Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Fluoroquinolones: Action, Synthesis and Applications in Antimicrobial Therapy
Authors: Hansika Bansal, Anita Singh, Priya Gupta, Manisha Joshi
DOI Link: https://doi.org/10.22214/ijraset.2024.60354
Certificate: View Certificate
Abstract
In the late nineteenth century, diseases were assumed to be linked to the presence of microbes. The mortality rate dropped after the antiseptic approach was used. Good cleanliness and sanitation helped to drastically cut the death rate from infectious diseases. Antimicrobials were produced, and numerous pathogenic diseases were eradicated. Essential antibacterial drugs that are frequently utilized in clinical therapies include fluoroquinolones. Their synthetic pathway, action and therapeutic uses are described in this review. Their primary mechanism of action is the inhibition of bacterial DNA gyrase and topoisomerase IV, which are essential enzymes involved in transcription and DNA replication. Fluorination is one of the important structural change that improves their efficacy and spectrum of activity against a variety of infections. To enhance pharmacokinetics, substituent modification, fluorination, and cyclization of heterocycles are used in synthesis. Advances in synthetic chemistry have produced compounds that exhibit increased activity and decreased resistance. Clinical applications include treating respiratory, urinary tract, cutaneous, and gastrointestinal diseases. These drugs also show activity like antitumor, wound healing, antimycobacterial, etc. This review aims to provide researchers and academicians a brief account about the bacterial action, synthesis and clinical applications in antimicrobial therapy.
Introduction
I. INTRODUCTION
Since ancient times, antibiotics have been used to treat illnesses. Through Paul Ehrlich's invention of salvarsan to treat syphilis, the era of antibiotics was ushered in. Alexander Fleming made the serendipitous discovery of the antibiotic penicillin in 1928.[1] But the necessity for developing new antimicrobials has grown in light of the rise in antimicrobial resistance over the past several years and the numerous deaths brought on by the emergence of antimicrobial-resistant strains.[2] The microbes infiltrate our tissues by destroying cellular processes. They exude toxins that harm the cells of the host. Antibiotics are used to treat both acute and chronic illnesses. They are primarily classified as either cidal or static, meaning that they either kill or stop the development of bacteria.[3]
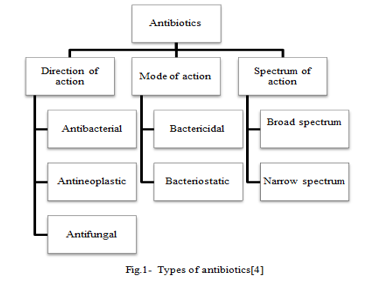
Antimicrobials frequently target the rupture of the cell wall, blockage of protein synthesis at the 50s and 30s ribosomal subunit, cause the inhibition of RNA polymerase-level nucleic acid synthesis, targeting topoisomerases to damage DNA, deactivation of the folate synthesis pathway, etc.[5] Beta lactams, macrolides, oxazolidinones, streptogramins, lincosamifdes, tetracyclins, aminoglycosides, ansamycins, sulfonamides, quinolones, fluroquinolones, azoles, and others are examples of antimicrobial agents.[6]
Table.1- Classification of antibiotics[7], [8]
|
Antibiotics |
MOA |
Target bacteria |
Examples |
Uses |
|
Penicillin |
Cell wall synthesis inhibitor |
Gram positive |
Amoxicillin , ampicillin |
ENT, Skin infections |
|
Cephalosporin |
Cell wall synthesis inhibitor |
Gram positive |
Cefixime, cephalexin |
Skin , urinary, respiratory infections |
|
Fluoroquinolones |
Type II topoisomerases inhibitor |
Broad spectrum |
Ofloxacin , moxifloxacin |
Respiratory and UTI |
|
Quinolones |
Inhibits DNA synthesis |
Gram negative |
Nalidixic acid |
UTI |
|
Tetracyclins |
Protein synthesis inhibitor |
Broad spectrum |
Doxycycline |
PID, STD, Lyme disease |
|
Macrolides |
Inhibit protein synthesis ( 50s) |
Gram positive |
Azithromycin, clarithromycin |
Sinus, ENT, Pneumonia |
|
Aminoglycosides |
Inhibit protein synthesis (30s) |
