Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Flavonoids and Fruit Coloration: A Comprehensive Review of Biochemical Pathway and Environmental Interactions
Authors: Ankita Sunil Jadhav, Anil G. Jadhav, Nisha Kamalakar Dandage, Rupali Madhav Thakare, Prajakta Devrao Deshmukh, Vaishnavi Ashok Aware
DOI Link: https://doi.org/10.22214/ijraset.2024.65950
Certificate: View Certificate
Abstract
Flavonoids play a crucial role in the coloration of fruits, contributing to a broad spectrum of hues that range from yellow to red and blue. This comprehensive review examines the correlation between flavonoid biosynthesis and fruit color transitions, focusing on key pigments like anthocyanin, flavonol and chalcone that are responsible for producing a wide range of colors, including yellow, orange, red, purple and blue. The review highlights the biochemical pathways involved in flavonoid production and how these pathways correspond to changes in fruit pigmentation during different development stages. Additionally, it explores how environmental factors such as light intensity, temperature, and soil nutrients influence these pathways directly affecting flavonoid concentration and frequently fruit color.
Introduction
I. INTRODUCTION
Flavonoids are important secondary metabolites in plants which determine fruit quality due to their essential contribution to fruit color, antioxidant capacity and nutritive value. Flavonoids are associated with a broad spectrum of health-promoting effects and are an indispensable component in a variety of nutraceutical, pharmaceutical, medicinal and cosmetic applications. In nature, flavonoid compounds are products extracted from plants and they are found in several parts of the plant. Flavonoids are used by vegetables for their growth and defense against plaques. In plants, flavonoids have long been known to be synthesized in particular sites and are responsible for the color and aroma of flowers, and in fruits to attract pollinators and consequently fruit dispersion to help in seed and spore germination. The MYBs controlling the flavonoids biosynthesis in fruits. The expression of flavonoids biosynthetic genes is regulated by MYB-Bhlh-wd40 (MBW) complex, with crucial contribution of R2R3-MYB transcription factor (RF) (Broun, 2005).
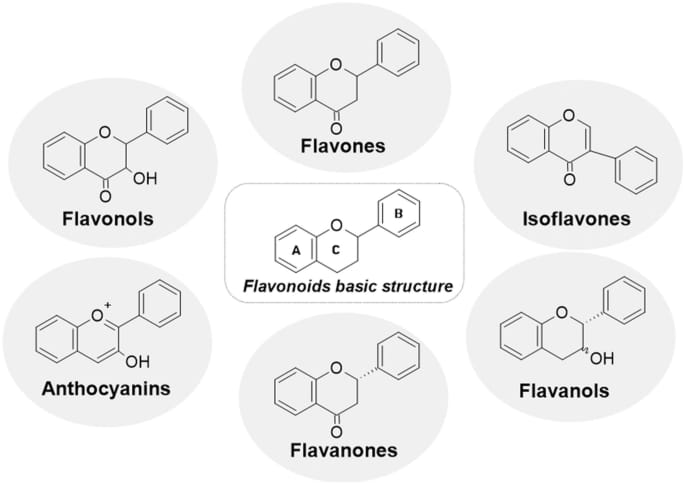
Figure. Structure of flavonoid classes.
Table 1. The various classes, subclasses, sources and pharmacological activity of flavonoids.
|
Sr. No. |
Class of flavonoids |
Subclass |
Sources |
Pharmacological activity |
|
1 |
Flavonols |
|
Green tea; Fruits; Vegetable; Spices; Medicinal plants. |
Antiviral; Anti-aging; Cardioprotectors; Anti-diabetic. |
|
2 |
Flavones |
|
Fruits; Milk; Olive oil; Medicinal plants. |
Antifungal; Antioxidant; Anti-carcinogenic; Detoxification. |
|
3 |
Flavonones |
|
Rose hips; Fruits; Vegetables. |
Chemoprotective effect; Anti-atherosclerotic. |
|
4 |
Anthocyanins |
|
Nuts and dried fruits; Sweet potatoes; Vegetables.
