Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Formulation and Evaluation of Medicated Lollipop
Authors: Ms. Nikita S. Patil, Mr. Pankaj N. Patil, Ms. Nikita L. Patil, Mr. Divakar R. Patil, Mr. Akash S. Jain, Mr. Azam Z. Shaikh, Mr. Sandip A. Tadavi
DOI Link: https://doi.org/10.22214/ijraset.2023.52302
Certificate: View Certificate
Abstract
The benefits of medicated lollipops include better drug retention duration in the mouth, increased bioavailability, decreased gastrointestinal pain, and omission of first-pass metabolism. Both adults and children accept lollipops as a dose form with a high level of acceptance. The primary ingredients found in lollipops include sweetening, flavoring, coloring, opacifiers, and stabilizing agents. Medication-delivery systems in the form of lollipops dissolve slowly within 1 to 10 minutes, they disintegrate in the mouth. They are easy to swallow because there will be attractive for children. The absorption of the drug mouth to the systemic circulation, the effective dose of the drug should be provided to the patient. The Medicated Lollipops are administered by the oral route. Children’s patients frequently utilize paracetamol as an over the counter analgesic (pain reliever) and antipyretic (fever reducer). It is a key component in many cold and flu cures and is frequently used to treat headaches and other mild aches and pains. Dysphagia (trouble swallowing) is a prevalent condition in Children individuals. As a result, there is a need for a solid medication that is simple to use and swallow dosage like medicated lollipops.
Introduction
I. INTRODUCTION
Oral drug distribution is surrounded by several scientific issues that could be studied for years to come. There are new technologies also required to develop drug delivery methods and produce innovative dosage forms (1). Tablets are the most extensively used dosage form, and oral drug delivery is the most flavorful method of pharmaceutical administration. Solid dosage forms are widely used because they are simple to administer, precise in their amount, allow for self- medication reduce pain, and most importantly ensure patient compliance.
The inability to take typical pill dosage forms is among the main issues that many patients. This issue becomes more obvious when a patient taking medication cannot easily access drinking water. The dispersible pill distribution technology is distinguished by rapid release, rapid disintegration, and increased patient compliance. Dysphagia or trouble swallowing affects people of all ages, but it is most prevalent in the elderly and young children due to physiological changes in those populations (2). The benefits of medicated lollipops include better drug retention duration in the mouth, increased bioavailability, decreased gastrointestinal pain, and omission of first-pass metabolism.
Both adults and children accept lollipops as a dose form with a high level of acceptance. There are many lollipops, including compressed, firm, and soft lollipops. The several kinds of lollipops that are sold today, how they are made and what components are utilized (3). The medication is contained in a sweetened and flavored foundation in lollipop-style solid dosage forms that are meant to dissolve gradually in the mouth.
The primary ingredients found in lollipops include sweetening, flavoring, coloring, opacifiers, and stabilizing agents. Medication-delivery systems in the form of lollipops dissolve slowly. They disintegrate in the mouth within 1 to 10 minutes (4). Lollipops are large, boiled sugar confections in various flavors attached to a plastic stick and can be eaten slowly by licking them. The medicament is held together by a plastic stick. The medication found in lollipops is a solid unit dosage form that dissolves in the mouth or pharynx.
Lollipops were first created in the 20th century and are still manufactured for sale. The majority of the preparations for lollipops are accessible as OTC drugs. Due to their many advantages, lollipop administration is a delectable option that is well-liked in the pharmaceutical sector. However, the lollipop administration has certain disadvantages. They often contain one or more medications in a base that is typically flavored and sweetened. Typically, lollipops are utilized to make an immediate impression in the mouth. The drug may also be utilized for systemic effect provided that it is effectively absorbed via the buccal lining. (5).