Gram negative |
Gentamicin |
Abdominal infections |
|
Glycopeptides |
Cell wall synthesis inhibitor |
Gram positive |
Vancomycin |
MRSA, endocarditis |
|
Lincosamides |
Inhibit 50s protein synthesis |
Gram positive |
Clindamycin |
Skin, bone, lung infections |
|
Sulfonamides |
Inhibit folate synthesis |
Broad spectrum |
Sulfasalazine |
UTI, burns, eye infections |
Thirty years have passed since the discovery of the first generation of quinolones, or nalidixic acid derivatives. Subsequently, fluorine was included into the quinolone structure, leading to the introduction of fluoroquinolones, including norfloxacin, pefloxacin, ofloxacin, and ciprofloxacin, onto the allopathic market. This development was extremely beneficial for the pharmaceutical industry.[9] Quinolones are naturally broad-spectrum antibiotics with a bicyclic scaffold that includes a nitrogen atom and other substituents that are essential to increasing their bioavailability and antimicrobial effectiveness.[10] Nalidixic acid is the first member of the quinolones family; nonetheless, it is a restricted spectrum antibiotic that was exclusively used to treat urinary tract infections.[8]
The second generation of this family included a fluorine atom at the sixth carbon atom to boost activity. One such example is ofloxacin, a broad range antibiotic that works against both gram positive and gram negative bacteria. Yet, a lot of bacterial strains quickly developed an ofloxacin resistance.[11] As a result, several fluoroquinolone analogues were created and their antibacterial efficacy evaluated in an effort to counteract the bacterial resistance.[9] The introduction of the fluorine atom during the creation of fluroquinolones was motivated by its QSAR properties, namely electronic, steric, and lipophilic, as fluorine possesses strong electronegativity, small size, and less steric hindrance, as well as very low polarizablity and solubility. These fluorine qualities influence the drug's biological activity as well as its pharmacokinetics and pharmacodynamics.[12]

Following this significant discovery, SAR was investigated for the novel class of drugs known as fluoroquinolones. A variety of substituents were added to the original compounds to create new analogues with improved activity, such as antimicrobial activity, solubility, reduced side effects, improved serum half-life, favorable dosage and dosage form, etc.[13] Because of their efficacy in treating a variety of infectious diseases and in halting the development of drug resistance, fluoroquinolones have remained a crucial class of antibiotics in clinical practice to this day.[14] In 1963, quinolones were introduced to the allopathic division. Their classification is provided below:
Table 2: Quinolone drugs and their generations[8], [15]
|
Generation |
Name |
Antimicrobial spectrum |
Clinical indications |
|
I |
Nalidixic acid Cinoxacin |
Gram negative (except Pseudomonas species) |
Uncomplicated UTI |
|
IIa |
Norfloxacin Enoxacin Ofloxacin Ciprofloxacin |
Gram negative pathogens |
All types of UTI, STDs, prostatitis, skin and soft tissue infections |
|
IIb |
Lomefloxacin |
All gram negative and some gram positive pathogens |
Same as previous generation |
|
III |
Levofloxacin Sparfloxacin Gatifloxacin Grepafloxacin Clinafloxacin |
Retains the activity of previous generation and covers more gram positive pathogens |
Community acquired pneumonia, chronic bronchitis |
|
IV |
Moxifloxacin Gemifloxacin Trovafloxacin |
Covers all III generation activities plus anaerobic activity |
Same as previous generations plus intra-abdominal infections, pelvic infections, nosocomical pneumonia. |
II. STRUCTURE-ACTIVITY-RELATIONSHIP
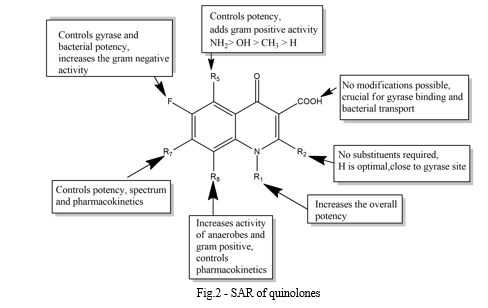
III. BACTERIAL CLASSIFICATION
In this review, we will talk about the bacterial classification based on their gram staining. Hans Christian Gram first invented this microbial staining technique in 1884.
