Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
From Liquid to Solid: Unravelling the Magic of In-Situ Gels
Authors: Maithili Brahmavale, Pooja Bhopi, Shraddha Bhavsar, Sakshi Bramhane, Murshid Bubere, Nilesh Bonde
DOI Link: https://doi.org/10.22214/ijraset.2024.62471
Certificate: View Certificate
Abstract
In situ gelling formulations are liquid at room temperature and transform into gels upon application to the body in response to various stimuli (e.g., pH, temperature, ionic composition). Incorporating drug nanoparticles into these systems enhances their biomedical utility by prolonging drug release, reducing dosing frequency, and improving patient outcomes. These formulations, particularly those with mucoadhesive polymers, offer promising potential for targeted drug delivery and prolonged drug retention at specific sites. A review of clinical properties of in situ gelling systems investigated over the past decade underscores their effectiveness and challenges in achieving optimal dosage forms.
Introduction
I. INTRODUCTION
This Systems for in situ gelling are liquid formulations that, when injected into the body or applied topically, solidify into a depot [1]. Over the last two decades, there has been increasing interest in responsive drug delivery systems as an attractive category of devices for various pharmaceutical and biological applications. They are preferred over traditional injectable depot systems like wafers or implants because they are easier to prepare and require less capital investment because they can be self-administered with autoregulators, be injected using smaller gauge needles, and allow for the controlled release of incorporated therapeutic agents, which lowers the frequency of dosing and prevents therapeutic failure or unwanted side effects [2]. Using these systems for topical administration is especially appealing since the gel state is retentive and controls distribution, while the low viscosity state at ambient settings facilitates administration and permits procedures like spraying.
The behavior of the sol–gel phase transition seen by in situ gelling formulations is dependent on one or more stimuli, including changes in pH, temperature, solvent exchange, UV radiation, and the presence of certain ions or molecules [3] . Polymer solutions are usually used to generate this gelation, while some inorganic and tiny organic systems do exist and are becoming more and more interesting [4], [5]. Molecular weight (MW), architecture, temperature, and physiological media composition all affect the gelation of in situ gelling formulations in polymer systems [6]. The focus of this paper will be on formulations of polymer systems that are attractive for transport to the body yet are sensitive to pH, ions, and temperature [1].
Mucoadhesive polymers and in situ gelling have shown to be necessary excipients for extending the mucosal residence time of drug delivery systems. Numerous medications' systemic and local therapeutic efficacy can be significantly enhanced by this extended residence time [7]. Many mucoadhesive polymers have been investigated for mucosal drug delivery, including poly(acrylic acid), gellan gum, hyaluronic acid, chitosan, cellulose derivatives, and thiolated polymers, sometimes known as thiomers [7].
II. SYSTEMS USED IN IN SITU GELLING FORMULATION
A. pH Sensitive System
Variations in the physiological environment's pH may allow for modifications to the polymer's ionization state, conformation, and solubility, which could lead to the drug carrier gelling [8]. The gelation behavior of pH-responsive formulations is influenced by the pKa of the constituent polymer [9].
pH-sensitive polymeric solutions that are weakly acidic or basic usually gel at pH values that are higher than their pKa values, respectively [8]. pH-sensitive formulations include xanthan-based systems for controlled release of bovine serum albumin at physiological pH, polyacrylic acid-based in situ nasal gels (Carbopol 934) that demonstrate in situ gelling properties by deprotonation at nasal pH ≈ 8.3 in patients with rhinitis, and formulations based on polyacrylic acid for colonic delivery of 5-aminosalicylic acid [10], [11], [12] .
B. Ion-Sensitive System
By combining simulant biological fluid (tear, nasal, or vaginal fluid) with the in-situ gelling samples, the gelation potential of an ion-sensitive delivery system can be assessed [1]. Changes in the rheological profiles of these samples can then be examined using viscometry or turbidimetric analysis [13].
The viscosity of the cross-linked polymer and the rate at which the polymeric system transitions from a sol-gel depends on the kind and concentration of cation that cross-links the ion-sensitive polymers [14]. Furthermore, pathological circumstances that alter the ionic composition and strength of the target sites may aid in the sol–gel transition of ion-sensitive dosage forms [14].