|
Cytoprotectors; Antibacterial; Anti-angiogenic.
|
|
5 |
Isoflavonoids |
|
Legumes; Medicinal plants; Tofu’ |
Anti-estrogenic effect; Regulation of menstrual cycle. |
|
6 |
Chalcones |
|
Fruits; Vegetable; Wheat products; Medicinal plants.
|
Antimicrobial; Antioxidants. |
A. Yellow mango pulp colors are associated with carotenoid and flavonoid accumulation
One of the most significant tropical fruits, the mango (Mangifera indica L.), is grown in more than 94 nations worldwide, yet commercial production of mangoes did not begin until the 1960s. China is the world’s second-largest producer of mangoes; as of 2022, 3494 thousand hectares of mango planting area generated 3.306 million tons, valued at 2.96 billion USD (https://www.tridge.com/;viewed on October 07 , 2022). Chinese markets continue to see a rise in the demand for imported mangoes, though. China bought 0.77 million tons of mangoes (worth 5.80 million US dollars) from neighboring countries such as Vietnam, Thailand, Cambodia, Pakistan, and Myanmar in May 2022. Rich in colors, volatile components, and secondary metabolites, mango pulp is also high in micronutrients and macronutrients. It also includes amino acids, lipids, and structural carbohydrates like cellulose and pectin.
Fruits undergo biochemical, physiological, and structural alterations that alter their nutrient and phytochemical composition, hence affecting their texture (softness), flavor, aroma, color, and antioxidant activity as they develop and ripen. These traits differ between genotype. A few other variables that affect these qualities are the fruit’s age, the production region, and the climate.
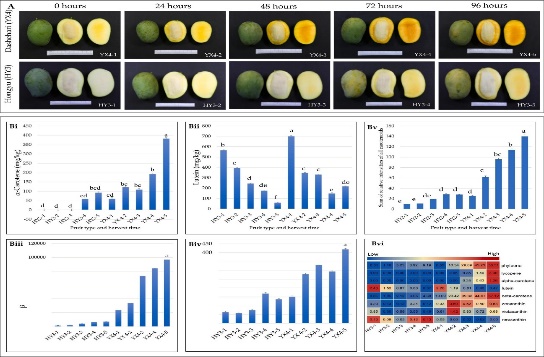
Mangoes (Mangifera indica L.) are a popular tropical fruit widely used due to their rich color and nutritional value. But there is little information available regarding the molecular causes of color variance. Mango pulp is however, a good source of phytochemicals, micro- and macronutrients, and vitamins. Mango pulp contains a variety of phytochemicals, including carotenoids, flavonoids, phenolic acids, and alkaloids, all of which are potent antioxidants. Mango peel and pulp color is caused by a few of these phytochemicals, including flavonoids and carotenoids (together with xanthophylls) in addition to their antioxidant properties. In particular, the primary pigments in the pulp that give peel and pulp their yellow color are called carotenoids, whereas anthocyanins are responsible for other mango species’ crimson color. The yellow pulp (YX4) and the yellowish-White pulp (XY3) that were harvested 24 hours apart from the usual harvesting time were the subjects of our study. As harvest time approached, the amount of carotenoids and total flavonoids increased (YX4 > HY34). Higher expression of the genes responsible for the flavonoids and core carotenoids production are associated with their respective contents, as demonstrated by transcriptome sequencing. With a longer harvesting period, the endogenous levels of ethylene and abscisic acid and jasmonate acid declined while those of abscisic acid and ethylene increased (YX4 > XY34). Comparable patterns were noted for the matching genes. Our findings suggest that the variations in hue are associated with the levels of carotenoids and flavonoids, which are impacted by the accumulation and signaling of phytohormones.