The principle behind this methodology is the electrostatic interaction which takes place between the microbe and the dye which causes the microorganism to stain. This method is quite simple and has additional advantages like highly efficient staining. There are four steps in the conventional gram staining i.e. primary stain, mordant, decolourization and counter stain.
According to this method, the bacteria are classified into two groups which are named as gram positive and gram negative according to their colors. Gram positive microbes are those which are stained purple in the primary stain due to the strong electrostatic bond formation with the dye. Their ph values ranges from 1.75 to 4.15. Gram-negative bacteria are those which are stained pinkish in color due to the weak electrostatic interaction with the dye and also dye-losing after decolourization. Their ph values ranges between 2.07 to 3.65.[7]
The ph value also affects the gram stain because the intercellular environment of the microbes directly effects the electrostatic interaction.
The cellular structure of the gram positive and gram negative microbes are different in several aspects. If we look deeply, we will find out that the cell wall of gram positive microbe is thick and homologous where as that of gram negative is thin and distinctly layered, it also possess an outer membrane which is similar to the typical trilaminar cytoplasmic membrane.[4]
In the above two groups i.e. gram positive and gram negative, the polymers present in the cell walls are also different. The cell walls of gram negative bacteria have lipopolysaccharide, protein, phospholipids, lipoprotein and less than 10% peptidoglycan. On the other hand, the cell wall of gram positive bacteria contains more than 30% peptidoglycan, polysaccharide, echoic acid, teichuronic acid as major components, thus they hardly contain any lipids in their cells. The only exception to this are acid fast bacteria which are gram positive in nature but still has large amount of lipids in their cell walls especially mycolic acids.[16]
The only cell wall polymer which is common in both gram positive and gram negative organisms is peptidoglycan. Gram negative bacteria has a markedly uniform peptidoglycan where as gram positive has multilayered peptidoglycan.
Table.3- Gram staining techniques[7]
|
Characteristics |
Gram positive |
Gram negative |
|
Gram reaction |
Retain crystal violet dye and stain purplish in color |
Stain red and can be decolorized to accept counter stain. |
|
Teichoic acid |
Present |
Absent |
|
Peptidoglycan |
Thick and multilayered |
Thin and single layered |
|
Flagellar structure |
2 rings in basal bodies |
4 rings |
|
Periplasmic space |
Absent |
Present |
|
LPS content |
None |
High |
|
Outer membrane |
Absent |
Present |
|
Lipid and lipoprotein content |
Low |
High |
|
Toxins produced |
Exotoxins |
Endotoxins |
IV. BACTERIAL ACTION
To define the bacterial action, the two commonly used terminologies are bacteriostatic and bactericidal. They are the in-vitro terms used to describe the antibiotic concentrations and their effect on bacterial effect. Bactericidal drugs are usually used to kill the bacteria more easily than the bacteriostatic agents. Thus, bactericides are those agents which can actually kill the bacteria whether they are antibiotics, antiseptics or disinfectants. On the other hand, the static agents require a much higher concentration to kill the bacteria. The bactericides can also show the bacteriostatic activity at lower concentrations.[3]
So, antibiotics that prevent the bacterial growth and proliferation without killing it in-vitro are bacteriostatic agents. They also have the capability to kill the bacteria but at a slower rate. There is a myth that the static agents require more activity from the immune system to eradicate the bacteria.
Factors influencing the bacteriostatic and bactericidal effects of a drug are –
- The amount of bacterial inoculums and the mass of the pathogens can affect the properties of antibiotics.
- To certain microorganisms, some antibiotics are cidal where are some are static, for e.g. Azithromycin is cidal against streptococcus but it is static against staphylococcus, vancomycin is static against enterococcus but it is cidal against staphylococci and streptococci.