It is very likely that the lollipops have been created and reinvented numerous times because the concept of an edible candy on a stick is so straightforward. When the aristocracy frequently used sticks or handles to consume boiled sugar, the first candies that closely resembled what we now refer to as lollipops emerged in the middle Ages. Although the contemporary lollipop’s origins are still a mystery, several American businesses have claimed to have invented them in the the beginning of the twentieth century. According to the book “Food for Thought: extraordinary little chronicles of the World”, they were created by George Smith of New Haven, Connecticut, who began manufacturing big boiled sweets mounted on sticks in 1908. He gave them the moniker "lollipop" in honor of a popular racehorse at the time, and in 1931 he trademarked the term. Francis Grose, an English lexicographer, first noted the word "lollipop" in 1796. The word is taken from "lolly," which means tongue, and "pop," which means slap. The 1920s saw the earliest mentions of the lollipops in their current form. As an alt ; ernative, it might be a term with Romany roots that refers to the Roma custom of selling toffee apples on sticks. he Romany word for red fruit is loli phobia (6
II. DRUG PROFILE
Paracetamol children frequently take it as a pain reliever. It is frequently used to alleviate symptoms of colds, stomachaches, headaches, and earaches. it can also be applied to lower an elevated temperature. The over-the-counter drug paracetamol is often used by kids.
Most commonly, paracetamol is used to reduce fever and ease pain. The children typically experience pain and fever. Due to parents' fear and fever phobia, fever management is frequently characterized by over-treatment. The characteristics of pain management include under treatment, especially in very young children's youngsters with severe, excruciating injuries. Often used as an analgesic and antipyretic medication to treat fever and children who experience mild to moderate pain. It is the primary option for both treatment discomfort and fever by national and international recommendations and guidelines and it is also mentioned in the list of important things. Medication of children in world health organization (WHO) (14-32).
The class of drugs known as analgesics, or painkillers, includes paracetamol. Pain that is mild to moderate can be relieved with paracetamol. It is also helpful for bringing down a fever, which may be elevated due to a cold or the effects of childhood vaccinations. A typical pain reliever, paracetamol is sold in tablet/capsule and liquid form at numerous retail locations.
Many Paracetamols are an ingredient in several 'over-the-counter' combination painkiller brands as well as numerous cold and flu medications (8). Two liquid dosage levels of paracetamol can be used orally: 250mg/5ml for children over 6 years old and 120mg/5ml for children under 6 years old. It is crucial to choose the appropriate strength because it is also offered in melt-tab and rectal formulations (9).
A. Signs That your Child is in Pain
Younger children may demonstrate their discomfort to you by doing one of the following: older children may frequently tell you that they are in pain
- crying or screaming
- pulling a face
- changes in their sleeping or eating patterns
- becoming quiet and withdrawn (10)
B. Dose Calculation for Children
The recommended dosage for children is 10–15 mg of paracetamol per kilogram of body weight, which is about half the weight of a grain of rice. In other words, a child who weighs 20 kg needs to consume 200–300 mg (about the weight of ten grains of rice), or 10-15 mg (around the weight of one grain of rice) multiplied by 20. If necessary, this dose may be administered up to four times in 24 hours, once every four to six hours. (9).
C. Mechanism of Action of Medicated Lollipop
In contrast to when a drug is swallowed and absorbed by the digestive system, a drug is more quickly absorbed through the oral mucosa when it is administered using our lollipop delivery technology by giving a lollipop until the desired result is obtained, the dose can be readily regulated. Consuming anything is entertaining. Also, they don’t need water, so they may be used anytime and anyplace for children.
D. How Does it Work?
As a patient suckers or twists a lollipop in their mouth, the chemical slowly releases. After being absorbed by the buccal mucosa, the medication may have local or systemic circulation (6).
E. Recommended doses of Paracetamol (120mg/5ml) for infants and children (3 months 6 years)
Paracetamol for the treatment of mild to moderate pain and as an antipyretic.
|
Children Age |
How Much |
How Often (In 24Hour) |
|
3-6 months |
2.5mL |
Four Times |
|
6-24 months |
5mL |
Four Times |
|
2-4 years |
7.5mL (5mL +2.5mL) |
Four Times |
|
4-8 years |
10 mL (5mL +5mL) |
Four Times |
- For the treatment of mild to moderate pain and as an antipyretic, use paracetamol (120 mg/5 ml). Used to treat fever and discomfort, it is connected to post-immunization pyrexia, colds, flu, toothaches, and teething.
- Give no more than four doses in 24 hours.