Gellan gum , methacrylated gellan gum, pectin, and sodium alginate are a few examples of ion-sensitive drug delivery methods. Gellan gum, an anionic polysaccharide, comprises repeating units of 1,3-β-D-glucose, 1,4-β-D-glucuronic acid, 1,4-β-D-glucose, and 1,4-α-L-rhamnose. It is noteworthy that gelation is produced by ions and is dependent on temperature and pH [15], [16], [17].
C. Temperature-Sensitive system
Drug formulations that gel in situ at temperatures higher than 37 °C may not be appropriate for transmucosal drug delivery to humans because they may remain liquid at physiological temperature, causing biological fluid dilution and drug formulation wash-off, as well as a brief period of mucosal retention of the drug formulation and decreased therapeutic efficacy [18].
Typically, single phase, sol-like thermosensitive in situ gelling systems are used in aqueous media below the lower critical solution temperature (LCST) [1]. The entropy of mixing decreases above LCST, increasing hydrophobic interactions and allowing the solvated polymer chains to dehydrate quickly. This can result in polymer–polymer interactions and the sol–gel transition [1].
Chitosan/beta-glycerophosphate systems, polyethylene oxide-polypropylene oxide-polyethylene oxide (Poloxamers), and poly(N, N-diethyl acrylamide)-b-poly (ethylene glycol)-b-poly (N, N-diethyl acrylamide) are a few examples of temperature-sensitive drug delivery systems[1], [19], [20].
III. POLYMERS USED IN IN SITU GELLING SYSTEM
When administered as liquids at room temperature, poloxamers are attractive thermo-responsive in situ gelling systems for drug delivery; at physiological temperature, they convert into a gel state for sustained release [21]. Chitin, a naturally occurring polymer present in crab shells, is the source of chitosan, a biodegradable and biocompatible polysaccharide. Because of its special qualities, which include mucoadhesion, low toxicity, and enhanced permeability, chitosan has found extensive use in pharmaceutical and biological applications [22]. An in-situ gelling system using the stable, isotonic, non-irritating Carbopol 940 and HPMC E50 has strong antibacterial activity [23].
A. Pectins
Pectins are polysaccharide groups whose main constituent is a galacturonic α--(1-4)--D- corrosive buildup found in the polymer. The egg carton model describes how the gels are connected to the galaxy-corrosive chains in an aqueous arrangement with low methoxy gelatins (esterification level < 50%) when free carbon particles are present. Gelatinization of gelatine, a source of divalent particles, can happen with the H + particles in mind; primarily, calcium particles are needed to provide the proper gels for transportation. Since pectin is soluble in water, natural solvents are not needed when using it for these flooring. When taken orally, the divalent cations help the pectin reach the gel form in the stomach [24].
B. Guar gum
Another name for guar gum is natural rubber, or guaran, which is made from endosperm seeds. When dissolved in water, lipids, alcohols, esters, ketones, and hydrocarbons, guar becomes insoluble. They spread in both hot and cold climates and are water-soluble in both, helping to minimally frame the colloidal arrangement in both cold and boiling water. The branches of guar gum are employed to form hydrogels in transport systems, nano-microparticles, and network architectures. In addition, branches of guar gum, for instance, bind polymers similar to guar gums, which are linked with polyacrylamide and have superior colon selection qualities [25].
C. Carbopol
The pH of the corrosive polyacrylic polymer (PAA) carbopol has changed from 4,0 to 7,4 [26]. Carbopol turns into a poor consistency gel with soluble pH, but it maintains its structure that makes it useful for acid pH. When HPMC is used with carbopol, the acidity of the layout is reduced and the consistency of the carbopol is improved. After examining several poly (corrosive acrylic) (Carbopol 940-934-941 and 910) 47 forms, he came to the conclusion that Carbopol 940 showed greater look and clarity [27].
D. Gellan gum [28]
Gellan gum, an anionic straight polysaccharide, is produced by the bacterium Sphingomonas elodea. It consists of glucuronic acid, rhamnose, and glucose, which combine to form a tetrasaccharide molecule [28]. Gelrite, a deacetylated form of gellan gum, is obtained by treating the molecule with an alkaline solution to remove the acetyl group. Gelrite exhibits gel-like properties due to the presence of calcium ions[26]. Gelation occurs through complexation with cations and hydrogen bonding with water, resulting in the formation of double-helical structures that aggregate into three-dimensional networks. In the food industry, gellan gum is commonly used as a stabilizing and suspending agent. [26], [29].