- The location or the medium of infection is important because certain antibiotics are only effective in some parts of the body and not in other for e.g. tigecycline cannot achieve high concentrations in the bloodstream.[17]
The fluroquinolones represent a vast class of bactericides which are potent against Enterobacteriaceae, Neisseria spp., Haemophillus spp., and possess great activity against Pseudomonas aeruginosa and staphylococci and are less active against streptococci and fair to poor against anaerobes.

VI. SPECTRUM OF ACTIVITY IN-VITRO
In general, fluoroquinolones are found to have excellent in-vitro activity against Enterobacteriaceae family, H. influenza, N. gonorrhoeae, N. meningitides, Branhamella catarrhalis, P. aeruginosa, Aeromonas hydrophilia, Legionella pneumophilia and staphylococcal species. They are less potent towards streptococci and works only moderate against anaerobic species. Their activity is found to be most potent against gram-negative bacilli and cocci but is less active for Pseudomonas species except P. aeruginosa. They work well for the bacterial infections of the GI tract caused by E. coli, Salmonella species, Shigella species, Yersinia enterocolitica, Vibrio species and Campylobacterjejuni.[10]
For the gram positive organisms, the minimum inhibitory concentrations are generally higher. Fluoroquinolones are also used for urinary tract infections caused by pathogens like S. saprophyticus. Fluoroquinolones vary in their in-vitro potency for example, on the molar basis, ciprofloxacin is more potent than ofloxacin in-vitro but it is counterbalanced by high serum levels of ofloxacin. Cross resistance is common in the case of fluoroquinolones.[12]
They are also active against MRSA, multiply-antibiotic-resistant gram negative bacilli, β-lactamase-producing Neisseria gonorrhoeae. At concentrations which are one to four times the minimum inhibitory concentration, the drugs are found to be bactericidal. Derivatives such as ofloxacin and enoxacin are found to be active against Mycoplasma pneumoniae; ofloxacin and ciprofloxacin show activity against mycobacteria. These drugs have less or no activity against fungi.
In order to minimize the resistance, these drugs may be used in combination with the other drugs; they can also be used to treat the patients affected by multiple bacterial infections. The combination of several fluoroquinolones with coumermycin A1 showed synergistic effect against S. aureus. Quinolones are found to be inactive against C. albicans.
Ofloxacin and pefloxacin are found to be active against M. leprae in in-vivo animal models. Also, ofloxacin, ciprofloxacin and tosufloxacin possess activity against Chlamydia trachomatis and Mycoplasma hominis. They are also potent against Rickettdia species and Plasmodium falciparum.[16]
As a summary, fluoroquinolones work well against almost all the bacterial pathogens causing UTI and gastroenteritis. They work against some microbes which cause STDs and respiratory tract infections. The newer generations covered the broad spectrum bacterial infections such as diahhorea, pneumonia, fever etc.
VII. THERAPEUTIC AND CLINICALUSES OF FLUOROQUINOLONES
Fluoroquinolones are associated with a variety of therapeutic and clinical uses. They are used for a wide variety of bacterial infections some of which are genital, urinary tract infections, respiratory disease, gastroenteritis, STDs etc. [21]
In the patients with stones or uropathies, they are used for UTIs because they have a high renal clearance and greater renal concentration, thus are the widely used for urinary tract infections. Levofloxacin and ciprofloxacin are used in the treatment of complex UTI and pyelonephritis. However, reoccurrence and resistance are two factors which have no control.
Fluoroquinolones are quite effective in the treatment of prostatitis because they are well penetrated into the prostatic tissue when taken as a long term treatment of four to six weeks. For shorter treatment courses, treatment failures have been observed. The first line drug for prostitis is levofloxacin. In case of resistant gram negative, pseudomonal and enterococcal prostitis, ciprofloxacin is reserved.