- Leave at least 4 hours between doses (11)
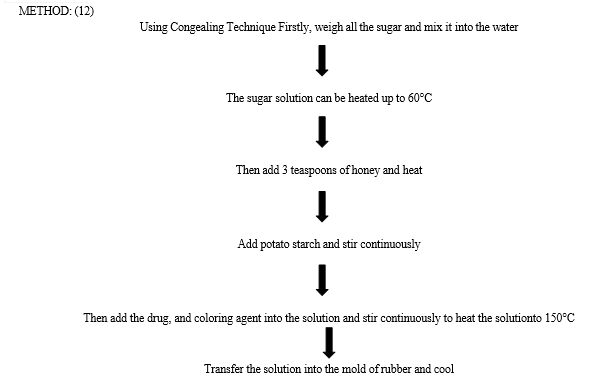
MATERIAL: (12)
Formula for 10 Lollipop
|
Sr. No |
Ingredients |
Amount |
Uses |
|
|
Sucrose |
70 g |
Sucrose has been used since antiquity for its sweetness. It is often used in medication to impart a more pleasant taste to often unpalatable chemicals. |
|
|
Natural Potato Starch |
10 g |
The Pharmaceuticals industry for a wide variety of reasons, such as an excipient tablet, capsule disintegrant, glidant, or as a binder. |
|
|
Paracetamol |
2.5 g |
Paracetamol is a commonly used medicine that can help treat pain and reduce a high temperature (fever). |
|
|
Flavoring agent (Honey) |
3 Teaspoon |
Flavoring agents are addictive substances that give a tablet an additional taste or flavor. In particular, they help in masking the unpleasant taste (e.g. bitter or pungent taste) of drugs/excipients and instead improve the quality of their taste. |
|
|
Coloring agent |
0.2 g |
Colors for pharmaceutical products are used to impart organoleptic properties or for technical purposes. |
|
|
Water |
q. s |
Water for pharmaceuticals is used for the cleansing and rinsing processes required in the processing plant, being also a regular agent for cleansing of reactors and other pharmaceuticals. |
III. EVALUATION STUDIES
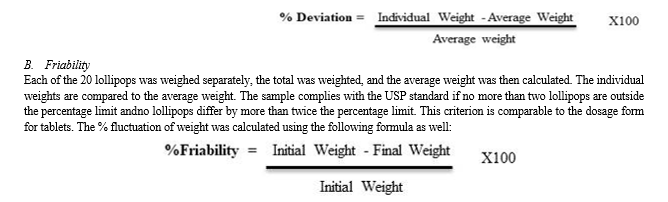
A. Weight Variation
Twenty lollipops were weighed individually, the total number was weighted, and then the average weight was determined. The average weight is contrasted with the individual weights. This criterion is comparable to the dosage form for tablets: the sample complies with USP standards if no more than two lollipops are outside the percentage limit and if no lollipop differs by more than twice the percentage limit. The following formula was also used to compute the percent variation of weight:
C. Hardness and Thickness Test
Four lollipops were selected from each batch of production, their hardness was assessed using a tablet hardness device, and an average was determined. Vernier calipers were also used to measure the thickness of the created lollipops.
D. Dissolution Studies
Using a USP dissolution test apparatus type II, in-vitro dissolution investigations were carried out in 900 mL of phosphate buffer (pH 6.8) at 37 0.5 °C with the paddle speed fixed at 25 rpm. To maintain sink conditions, identical amounts of the sample were replenished. Sampling was done every 10 minutes for 40 minutes, and the samples were detected using a UV-spectrophotometer at 243 nm. The samples were filtered, and after being appropriately diluted, the drug content of paracetamol in each sample was determined using a UV spectrophotometer at 243 nm.
E. Determination of Drug Content
An amount equal to 100 mg of paracetamol was weighed, placed in a volumetric flask of 100 mL, dissolved in 100 mL of phosphate buffer with a pH of 6.8, and put on a shaker overnight before being filtered. The homogeneity of the content was checked by crushing 20 lollipops. From there, 1 mL of the solution was removed and placed in a 100 ml volumetric flask after the volume had been brought up to 100 ml with phosphate buffer (pH 6.8). Using phosphate buffer (pH 6.8) as a blank, the absorbance was calculated using a UV spectrophotometer at a wavelength of 243 nm.