E. Alginic acid
When consecutive glucuronic deposits on the alginate chain glucuronic α-L squares are covered by an appropriate approach that weakens the aqueous alginate arrangements, strong gels are produced [30]. Alginic corrosive, which exhibits potent natural properties such biodegradability and non-toxicity, is utilized as an ophthalmic plane vehicle [31].
F. Xanthum gum
Mature Xanthomonas campestris, a gram-negative bacterium, forms the extracellular polysaccharide atomic weight of xanthum gum. The crucial cellulose structure of this subsidiary consists of a β-D-mannose, a β-D-glucuronic α-D-corrosive lateral chain of corrosive glucose chain, and a cellulosic backbone (such as βD-glucose accumulations) [26]. The thickener dissolves in cold, boiling water due to the acidic conditions present. It has exceptional stability under basic circumstances [32].
G. Hydroxy Propyl Methyl Cellulose
Cellulose is a glucan chain with β-(1, 4) -D-glucopyranose as its unit. Certain common polymers, like HPMC, MC, and EC, demonstrate the sensitive evolution of the sol-gel temperature [33]. The cellulose substance thickens with decreasing temperature, while its branches—HPMC and MC, for example—get more consistent with increasing temperature. MC is a typical local polymer based on cellulose that has a chain that exchanges methyl substitution groups. At low temperatures (around 30 °C), the structure becomes fluidized; when the temperature rises to 40–50 °C, gelation takes place [34].
H. Poloxamer
A three-block water-soluble copolymer is called poloxamer. It is composed of two ABA-configured polypropylene oxide (PPO) and two polyethylene oxide (PEO) cores [35].
I. Chitosan
Two types of alterations, such as temperature changes and pH-sensitive changes, can cause chitosan gel formation. A distinctive feature of shrimp and crab shells, chitosan is a thermosensitive, biodegradable polycationic derived from the straightforward deacetylation of chitine [26]. Chitosan is a cationic polymer that can be processed in aquatic environments up to pH 6.2. It is subject to the pH of biocompatibility. Precipitation is induced by removing the hydrated gel and balancing the chitosan aqueous reaction to a pH greater than 6.2 [36].
J. Applications
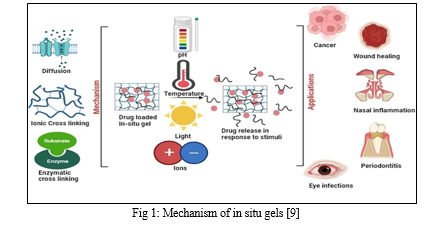
Hydrogels for in situ gelling are adaptable materials having applications in wound healing, tissue engineering, drug delivery, and other medical domains [37]. Chitosan-based in situ gel-forming delivery solutions are preferred because they show little to no toxicity and are both biocompatible and biodegradable [38]. The ability to precisely control the mucoadhesive qualities, drug release profiles, and sol–gel transition temperature of poloxamer-based in situ gels makes it possible to use them as delivery systems for both big and small drug molecules to treat conditions affecting the anterior and posterior portions of the eye [39]. Alginic acid, chitosan, gellan gum, pectin, xyloglucan, carageenan, HPMC, and other hydrophilic and biodegradable polysaccharides have been used to create a variety of better and more palatable in situ topical gels that administer medications in controlled and efficient ways over an extended period of time. These topical gels made of in situ polysaccharides are usually applied via transdermal, ophthalmic, rectal, vaginal, etc. channels [40]. The in situ gel may prove to be an efficient intranasal formulation for Tacrine, as evidenced by its extended nasal residence duration, greater bioavailability, increased brain uptake of the parent drug, and decreased exposure to metabolites [41].
IV. MECHANISM OF IN SITU GEL SYSTEM
Two considerations, such as chemical and physical mechanisms, prepare the in situ gel system for creation [42].