Acute bacterial sinusitis is caused by S. aureus, S. pneumoniae, H. influenza and Moraxella catarrhalis. According to the USFDA, gatifloxacin, moxifloxacin, sparfloxacin and levofloxacin are used in the treatment course; yet, some researchers claim that due to increasing bacterial resistance, fluoroquinolones should not be used as first line drugs.[22]
Fluoroquinolones are also preferred in the case of community-acquired pneumonia, which is one of the leading causes of death. The pathogens causing this are S. pneumoniae, H. influenza, M. pneumoniae etc. due to ADRs and drug interactions, care has to taken before prescribing fluoroquinolones in the elders and they should not be used as the first line agents until there is an emergency. Ciprofloxacin and trovafloxacin have been studied for the treatment of nosocomical pneumonia; combination antimicrobial therapy has also been widely accepted.[23]
Sexually transmitted diseases caused by N. gonorrhoeae are treated by ofloxacin and gatifloxacin. The infections caused by C. trachomatis are treated by seven day course of ofloxacin or sparfloxacin. Pelvic inflammatory diseases are treated by various treatment therapies like ofloxacin plus metronidazole or cefoxitin, ciprofloxacin plus clindamycin.[24]
In the treatment of traveler’s diarrhea, norfloxacin or ciprofloxacin are found to be useful, which is caused by Shigella species, E. coli, C. jejuni etc and for enteric typhoid fever, ciprofloxacin and ofloxacin are used. Fluoroquinolones are not preferable for skin and soft tissue infections due to their limited gram positive activity.[25]
VIII. OTHER ACTIVITIES OF FLUOROQUINOLONES
According to the latest researches, it has been found that this moiety is not only capable of killing microbes but is also effective as antiviral, antitumor, antimycobacterial drug, etc. [26]
Polycyclic compounds of the fluoroquinolone family are found to be capable of inhibiting topoisomerase II which is responsible for DNA replication and thus they are promising structures as antineoplastic agents. If isoniazide and pyrazinamide residues are introduced into the piperazine ring at 7th position, then the fluoroquinolones become active against mycobacteria and they can be used for rifampicin-resistant tuberculosis. If (triazolylmethyl)phenyl fragment is placed at 1st position and an aryl substituent is introduced at position 4th of piperazine, then they are capable of working as Anti-HIV agents and they can be used to cure the viral infections caused by HIV retroviruses. Some derivatives are also capable of inhibit the reverse transcriptase of HIV-1. Some of the derivatives are antifungal and antiparasitic in nature for example the mannic derivatives of norfloxacin. The newer generation of fluoroquinolones like moxifloxacin and gatifloxacin show activity against Toxoplasma gondii and Plasmodium falciparum.[27]
They are also known to exhibit antithrombocyte, hypertensive and cardiovascular actions. Some derivatives are GSK inhibitors and some are modulators of M1 receptors. Thus, this moiety is known to exhibit multiple properties.
IX. ACKNOWLEDGEMENTS
I would like to express my gratefulness to my institution, “Department of Pharmaceutical Sciences, Sir J.C. Bose Technical Campus, Bhimtal, Kumaun University, Nainital” for the successful completion of my work.
X. CONFLICT OF INTEREST
The author has no conflict of interest in this article.
Conclusion
In summary, this review offers a comprehensive understanding of fluoroquinolones, covering their mechanisms of action, synthesis, and therapeutic applications in antimicrobial therapy. Fluoroquinolones exert their antimicrobial effects primarily by inhibiting bacterial DNA gyrase and topoisomerase IV, crucial enzymes involved in DNA replication and transcription. Structural modifications, notably fluorination, have led to the development of derivatives with enhanced potency and broader spectrum activity against various bacterial strains. Synthetically, fluoroquinolones undergo a series of steps including heterocyclic cyclization, fluorination, and substituent modulation, resulting in compounds with improved pharmacokinetic properties. Recent advancements in synthetic methodologies have facilitated the production of novel derivatives with enhanced antimicrobial activity and reduced resistance profiles. In clinical practice, fluoroquinolones are widely used for treating respiratory, urinary tract, skin and soft tissue, and gastrointestinal infections. However, the rise of bacterial resistance presents a significant challenge to their efficacy, prompting the need for strategies such as combination therapy and antimicrobial stewardship programs. Furthermore, fluoroquinolones exhibit potential beyond antimicrobial therapy, including anti-inflammatory and immunomodulatory effects. Nevertheless, concerns regarding adverse effects, such as tendon rupture and peripheral neuropathy, underscore the importance of cautious clinical use and ongoing surveillance. In conclusion, fluoroquinolones represent indispensable antimicrobial agents with diverse mechanisms of action and broad-spectrum activity. Continued research efforts focused on understanding their mechanisms, refining synthesis, and exploring new therapeutic applications are vital for addressing emerging challenges in antimicrobial resistance and optimizing patient outcomes in antimicrobial therapy.