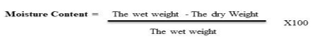
F. Moisture Content
A mortar and pestle were used to weigh and crush one lollipop from each formulation. From then, 1 g of the sample was weighed and dehydrated for 24 hours. The sample is weighed after 24 hours. By subtracting the final weight from the moisture content, the original weight of the lollipops sample
G. Stability Studies
Four lollipops were selected from each batch and treated to various conditions, such as varying humidity and temperature. 45 days of stability tests were conducted at 30 °C with 65% relative humidity. To demonstrate the impact of these circumstances on the dosage form, studies on the drug content and organoleptic properties (physical appearance) were carried out. The UV spectrophotometer was set at a max of 243 nm to determine the drug content. (12).
Observation Table No: 1
|
Parameter |
Standard limits |
Value |
|
Weight variation |
5 ± 0.41 |
1.6 |
|
Friability |
0.6 ± 0.033 |
0.1 |
|
Hardness |
11 ± 0.23 |
5 |
|
Thickness |
12.6 ± 0.33 |
10 |
|
%Drug content |
95.98 ± 1.09 |
95.79 |
|
Moisture content |
0.51 ± 0.04 |
0.50 |
IV. DISCUSSION
Patient compliance is one of the most important aspects of medication administration. The development of paracetamol-sweetened lollipops for the efficient management of pain and fever in pediatric and geriatric patients was the goal of the current investigation. Paracetamol, commonly known as paracetamol, is frequently used by children's patients as an over-the-counter analgesic (pain reliever) and antipyretic (fever reducer). It is widely used to relieve headaches and other moderate aches and pains, and it is an essential ingredient in many remedies for the cold and flu. Dysphagia, or difficulty swallowing, is a common condition among kids. Therefore, a solid drug that is simple to use and swallow, like lollipops, is required. The prepared lollipops had a nice flavor, a decent color distribution, and an appealing physical appearance in each composition. All batches’ friability shows that the lollipops are of good strength. Hardness results suggest that all batches of lollipops have good strength. The ICH defines an acceptable deviation as percent guidelines. The samples meet USP standards because there is the percentage cap is exceeded if there are more than two tablets. (The discrepancy may not exceed 5%) hence, lollipops pass the test for weight consistency and weight fluctuation.
The all-lollipops’ average percent deviation was found to be all formulations pass the test since they fall within the allowed range (5%) weight difference Shown in Table number 1.
The supplied lollipops' moisture content and medication content were confirmed to be consistent and acceptable across all formulations. Since all lollipops were discovered to be within the limit, all formulations passed the various testing. The physicochemical properties do not change after conducting stability analyses, or just slightly altering the drug's ingredients. All formulations were found to contain the same amount of medication Shown in Table number 1.
Dissolution is the mass transfer from the dosage form's surface to the main body of the solution. Dissolution is crucial for all conventional, solid, and graded limiting steps for oral dose formulations and the assimilation of oral medications, particularly for lipophilic medicines. In vitro dissolving tests can be used to help create formulations and pinpoint important manufacturing factors. So, using phosphate buffer, the rate of the lollipops' dissolution was investigated (pH 6.8) for the final hours in a sink environment utilizing a type of USP dissolving device. A theoretical calculation of release profiles is crucial to assessing the formula for calculating release rates and determining whether the medicine is released in a planned way. When beginning to prepare the formula for a new dosage form, there are a few measures that must be taken into consideration, notably to get rid of any potential interference from particular elements. Purity testing, melting point determination, and fundamental analytical requirements like the UV calibration curve are some of these stages. As the melting point of paracetamol, as previously indicated, was determined using the capillary method and was shown to be between 168 and 170 °C, these experiments served as confirmation. The calibration curve for the various tests and investigations was built by dissolving a calculated amount of paracetamol in phosphate buffer (pH 6.8) to create a standard solution for the medication. Paracetamol's UV spectrum in phosphate buffer (pH 6.8) demonstrated the greatest absorption, as stated, at 243 nm. Hence, it was determined that the medicine employed in the formulation was pure, and the requirements were also identical. The paracetamol's UV spectrum is Using phosphate buffer (pH 6.8) shown in Table number 2.