A. Physical mechanism
Based on physical mechanisms, in situ formation consists of the following
- Diffusion
One physical kind that is utilized in the creation of in situ gel is diffusion. By dispersing the solvent from the polymer into the surrounding tissue, the polymer matrix is precipitated or cemented using this method [26]. A common polymer utilized in the creation of in situ gelation systems was N-methylpyrrolidone (NMP) [26].
Matrix system: Hydrogel inert biodegradable polymer matrix with homogeneously disseminated solid active agent
Reservoir devices: The drug is contained in a core (often termed as reservoir) which is surrounded by a rate-controlling polymeric membrane of hydrogel which allows the diffusion of drug
2. Swelling
Swelling is a physical in-situ formulation technique that involves the absorption of fluids by polymers. These polymers swell from the inside to the outside, creating a localized effect [43].
Solvent-activated system When drug diffusion outpaces hydrogel swelling, water molecules infiltrate the polymer network, occupying space within the hydrogel submerged in an aqueous solution. As a result, certain mesh structures within the network expand, allowing additional water molecules to enter [44]. However, swelling is not an indefinite process; the elasticity of the covalently or physically cross-linked network counterbalances its potential for limitless stretching, preventing collapse. This mechanism is commonly employed to simulate drug release from hydroxypropyl methylcellulose (HPMC) hydrogels [43]
Osmotic swelling: In hydrogels, the overall gel swelling pressure remains unaffected by gel pH and swelling duration. However, it may exhibit correlation with factors such as volume fraction, network’s relaxed volume, and cross-link density [45].
B. Chemical Mechanism
The production of in situ gelation is reliant on chemical reaction processes. Additional processes will arise from the chemical reaction that results in gelling in situ [46].
- Enzymatic Bonding: The most effective method for developing the in-situ gelation system is enzyme cross-linking. This procedure usually results in the gel being generated by cross-linking enzymes in bodily fluids
- Cross-linking of Ions: This procedure makes use of the ion-sensitive polymer. Ion-sensitive polymers can undergo a phase shift in the presence of various ions, including Na?, K?, Ca?, and Mg? [28]. Additionally, certain polysaccharides also belong to the ion-sensitive category [28]. Although k-carrageenan forms firmly and modest levels of K+ react to generate fragile gels, elastics form, especially when Ca2 + is present. Gelrite is the main product that Gellan pneumatics are offered in. This anionic polymer is put through an in situ gelation apparatus with both mono- and bivalent cations present [47], [48].
V. ADVANTAGES AND DISADVANTAGES OF IN SITU GELS
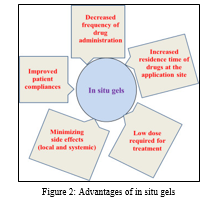
A. Advantages:
- Controlled and Sustained Release
- Ease of Administration
- Suitable for Unconscious Patients
- Reduced Dose Frequency and Toxicity
- Improved Bioavailability [49]
- Biocompatibility and Biodegradation [50], [51], [52]
- Natural polymers have characteristic properties of biocompatibility, biodegradability, and biologically recognizable moieties that support cellular activities
- Synthetic polymers typically possess precisely defined structures that can be tailored to achieve acceptable degradability and desired functionality.
- In situ gels can be designed to demonstrate bio adhesiveness, enabling targeted drug delivery, particularly through mucous membranes, for non-invasive administration [26]
B. Disadvantages: [53], [54]
- High Fluid Requirement
- Susceptibility to Degradation
- Stability Challenges
- Post-Administration Restrictions
- Limited Drug Loading
- Small Dose Compatibility
- Mechanical Weakness [33]
VI. STUDIES ON THE ADMINISTRATION OF BIOACTIVE AGENTS CONTAINING IN SITU GELLING
In-situ gel nasal medication delivery systems because they have a higher systemic bioavailability when administered via the nasal route as opposed to the oral route. A unique dosage form for the nasal distribution of several medications is in-situ gel [55]. When administered orally, medications with a restricted window of absorption in the gastrointestinal tract (GIT) are frequently constrained by low bioavailability as a result of insufficient drug release and brief residence times at the absorption site [56]. In order to provide regulated distribution of pharmaceuticals with extended stomach residence time, novel drug delivery systems in the form of gastro retentive systems, such as floating systems, mucoadhesive, high-density, and expandable, have been created [57]. The effectiveness of local or systemic medications delivered vaginally can be enhanced by mucoadhesive and thermogelling delivery systems, which also extend the formulation's residence time in the vaginal lumen [58]. The next phase in the long tradition of "smart" ocular delivery is starting with the development of in situ gel systems. These systems, as typical eye drops, are easily given into the eye due to their stimuli-responsive phase transition features [59]
The buccal, ocular, nasal and vaginal routes of drug administration have all been used to provide different in situ gelling formulations to different body sites.