II. ANTHOCYANIDINS
Anthocyanidins are phytochemical pigments and a subclass of flavonoids, which are responsible for many different colors in plants. Anthocyanidins found in various fruits and vegetables. Anthocyanidins found predominantly in tea, honey, fruits, vegetables, nuts, olive oil. Common anthocyanidins found in fruits and vegetables are cyanidin, pelargonidin, delphinidin, malvidin, petunidin and peonidin, which are having a different number and position of the hydroxyl and methoxyl group substituents.
Anthocyanins are glycone form of anthocyanidins, when the addition of carbohydrate residue occurs. Anthocyanins are responsible for the colors, red, purplr, and blue, are in fruits and vegetables. Among the anthocyanin pigments, cyanidin-3-glucoside is the major anthocyanin found in most of the plants. Anthocyanins are the main compounds responsible for the red coloring of mango and apple fruit peel. Anthocyanins and flavonoids are natural compounds synthesized via the phenylpropanoid pathway Phenylamine (Phe), the precursor of the phenylpropanoid pathway, is an aromatic amino acid existing naturally in plants and derived from the shikimate pathway.
A. UFGT genes’ roles in fruits species’ color development
In several fruits-bearing plant species, the function of the UFGT genes in color development has been discovered and studied. In order to examine the cooperative function of the UPGT genes with other genes in stone and non-stone fruit-bearing plants, we separated fruit species into two groups. The following discusses the combined role of UFGTs in the production of color in certain fruit species.
B. Multi-Omics Technologies’ Impact On Fruit And Vegetable Postharvest Physiology
Understanding the physiological processes of fruits and vegetables after harvest is largely dependent on multi-omics analysis (fig. ). Utilizing these technologies offers insightful information about the intricate molecular interactions that occur during postharvest handling processing, and storage (Chen et., 2023). In postharvest physiology, multi-omics can aid in clarifying the metabolite pathways, protein synthesis, and gene expression that control nutritional composition, quality, and flavor of fruits.
C. Multi-Omics Analysis In Fruit And Vegetable Postharvest Quality
After harvesting fruit and vegetable quality must be preserved to maintain market competitiveness. The process underlying these aspects, which include visual appearance, physiochemical parameters, nutritional value, texture flavor, sugar content, and acidity, can be discovered through molecular research on quality attributes (Belay and Caleb, 2022). The main genes, proteins, and metabolites involved in the continuous molecular processes that can be found via multi-omics studies.
D. Multi-Omics Analysis In Fruit And Vegetable Senescence
The term “postharvest senescence” describes the again process that naturally takes place in fruit and vegetables following harvest (Meitha at al., 2020). The intricate sequence of physiological and biochemical alterations known as the senescence process causes the harvested product to deteriorate over time by progressively altering its components, structure, and metabolism. Because of the differences in their physiologies, fruits and vegetables might differ in terms of when and how quicky they age.
E. Fruit Cuticle Metabolism
According to Konarska (2014), Trivedi et al., 2019b, chai et al., 2020, Huang et al., 2022, Moreno et al., 2022, Reynoud et al., 2022, and Ramakrishna (2022), fruit cuticle metabolism is a dynamic process that tracks the development, maturity, postharvest storage, and senescence stage of fruits. It is regulated by both genetic and environmental factors and is essentially the outcome of the spatial and temporal specific expression of genes linked to fruit cuticle metabolism, as well as the expression level of these genes.
F. Fruit Cuticle’s Postharvest Purposes
Fruits’ own genetic makeup and the surrounding environment have an impact on their postharvest quality. The fruit cuticle has also gained attention in recent years as a key regulator of postharvest fruit quality, which is influenced by factors such water loss, softening, resistance to pathogens, and fruit integrity against inspect pests (Fernandez-Munoz, Heredia and Dominguez, 2022).
G. Role of Ethylene and ABA during the Ripening of Fleshy Fruits
Ripe fruit undergoes the intricate physiological, biochemical, and sensory alterations during the ripening process. In plants, ethylene encourages the breakdown of chloroplasts, the deterioration of cell walls, and the production of carotenoid pigments. Ethylene is therefore thought to have a major role in fruit ripening (Li et al., 2019b; Tucker et al., 2017). Ethylene production mainly occurs at the early stage of the fruit development, whereas ABA accumulation mainly occurred at the ripening stages, highly associated with the fruit ripening process. According to Pech et al. (2008), fleshy fruit has two different ethylene production mechanism.