References
[1] L. Zaffiri and J. Gardner, “History of antibiotics. from salvarsan to cephalosporins,” J. Invest. Surg., vol. 25, no. 2, pp. 67–77, Apr. 2012, doi: 10.3109/08941939.2012.664099. [2] L. Swellmeen, A. Uzrail, R. Shahin, and Y. AL-Hiari, “Synthesis of Fluoroquinolones Derivatives as Antimicrobial Agents,” Orient. J. Chem., vol. 35, no. 4, pp. 1248–1253, Aug. 2019, doi: 10.13005/ojc/350401. [3] N. Wald-Dickler, P. Holtom, and B. Spellberg, “Busting the Myth of ‘Static vs Cidal’: A Systemic Literature Review,” Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., vol. 66, no. 9, pp. 1470–1474, Apr. 2018, doi: 10.1093/cid/cix1127. [4] K. H. Schleifer, “Classification of Bacteria and Archaea: Past, present and future,” Syst. Appl. Microbiol., vol. 32, no. 8, pp. 533–542, Dec. 2009, doi: 10.1016/j.syapm.2009.09.002. [5] D. J. Mason, E. G. M. Power, H. Talsania, I. Phillips, and V. A. Gant, “Antibacterial action of ciprofloxacin,” Antimicrob. Agents Chemother., vol. 39, no. 12, pp. 2752–2758, 1995, doi: 10.1128/AAC.39.12.2752. [6] S. K?rmusao?lu, N. Gareayaghi, and B. S. Kocazeybek, “Introductory Chapter: The Action Mechanisms of Antibiotics and Antibiotic Resistance,” in Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods, IntechOpen, 2019. doi: 10.5772/intechopen.85211. [7] K. Liu et al., “Classification of two species of Gram-positive bacteria through hyperspectral microscopy coupled with machine learning,” Biomed. Opt. Express, vol. 12, no. 12, p. 7906, Dec. 2021, doi: 10.1364/boe.445041. [8] T. D. M. Pham, Z. M. Ziora, and M. A. T. Blaskovich, “Quinolone antibiotics,” MedChemComm, vol. 10, no. 10, pp. 1719–1739, 2019, doi: 10.1039/c9md00120d. [9] V. N. Charushin, E. V. Nosova, G. N. Lipunova, and O. N. Chupakhin, “Fluoroquinolones: Synthesis and application,” in Fluorine in Heterocyclic Chemistry: Volume 2: 6-Membered Heterocycles, Springer International Publishing, 2014, pp. 111–179. doi: 10.1007/978-3-319-04435-4_3. [10] J. S. Wolfson and D. C. Hooper, “The Fluoroquinolones: Structures, Mechanisms of Action and Resistance, and Spectra of Activity In Vitro,” 1985. [Online]. Available: https://journals.asm.org/journal/aac [11] Z. A. Kanafani and J. R. Perfect, “Resistance to antifungal agents: mechanisms and clinical impact,” Clin. Infect. Dis., vol. 46, no. 1, pp. 120–128, 2008. [12] M. F. Medellín-Luna et al., “Fluoroquinolone Analogs, SAR Analysis, and the Antimicrobial Evaluation of 7-Benzimidazol-1-yl-fluoroquinolone in In Vitro, In Silico, and In Vivo Models,” Molecules, vol. 28, no. 16, p. 6018, Aug. 2023, doi: 10.3390/molecules28166018. [13] T. D. Gootz and K. E. Brighty, “Fluoroquinolone Antibacterials: SAR, Mechanism of Action, Resistance, and Clinical Aspects.” [14] A. Roy, S. M. Sardar#, B. U. Salve#, and D. D. Rishipathak, “STUDIES ON SYNTHESIS AND BIOLOGICAL EVALUATION OF SOME SUBSTITUTED FLUOROQUINOLONES’.” [15] D. Baggio and M. R. Ananda-Rajah, “Fluoroquinolone antibiotics and adverse events,” Aust. Prescr., vol. 44, no. 5, pp. 161–164, Oct. 2021, doi: 10.18773/austprescr.2021.035. [16] C. M. Oliphant, G. M. Green, K. Permanente, and R. W. Sloan, “Mechanism of Action Quinolones rapidly inhibit DNA synthesis by promoting cleavage of bacterial DNA in the DNA-enzyme complexes of DNA gyrase and type IV topoisomerase, resulting in rapid bac-terial death. 