TABLE I: Route of Administation of in SITU Gels
|
Route |
Medicinal substance and delivery method |
Clinical outcomes |
Potential Symptoms |
Ref |
|
Buccal |
Systems for thermo- and ion-sensitive in situ gelling that use a combination of hydroxypropyl (HPC) and k-carrageenan (k-CG) and Hibiscus sabdariffa extract |
Based on their rheological synergism and loss tangent values, formulations containing Hibiscus sabdariffa extract demonstrated excellent gelation potential and elasticity; the bioactive extract's presence had no discernible impact on the formulation's mucoadhesiveness. |
treating esophagitis and oral mucositis
|
[60]
|
|
PLGA nanoparticles loaded with moxifloxacin HCl and poloxamer-based thermosensitive gel |
The optimized formulation exhibited improved gelation ease and gel strength, essential for enhanced periodontal retention. In vivo histological analyses of periodontal tissues in treated patients revealed that the novel medication carrier was more biocompatible than the commercially available moxifloxacin product. |
Localized intra-pocket therapy for periodontitis |
[1], [61] |
|
|
Occular |
Thermosensitive gels based on P407/P188 with Dexamethasone nanoparticle loading
|
The gelation temperature of the thermosensitive gel was 32.7 °C, while that of the gel-nanoparticulate samples was 34.3 °C. These formulations exhibited improved corneal permeability, enhanced retention, and controlled drug release. In vivo rabbit trials using the composite nanoparticulate gel demonstrated its superior effectiveness compared to commercial TobraDex eye drops |
Ocular anti-inflammatory activity
|
[62]
|
|
Gelatin-graft-poly(N-isopropylacrylamide) gels loaded with pilocarpine |
The LCST (lower critical solution temperature) of the gelatin-PNIPAAm copolymer was influenced by the number-average molecular weight of the thermoresponsive polymer. Furthermore, the ratio of N-isopropylacrylamide to mercaptoacetic acid played a role in controlling breakdown and managing the pilocarpine release profile. |
Glaucoma treatment
|
[63] |
|
|
Nasal |
A sprayable in situ gelling system has been formulated using fluticasone, pectin, gellan gum, sodium hyaluronate, and Tween 80
|
The optimized formulation demonstrated increased turbinate deposition to the posterior portions of the nasal cavity, a narrower spray cone angle, and improved suspension stability. |
Nasal polyposis, chronic rhinosinusitis, and allergic and nonallergic rhinitis.
|
[1], [64]
|
|
A nasal spray with tranexamic acid with 1.4%–18% beta-glycerophosphate and chitosan |
The gelation temperature and time of the new formulation were 33 °C and 5 min, respectively. In comparison to the control tranexamic solution, it showed six times quicker wound closure and was non-irritating to human nasal epithelium. |
Nasal wound treatment |
[65], [66] |
|
|
Vaginal |
Thermosensitive gels based on Pluronic F127/F68 (20%/10%) containing metronidazole
|
Comparing the optimized Pluronic-based in situ gel to commercial metronidazole gel, it demonstrated a mucodhesive and prolonged drug release profile, was biocompatible, and had a gelation temperature of 28 °C and a viscosity of 2.22 × 105 mPas at 37 °C. |
Bacterial vaginosis treatment
|
[1], [67]
|
|
The glycerophosphate/chitosan lactate (CHILGP) and amoxicillin trihydrate including P407/chitosan lactate (CHILP407) systems |
At 37 °C, CHILGP, with or without simulant vaginal fluid, showed higher storage and loss moduli values than in situ gels based on CHIP407. Additionally, compared to P407-based formulations, CHILGP-based formulations demonstrated higher mucoadhesiveness, antibacterial activity, and wound healing capacity |