H. ABA Controls the Buildup of Pigment when Fruit Ripens
Fruit color varies as it ripens due to the accumulation of pigment. According to Luo et al. (2019), fruit pigments are classified into two groups: water-soluble pigments, which include anthocyanin and yellow pigment and fat-soluble pigments, which include chlorophyll and carotenoids.
The primary component of immature fruit is high levels of chlorophyll. The amount of chlorophyll steadily drops as the plant ages. Fruit color is determined by the ratio of anthocyanins to carotenoids.
I. Role of ABA in fruit softening
Cell wall alteration leads to the fruit softness, and throughout ripening, fruit cell walls underwent significant structural and compositional changes (Saladie et al., 2007; Tucker et al., 2017). According to Ashline et al., 2014, pectin, cellulose, hemicellulose, and a trace quantity od glycoprotein make up the cell wall.
According to the “tethered network” theory, cellulose microfibers are covered in xyloglucan or another hemicellulose. This is one of the two basic concepts of the cell wall structural model.
J. Polyamines, sugars and transcription factor ABA-stress-ripening (ASR) interact with ABA to influence fruit ripening
Specifically significant for the early stages of fruit development, polyamines are a type of molecules that includes two or more amino acids and closely involved in controlling the ripening of fruit (Chervin et al., 2004). Strawberries include a crucial regulator of polyamine synthesis, which is intimately linked to ethylene and ABA: S-adenosylmethionine decarboxylase, or SAMDC. Strawberries ripened more quickly when treated with RNA interference (RNAi) and when their expression of FaSAMDC was overexpressed.
K. Novel developments in the biology of the flavonoid pathways
Flavonoid production is mediated by the phenylpropanoid metabolic pathway, as was discussed in the sections that precede this one. Phenylalanine ammonia lyase (PAL), cinnamonate 4-hydroxylase (C4H), and 4-coumaroyl CoA ligase (4CL) are the enzymes used in the first step of the synthesis of p-coumaryl-CoA from the aromatic amino acid phenylalanine (Phe). The deamination of Phe to coumaric acid (by PAL), the subsequent oxidation to 4-coumaric acid (by C4H), and lastly the activation is all coordinated by these enzymes.
L. Functions of plant-derived hydroxylated flavonoids
Flavonoids are significant secondary metabolites that are produced throughout the plant in response to cues derived from interactions between the plant and its surroundings. They are crucial components for drawing insects, birds, or animals and for facilitating the spread of seeds because they are engaged in a wide range of physiological processes within the plant, including the development of color, aroma, and taste in fruits, vegetables, and flowers. Flavonoids also have a significant impact on how well plants defend themselves against herbivores and insects that feed on plants (Harborne and Williams,2000). According to Mierziak et al. (2014), they can function as poisons in certain situations or as pathogen development inhibitors in others (Harborne and Williams, 2000). In addition, they control symbiotic relationships with microbes (Abdel-Lateif et al, 2012). Legumes, for instance, synthesize luteolin and chrysin, which drive the process of root nodule development in the nitrogen-fixing bacteria (Cooper, 2004; Reddy et al., 2007; Jones et al.,2007) or initiate symbiosis by activating a signaling pathway for Rhizobacterium (Hartwig et al., 1990). In addition to being good modulators of plant-environment interactions, flavonoids can also have a detrimental effect by preventing the germination and growth of rival plants (Kong et al., 2004, 2007).