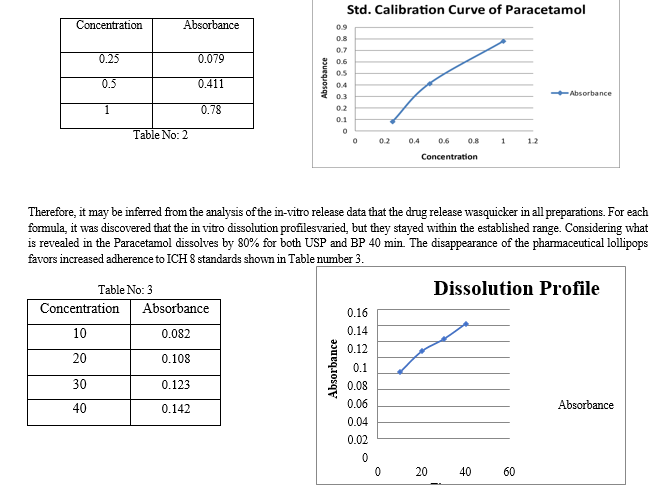
The shifting of paracetamol peaks in the FTIR spectrum indicates that there is no drug-drug interaction. There is a small exception in the case of the dissolution profile since earlier research employed 25 rpm while this study utilized 100 rpm to imitate the effect of tongue movement and 900 mL if the lollipops are the same. like regular pills. The appearance, flavor, and color were determined, although rigorous standard testing was not necessary to determine individual testing like what we did. The stability evaluations were conducted by calculating the number of drugs in each batch. to determine the impact of various circumstances and to make making certain that the formulations remain stable for a long time. At the time of the product's initial market debut, the stability samples of each batch may be taken, which just 2-5% of commercial batches at a time can be reduced. going forward. Consequently, all of the stability study formulations Lollipops make up 2% of the only formulations to investigate if the physicochemical properties, solubility, and in vitro release of the manufactured paracetamol lollipops are impacted by the variation between these formulations. Few differences existences existed, and formulation provided the most satisfying results factory outcomes, as already mentioned.
Thickness, weight variation, and hardness are established and evaluated metrics that demonstrate that they were within limitations. The consistency of drug content was also discovered to be within the horizon. Studies on the drug's in vitro release rate revealed that release met expectations FT- IR spectroscopy was used to investigate any potential interactions between the medicine and excipient, and the results showed that there were none. Ibuprofen and few chemicals. Therefore, paracetamol Medicated lollipops could be inexpensive and gradual release inside the mouth. In addition, it is a secure and efficient dose form for patients in children potentially more bioavailable. The prescription lollipops can be made with additional medications, including the popular patients in children to overcome the administrative issues additional research is required to determine the amount of medication absorption. The trickiest and most time-consuming part of this research was making the lollipops; the proportions of the ingredients, the temperature, and the timing were all essential. The formulation, as even a minor alteration to any of these parts could be parameters, and failing to follow the process and values as explained in the planning part will result in disappointed factory output (12).
References
[1] Review of Medicated Lollipop. Perumala Jagdesh, Padamasetty jyothi i,G Nehtra vani and S Dasthgiri. 2017, World Journal Pharmaceuticals, pp. 399-405. [2] Medicated Lozenges As An Easy To Use Dosage Form. Rathode Minakshi, Poharkar Sachin,Pandhre Yuvraj, Muneshwar Monali, Sul Sandesh. 2018, World Journal Of Pharmaceuticals Research, pp. 305-322. [3] MEDICATED LOZENGES: AS EASY TO USE FOR PEDIATRIC AND GERIATRIC PATIENTS. MR UJER N.MULLA, Mrs. A K.SALUNKHE. 2021, Vol. 9. [4] Formulation And Evaluation Of Medicated Candy Containing Albendazole For Pediatric. Chaudhari V. Punam, Chaudhari G. Nirma, Chaudhari S. Pooja, Patil M. Amruta, Pawar P. Sunil. 2019. 73-80. [5] Medicated Lollipop-A New Dosage For Pediatrics. Tejasavi, Bhandari Neeraj and Mahajan. 2018. 532-539. [6] A Review on Medicated Lollipops. Gupta Rashi, Mandloi Rampal, Pillai Sujit, Birla Nikhlesh. 1, 2021, Vol. 13. [7] A REVIEW ON MEDICATED LOLLIPOPS FOR PAEDIATRICS: A NEW DOSAGE FORM. Dhairyasheel Gund, Tushar Humbe, Prof. Vijaya Barge , Prof. Amit Kasabe. 6, 2021, Vol. 10. 2278-4357. [8] Patient. Michael Stewart, Reviewed by Sid Dajani |. 