Treating vaginal mucositis |
[1], [68] |
Conclusion
In conclusion, the realm of “in situ gelling formulations” represents a dynamic frontier in drug delivery. These systems respond to cues such as pH, temperature, and ionic composition, transforming from free-flowing liquids to cohesive gels. The transition occurs precisely where needed—within the body. The ability to precisely target drug release is a game-changer. In situ gels, when applied to specific sites (such as mucosal surfaces or wounds), undergo gelation. This localized transformation ensures that the drug remains concentrated at the intended location. For instance, an oral gel can adhere to the buccal mucosa, releasing the drug directly into the bloodstream. Similarly, wound gels can provide sustained drug release at the wound site, promoting healing. Mucoadhesive polymers play a crucial role in in situ gelling formulations. These polymers adhere to mucosal surfaces, prolonging drug retention. By enhancing contact time, they improve bioavailability. Imagine a dental gel that adheres to the gums, delivering an antimicrobial agent precisely where needed. Or an ocular gel that clings to the conjunctiva, releasing antibiotics directly onto the infected area. As researchers delve into the clinical properties of in situ gelling systems over the past ten years, we uncover insights that pave the way for innovative biomedical applications. The synergy between science and patient care continues to drive advancements in this field, ultimately benefiting individuals seeking effective yet challenging dosage forms
References
[1] O. M. Kolawole, W. M. Lau, and V. V. Khutoryanskiy, “Chitosan/?-glycerophosphate in situ gelling mucoadhesive systems for intravesical delivery of mitomycin-C,” Int J Pharm X, vol. 1, p. 100007, Dec. 2019, doi: 10.1016/j.ijpx.2019.100007. [2] [2] S. Supper, N. Anton, N. Seidel, M. Riemenschnitter, C. Curdy, and T. Vandamme, “Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications,” Expert Opin Drug Deliv, vol. 11, no. 2, pp. 249–267, Feb. 2014, doi: 10.1517/17425247.2014.867326. [3] M. Kouchak, “In Situ Gelling Systems for Drug Delivery,” Jundishapur J Nat Pharm Prod, vol. 9, no. 3, Jun. 2014, doi: 10.17795/jjnpp-20126. [4] X. Yang, G. Zhang, and D. Zhang, “Stimuli responsive gels based on low molecular weight gelators,” J. Mater. Chem., vol. 22, no. 1, pp. 38–50, 2012, doi: 10.1039/C1JM13205A. [5] K. Haraguchi, K. Murata, and T. Takehisa, “Stimuli-Responsive Nanocomposite Gels and Soft Nanocomposites Consisting of Inorganic Clays and Copolymers with Different Chemical Affinities,” Macromolecules, vol. 45, no. 1, pp. 385–391, Jan. 2012, doi: 10.1021/ma202114z. [6] B. Vigani, S. Rossi, G. Sandri, M. C. Bonferoni, C. M. Caramella, and F. Ferrari, “Recent Advances in the Development of In Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes,” Pharmaceutics, vol. 12, no. 9, p. 859, Sep. 2020, doi: 10.3390/pharmaceutics12090859. [7] F. Zahir-Jouzdani, J. D. Wolf, F. Atyabi, and A. Bernkop-Schnürch, “In situ gelling and mucoadhesive polymers: why do they need each other?,” Expert Opin Drug Deliv, vol. 15, no. 10, pp. 1007–1019, Oct. 2018, doi: 10.1080/17425247.2018.1517741. [8] M. Rizwan et al., “pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications,” Polymers (Basel), vol. 9, no. 12, p. 137, Apr. 2017, doi: 10.3390/polym9040137. [9] A. Garg, R. Agrawal, C. Singh Chauhan, and R. Deshmukh, “In-situ gel: A smart carrier for drug delivery,” Int J Pharm, vol. 652, p. 123819, Mar. 2024, doi: 10.1016/j.ijpharm.2024.123819. [10] I.