III. GLYCOSYLATION
One of the primary modification events that takes place in a variety of biological processes and given rise to a broad spectrum of natural products is glycosylation (Gachon et al., 2005; Tiwari et al., 2016b). The glycosyltransferase (GTs) enzymes play a major role in this modification by moving sugar moieties from the activator donor molecules to a variety of acceptor molecules, including sugars, lipids, proteins and small molecules like naturally occurring flavonoids (Dixon and Strack, 2003; Rai et al., 2017). As the donor molecule, they typically employ uridine 5-diphosphate sugars; these GTs are known as UDP-glycosyltransferases (UGTs) (Yonekura-Sakakibara and Hanada 2011).
The UGTs are primarily identified by a C-terminal signature motif that consists of 44 amino acid residues, known as the Plants Secondary Product Glycosyltransferase (PSPG) box (Mackenzie et al., 1997; Ross et al., 2001). These enzymes are typically found in the cytosol where they play a role in the biosynthesis of a variety of plant natural products, such as steroids, phenylpropanoids, and flavonoids (Bowles et al., 2006; Itkin et al., 2013).
GTs glycosylate flavonoids, and most naturally occurring sugars that are linked to flavonoids are glucose moieties (Hofer, 2016; Tiwari et al., 2016a). However, reports of galactose, xylose, rhamnose (Lim et al., 2004,2005). The most prevalent types are O- and C-glycosides. They often accumulate as mono-, di-, or triglycosides. The related aglycones’ reactivity and solubility, as well as their cellular location and bioactivity, are altered by these kinds of glycosylation (Augustin et al., 2011; Liu et al., 2013). According to Butelli et al.,(2019),the vacuole is the destination and storage location for numerous secondary metabolites, such as flavonoids and anthocyanins. Access to active membrane transport mechanisms that identify glucosylated molecules but not their aglycones is made possible by their glycosylation, which also improves their solubility and chemical stability (Hostel, 1981). Additionally, the glycosylation of naturally occurring compounds, like flavonoids, demonstrates remarkable complexity and structural diversity. Of particular interest are the glycosides of flavonoids, as they play a pivotal role in regulating numerous pharmacokinetic parameters, including but not limited to estrogenic, antibacterial, antiviral, anti-inflammatory, endocrinological, and anti-cancer properties (Hollman et al., 1995; Hollman and Katan, 1999).
Conclusion
Flavonoid are involved in a number of metabolic pathways that contributes significantly to the color of fruit. A vast range of hues, from the vivid reds and purples to the gentle yellows and blues seen in various fruits, are attributed to these substances, which also include anthocyanins, flavonols, and flavones. Light exposure, temperature, and the availability of nutrients are the main external influence on the complex network of genetic and environmental factors that tightly control the production of flavonoids. Variations in fruit color between climates and farming methods are a result of environmental interactions that profoundly alter flavonoid production. For instance, low temperatures can preserve anthocyanin pigments, boosting their coloration, whereas strong light intensity frequently boosts their accumulation, resulting in richer red or purple hues. Understanding the regulation of flavonoid biosynthesis offers potential uses in agriculture and the food sectors, in addition to offering insights into the natural mechanism that govern fruit colors. It is possible to improve the nutritional value and aesthetic appeal of fruits by selective breeding or environmental manipulation. The complex interplay between the flavonoids and fruit color highlights how biochemistry and environmental influences play a major role in determining the nutritional value and aesthetic appeal of fruits. Subsequent investigations focused on delving deeper into these interplays will further broaden our comprehension and facilitate more accurate regulation of fruit coloring in farming methods.
