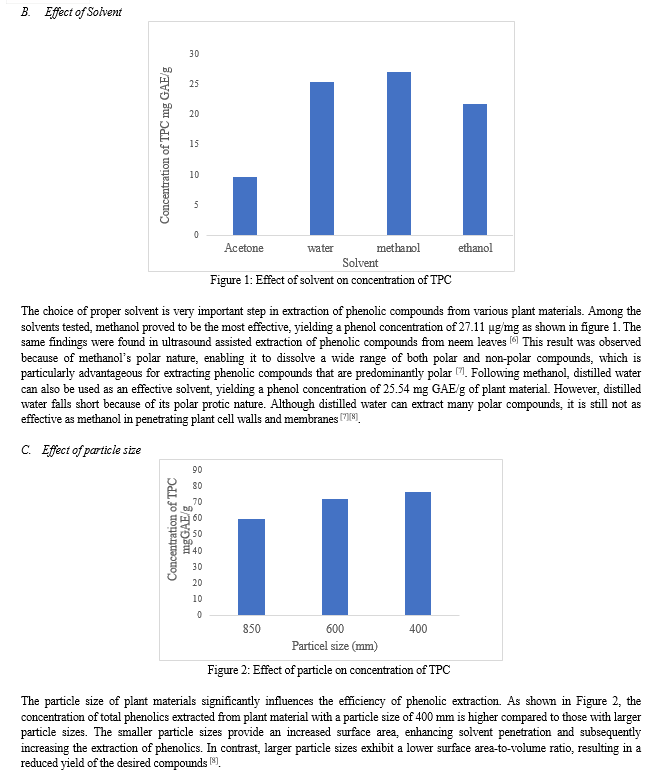
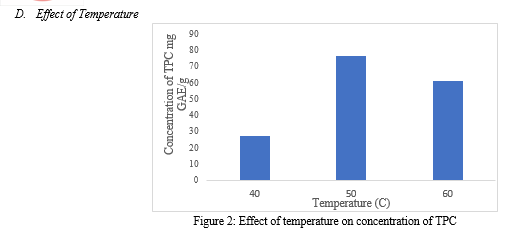
Temperature plays a crucial role in the process of extraction. It influences the solubility of the solvent and the efficiency of the process. As shown in figure 3, at 40 o C the extract concentration was observed to be 27.376 mg GAE /g of plant material suggesting, that lower temperatures generally result in slower diffusion rates of the solvent into the plant membrane, which can limit the efficiency of extraction. As the temperature was increased to 50 o C the concentration of phenolic compounds extracted increased up to 76.388 mg GAE /g of plant material. In this study, 50 o C appeared to provide an optimum balance between maximum yield and minimizing thermal degradation. Higher temperatures enhance the solubility of phytochemicals in the solvent and increase the diffusion rate, leading to a more effective and quicker extraction. However, at a high temperature of 60 o C, the yield decreased significantly suggesting that at high temperatures the solution is posed to risk of thermal degradation as well as solvent evaporation, reducing the efficiency of the process [7]
Conclusion
The key highlight of this research was to discover the potential of pomegranate peels, often discarded as waste, as a vital source of phytochemicals with significant health benefits. The yield of phenolic compounds is affected by the parameters such as temperature, choice of solvent and particle size. The results indicate that methanol is the most effective solvent in extracting phenolic compounds. They are polar in nature, economical, and feasible which makes them highly suitable for the process of extraction. Particle size is another crucial factor, the study suggests that smaller particle sizes provide a larger surface area, increasing the rate of solvent interaction, and resulting in a better and more efficient extraction process. As the particle size increases the surface decreases resulting in less extraction of phenolic compounds. Temperature of 50 o C was identified as the optimum temperature for the extraction of phenolics from pomegranate peels. The findings provide a foundation for further exploration and optimization of extraction methods, paving the way for the potential industrial application of pomegranate peel extracts in food, pharmaceutical, and cosmetic industry.
References
[1] Wang, Z., Pan, Z., Ma, H. and Atungulu, G.G., 2011. Extract of phenolics from pomegranate peels. The open food science journal, 5(1). [2] Li, Y., Guo, C., Yang, J., Wei, J., Xu, J. and Cheng, S., 2006. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food chemistry, 96(2), pp.254-260. [3] Jurenka, J., 2008. Therapeutic applications of pomegranate (Punica granatum L.): a review. Alternative medicine review, 13(2). [4] Sood, A. and Gupta, M., 2015. Extraction process optimization for bioactive compounds in pomegranate peel. Food Bioscience, 12, pp.100-106. [5] De Silva, G.O., Abeysundara, A.T. and Aponso, M.M.W., 2017. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. American Journal of Essential Oils and Natural Products, 5(2), pp.29-32. [6] Patil, S., Deshannavar, U, Ramasamy, M.and Hegde, P., 2021. Modeling and optimisation studies on the ultrasound-assisted extraction of phenolic compounds from Azadiracht indica. Chemical Engineering Communications, 209, pp 1423-1438 [7] Ben-Ali, S., Akermi, A., Mabrouk, M. and Ouederni, A., 2018. Optimization of extraction process and chemical characterization of pomegranate peel extract. Chemical Papers, 72(8), pp.2087-2100. [8] Hismath, I., Wan Aida, W.M. and Ho, C.W., 2011. Optimization of extraction conditions for phenolic compounds from neem (Azadirachta indica) leaves. International Food Research Journal, 18(3). [9] Jurenka, J., 2008. Therapeutic applications of pomegranate (Punica granatum L.): a review. Alternative medicine review, 13(2).
Copyright
Copyright © 2024 Sunita Patil, Shreya Joshi, Prapti Sutar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63244
Publish Date : 2024-06-11
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