1-3 As a general rule, gram-nega-tive bacterial activity correlates with inhibi-tion of DNA gyrase, and gram-positive bacterial activity corresponds with inhibition of DNA type IV topoisomerase. 1 Quinolones: A Comprehensive Review CLINICAL PHARMACOLOGY,” 2002. [Online]. Available: www.aafp.org/afpAMERICANFAMILYPHYSICIAN455 [17] M. L. Bayot and B. N. Bragg, “Antimicrobial Susceptibility Testing,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2023. Accessed: Dec. 04, 2023. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK539714/ [18] V. F. Batista, D. C. G. A. Pinto, and A. M. S. Silva, “Synthesis of Quinolines: A Green Perspective,” ACS Sustain. Chem. Eng., vol. 4, no. 8, pp. 4064–4078, Aug. 2016, doi: 10.1021/acssuschemeng.6b01010. [19] P. Lucia, “Quinolones: Synthesis and Antibacterial Activity.” [20] M. Mokaber-Esfahani et al., “Synthesis and Antibacterial Evaluation of New Pyrimidyl N-Ciprofloxacin Derivatives,” ChemistrySelect, vol. 4, no. 31, pp. 8930–8933, Aug. 2019, doi: 10.1002/slct.201901924. [21] R. C. Owens and P. G. Ambrose, “CLINICAL USE OF THE FLUOROQUINOLONES MEDICAL CLINICS OF NORTH AMERICA,” 2000. [22] D. Jiang, “4-Quinolone Derivatives and Their Activities Against Gram-negative Pathogens,” J. Heterocycl. Chem., vol. 55, no. 9, pp. 2003–2018, Sep. 2018, doi: 10.1002/jhet.3244. [23] A. D. Da Silva, M. V. De Almeida, M. V. N. De Souza, and M. R. C. Couri, “Biological Activity and Synthetic Metodologies for the Preparation of Fluoroquinolones, A Class of Potent Antibacterial Agents,” 2003. [24] C. Levine, H. Hiasa, and K. J. Marians Ay, “DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities.” [25] T. Khan, K. Sankhe, V. Suvarna, A. Sherje, K. Patel, and B. Dravyakar, “DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents,” Biomed. Pharmacother., vol. 103, pp. 923–938, Jul. 2018, doi: 10.1016/j.biopha.2018.04.021. [26] P. F. Chan et al., “Structural basis of DNA gyrase inhibition by antibacterial QPT-1, anticancer drug etoposide and moxifloxacin,” Nat. Commun., vol. 6, Dec. 2015, doi: 10.1038/ncomms10048. [27] I. A. Seliem et al., “Design, synthesis, antimicrobial, and DNA gyrase inhibitory properties of fluoroquinolone–dichloroacetic acid hybrids,” Chem. Biol. Drug Des., vol. 95, no. 2, pp. 248–259, Feb. 2020, doi: 10.1111/cbdd.13638.
Copyright
Copyright © 2024 Hansika Bansal, Anita Singh, Priya Gupta, Manisha Joshi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET60354
Publish Date : 2024-04-15
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