References
[1] Bowles et al., 2006 D. Bowles, E.K. Lim, B.Poppenberger, F.E. Vaistij
[2] Glycosyltransferases of lipophilic small molecules Annu. Rev. Plant Biol., 57 (2006), pp. 567-597
[3] Brazier-Hicks et al., 2009 M. Brazier-Hicks, K.?. Evans, M.C. Gershater, H. Puschmann, P.G. Steel, R. Edwards The C-glycosylation of flavonoids in cereals J. Biol. Chem., 284 (2009), pp. 17926-17934
[4] Burda and Oleszek, 2001 S. Burda, W. Oleszek Antioxidant and antiradical activities of flavonoids J. Agric. Food Chem., 49 (6) (2001), pp.2774-2779
[5] Bors et al., 2001 W. Bors, Y.L. Foo, N. Hertkorn,Ch Michel, K. Stettmaier Chemical studies of proanthocyanidins and hydrolysable tannins Antioxidants Redox Signal., 3 (6) (2001), pp. 995-1008
[6] Bors et al., 1990 W. Bors, W. Heller, C. Michel, M. Saran Flavonoids as antioxidants:Determination of radical-Scavenging efficiencies Methods Enzymol., 186 (1990), pp. 343-355
[7] Bernini et al., 2011 R. Bernini, F. Crisante, M.?.Ginnasi A convenient and safe O- methylation of flavonoids with dimethyl carbonate (DMC) Molecules, 16 (2011), pp. 1418-1425
[8] Bogs et al., 2006 J. Bogs, A. Ebadi, D. McDavid, S.P. Robinson Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development, Plant Physiol., 140 (1) (2006), pp. 279-291
[9] Bontpart et al., 2015 T. Bontpart, V. Cheynier, A.Ageorges, N. Terrier BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds, New Phytol., 208 (2015), pp. 695-707
[10] Badri et al., 2008 D.V. Badri, V.M. Loyola-Vargas,C.D. Broeckling, C. De-la-Pena, M.Jasinski, D. Santelia, E. Martinoia, L.W.Sumner, L.M. Banta, F. Stermitz Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants Plant Physiol., 146 (2008), pp. 762-771
[11] Benavente-Garcia and Castillo, 2008 O.Benavente-Garcia, J. Castillo Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity J. Agric. Food Chem., 56 (15) (2008), pp. 6185-6205
[12] Berim and Gang, 2013 A. Berim, D.R. Gang The roles of a flavone-6- hydroxylase and 7-0- demethylation in the flavone biosynthetic network of sweet basil
[13] Ardhaoui et al., 2004 M. Ardhaoui, A.Falcimaigne, S. Ognier, J.M. Engasser, P. Moussou, G. Pauly, M. Ghoul Effect of acyl donor chain length and substitutions pattern on the enzymatic acylation of flavonoids J. Biotechnol., 110 (2004), pp. 265-271
[14] Arora et al., 1998 A. Arora, M.G. Nair, G.M. Strasburg Structure-activity relationships for antioxidant activities of a series of flavonosids in a liposomal system Free Radic. Biol. Med., 24 (1998), pp. 1355- 1363
[15] Arroo et al., 2009 R.R.J. Arroo, V. Androutsopoulos, K. Beresford, K. Ruparelia, S. Surichan, N. Wilsher, G.A. Potter Phytoestrogens as natural prodrugs in cancer prevention: dietary flavonoids Phytochemistry Rev., 8 (2009), pp. 375-386
[16] Alseekh et al., 2015 S. Alseekh, T. Tohge, R. Wendenberg, F. Scossa, N. Omranian, J. Li, S. Kleessen, P. Giavalisco, T. Pleban, B. Mueller-Roeber, D. Zamir, Z. Nikoloski, A.R. Fernie Identification and mode of inheritance of quantitative trait loci for secondary metabolite abundance in tomato Plant Cell, 27 (2015), pp. 485-512
[17] Alseekh et al., 2017 S. Alseekh, H. Tong, F. Scossa, Y. Brotman, F. Vigroux, T. Tohge, I. Ofner, D. Zamir, Z. Nikoloski, A.R. Fernie Canalization of tomato fruit metabolism, Plant Cell, 29 (2017), pp. 2753-2765
[18] Amrutha et al., 2014 K. Amrutha, P. Nanjan, S.K. Shaji, D. Sunilkumar, L. Rajakrishna, A. Banerji Discovery of lesser known flavones as inhibitors of NF-KB signaling in MDA-MB-231 breast
[19] Abdel-Lateif et al., 2012 K. Abdel-Lateif, D.Bogusz, V. Hocher The role of flavonoids during legume root in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizoboa and Frankia bacteria Plant Signal. Behav., 41 (2012), pp. 2-62