2023. [9] NHS. How and when to give paracetamol for children. https://www.nhs.uk/medicines/paracetamol-for-children/how-and-when-to-give-paracetamol-for- children/. june 15, 2022-2025. [10] carers., The Royal Children\'s Hospital Emergency and Pharmacy departments. We acknowledge the input of RCH consumers and. [Online] July 2020. [11] Healthcare, Pinewood. Paracetamol. https://www.medicines.org.uk/emc/product/4108/smpc/print. [Online] July 29, 2022. [12] Hatem A. Hejaz, Ayat Kanan, Mahmood Al Mohtaseb and Ameer Ja’bari. Development and characterization of paracetamol. 2020. [13] Medicated Chocolate And Lollipops: A Novel Drug Delivery System For Pediatric Paitient. Pawar P.G., Darekar A.B.,Saudagar R.B. 2018. 677-696. [14] Chiappini E, Parretti A, Becherucci P, Pierattelli M, Bonsignori F, Galli L, et al. Parental and medical knowledge and management of fever in Italian pre-school children. BMC Pediatric. 2012; 12:97. [15] Purssell E. Fever phobia revisited. Arch Dis Child. 2004;89: 89–90. [16] Kanabar D. A practical approach to the treatment of low-risk childhood fever. Drugs R D. 2014;14: 45–55. [17] Betz MG, Grunfeld AF. ‘Fever phobia’ in the emergency department: a survey of children’s caregivers. Eur J Emerg Med. 2006;13: 129–33. [18] Poirier MP, Collins EP, McGuire E. Fever phobia: a survey of caregivers of children seen in a pediatric emergency department. Clin Pediatr. 2010;49: 530–4. [19] Alexander J, Manno M. Underuse of analgesia in very young pediatric patients with isolated painful injuries. Ann Emerg Med. 2003;41: 617–22. [20] Seupaul RA. Oligoanalgesia in very young pediatric patients. Ann EmergMed. 2004;43: 292(auth reply 3) [21] Cranswick N, Coghlan D. Paracetamol efficacy and safety in children: the first 40 years. Am J Ther. 2000;7: 135–41. [22] Green R, Jeena P, Kotze S, Lewis H, Webb D, Wells M. Management of acute fever in children: guideline for community healthcare providers and pharmacists. S Afr Med J. 2013;103: 948–54. [23] Guideline statement: management of procedure-related pain in children and adolescents. J Paediatr Child Health. 2006;42(Suppl 1): S1–29. [24] Stapelkamp C, Carter B, Gordon J, Watts C Assessment of acute pain in children: development of evidence-based guidelines. Int J Evid Based Healthcare. 2011;9: 39–50. [25] The National Institute for Health and Care Excellence (NICE). NICE clinical guideline 160 Feverish illness in children: assessment and initial management in children younger than 5 years. 2013. [26] Italian Ministry of Health. Pain in children: practical instruments for assessment and therapy 2013. [27] Italian Society of Pediatrics (Societa` Italiana di Pediatria). Gestione del segno/sintomo febbre in pediatria: Linee Guida della Societa` Italiana di Pediatria. 2013 [Management of the fever sign/symptoms in children: Guidelines of the Italian Society of Pediatrics, 2013]. [28] Savoia G, Coluzzi F, Di Maria C, Ambrosio F, Della Corte F, Oggioni R, et al. Italian Intersociety Recommendations (SIAARTI, SIMEU, SIS 118, AISD, SIARED, SICUT, IRC) on Pain Management in the Emergency Setting. Minerva Anestesiol. 2014. [29] De Martino M, Mansi N, Principi N, Serra A. Italian Guidelines: Management of Pharynotonsillitis in Children. 2011. [30] World Health Organization. WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. 2012. [31] Italian Society for the Study of Headaches Guidelines for the diagnosis and treatment of pediatric headaches. 2003. [32] Fields E, Chard J, Murphy MS, Richardson M. Assessment and initial management of feverish illness in children younger than 5 years: summary of updated NICE guidance. BMJ. 2013;346: 2866
Copyright
Copyright © 2023 Ms. Nikita S. Patil, Mr. Pankaj N. Patil, Ms. Nikita L. Patil, Mr. Divakar R. Patil, Mr. Akash S. Jain, Mr. Azam Z. Shaikh, Mr. Sandip A. Tadavi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET52302
Publish Date : 2023-05-15
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