[20] Agati et al., 2012 G. Agati, E. Azzarello, S.Pollastri, M. Tattini Flavonoids as antioxidants in plants: location and functional significance Plant Sci., 196 (2012), pp. 67-76
[21] Alseekh and Fernie, 2018 S. Alseekh, A.R. Fernie Metabolomics 20 years on: what have we learned and what hurdles remain? Plant J.: Cell Mol. Biol., 94 (2018),
[22] E.J. Brown et al. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties Biochem. J. (1998)
[23] F. Brugliera et al. Violet/blue chrysanthemums- metabolic engineering of the anthocyanin biosynthetic pathway results in novel petal colors Plant Cell Physiol. (2013)
[24] E. Butelli et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors Nat. Biotechnol. (2008)
[25] J. Callis The ubiquitination machinery of the ubiquitin system Arabidopsis Book/Am. Soc. Plant Biol. (2014)
[26] P. Bharti et al. AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco J. Exp. Bot. (2015)
[27] Bovy et al. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1 Plant Cell (2002)
[28] Bovy et al. Metabolic engineering of flavonoids in tomato (Solanum lycopersicum): the potential for metabolomics Metabolomics (2007)
[29] K.M. Brodowska Natural flavonoids: classification, potential role, and application of flavonoid analogues Eur. J. Biol. Res (2017)
[30] G. Agati et al. Multiple functional roles of flavonoids in photoprotection New Phytol. (2010)
[31] J.P. An et al. Apple RING E3 ligase MdMIEL1 inhibits anthocyanin accumulation by ubiquitinating and degrading MdMYB1 protein Plant Cell Physiol (2017)
[32] O.M. Andersen et al. Flavonoids: Chemistry, Biochemistry and Applications (2005)
[33] Bajpai et al.Molecular analysis of anthocyanin biosynthesis pathway genes and their differential expression in mango peel Genome (2018)
[34] Citric acid treatment inhibits fading of sorghum (Sorghum bicolor) by modulating the accumulation of flavonoids 2024, Food Chemistry
[35] Identification and analysis of flavonoid pathway genes in responsive to drought and salinity stress in Medicago truncatula 2024, Journal of Plant Physiology
[36] Therapeutic potentials and targeting strategies of quercetin on cancer cells: Challenges and future prospects 2024, Phytomedicine
[37] T. Ishikawa et al.Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification Am. J. Clin. Nutr. (1997)
[38] Y.B. Lee et al.Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats J. Nutr. (2004)
[39] G.Z. Lin et al.Expression and purification of His- tagged flavonol synthase of Camellia sinensis from Escherichia coli Protein Expr. Purif. (2007)
[40] F. Liu et al.Functional characterization of DnSIZ1, a SIZ/PIAS-type SUMO E3 ligase from Dendrobium
[41] J.R. Andersen et al.Characterization of phenylpropanoid pathway genes within European maize (Zea mays L.) inbreds BMC Plant Biol. (2008)
[42] M. Bemer et al.Cross-family transcription factor interactions: an additional layer of gene regulation Trends Plant Sci. (2017)
[43] T.T. Cushnie et al.Antimicrobial activity of flavonoids Int. J. Antimicrob. Agents (2005)
[44] R.A. Dixon et al.Metabolic engineering of anthocyanins and condensed tannins in plants Curr. Opin. Biotechnol. (2013).
[45] M. Hertog et al.Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly StudybSoil pH and organic matter: Key edaphic factors in sustaining optimum yield and quality of pomelo fruit 2024, Scientia Horticulturae
[46] CaC
Copyright
Copyright © 2024 Ankita Sunil Jadhav, Anil G. Jadhav, Nisha Kamalakar Dandage, Rupali Madhav Thakare, Prajakta Devrao Deshmukh, Vaishnavi Ashok Aware. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65950
Publish Date : 2024-12-16
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