-S. Kim and I.-J. Oh, “Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly(acrylic acid),” Arch Pharm Res, vol. 28, no. 8, pp. 983–987, Aug. 2005, doi: 10.1007/BF02973887. [11] T. Nandgude, R. Thube, N. Jaiswal, P. Deshmukh, V. Chatap, and N. Hire, “Formulation and Evaluation of pH Induced In-situ Nasal Gel of Salbutamol Sulphate,” International Journal of Pharmaceutical Sciences and Nanotechnology, vol. 1, no. 2, pp. 177–183, Jan. 1970, doi: 10.37285/ijpsn.2008.1.2.9. [12] V. B. Bueno, R. Bentini, L. H. Catalani, and D. F. S. Petri, “Synthesis and swelling behavior of xanthan-based hydrogels,” Carbohydr Polym, vol. 92, no. 2, pp. 1091–1099, Feb. 2013, doi: 10.1016/j.carbpol.2012.10.062. [13] P.-L. Destruel et al., “Novel in situ gelling ophthalmic drug delivery system based on gellan gum and hydroxyethylcellulose: Innovative rheological characterization, in vitro and in vivo evidence of a sustained precorneal retention time,” Int J Pharm, vol. 574, p. 118734, Jan. 2020, doi: 10.1016/j.ijpharm.2019.118734. [14] A. A. Al-Kinani, G. Zidan, N. Elsaid, A. Seyfoddin, A. W. G. Alani, and R. G. Alany, “Ophthalmic gels: Past, present and future,” Adv Drug Deliv Rev, vol. 126, pp. 113–126, Feb. 2018, doi: 10.1016/j.addr.2017.12.017. [15] S. Zhou et al., “Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment,” Gels, vol. 8, no. 8, p. 508, Aug. 2022, doi: 10.3390/gels8080508. [16] L. E. Agibayeva et al., “Gellan gum and its methacrylated derivatives as in situ gelling mucoadhesive formulations of pilocarpine: In vitro and in vivo studies,” Int J Pharm, vol. 577, p. 119093, Mar. 2020, doi: 10.1016/j.ijpharm.2020.119093. [17] A. Fernández-Ferreiro et al., “In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 94, pp. 342–351, Aug. 2015, doi: 10.1016/j.ejpb.2015.06.003. [18] H.-G. Choi, Y.-K. Oh, and C.-K. Kim, “In situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability,” Int J Pharm, vol. 165, no. 1, pp. 23–32, Apr. 1998, doi: 10.1016/S0378-5173(97)00385-2. [19] P. J. Haddow et al., “Polymer Architecture Effects on Poly(N,N?Diethyl Acrylamide)?b?Poly(Ethylene Glycol)?b?Poly(N,N?Diethyl Acrylamide) Thermoreversible Gels and Their Evaluation as a Healthcare Material,” Macromol Biosci, vol. 22, no. 3, Mar. 2022, doi: 10.1002/mabi.202100432. [20] B. Shriky et al., “Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development,” J Colloid Interface Sci, vol. 565, pp. 119–130, Apr. 2020, doi: 10.1016/j.jcis.2019.12.096. [21] H. Abdeltawab, D. Svirskis, and M. Sharma, “Formulation strategies to modulate drug release from poloxamer based in situ gelling systems,” Expert Opin Drug Deliv, vol. 17, no. 4, pp. 495–509, Apr. 2020, doi: 10.1080/17425247.2020.1731469. [22] S. A. A. A. Hard, H. N. Shivakumar, and M. A. M. Redhwan, “Development and optimization of in-situ gel containing chitosan nanoparticles for possible nose-to-brain delivery of vinpocetine,” Int J Biol Macromol, vol. 253, p. 127217, Dec. 2023, doi: 10.1016/j.ijbiomac.2023.127217. [23] S. P. Ahirrao, D. S. Bhambere, B. Agiwale, S. Algat, D. Zoman, and V. Chaudhari, “pH sensitive in-situ gel for ophthalmic delivery of ofloxacin and dexamethasone sodium phosphate: Formulation, development, and evaluation,” Mater Today Proc, Jul. 2023, doi: 10.1016/j.matpr.2023.07.161. [24] O. Séchoy, G. Tissié, C. Sébastian, F. Maurin, J.-Y. Driot, and C. Trinquand, “A new long acting ophthalmic formulation of Carteolol containing alginic acid,” Int J Pharm, vol. 207, no. 1–2, pp. 109–116, Oct. 2000, doi: 10.1016/S0378-5173(00)00539-1. [25] S. Cohen, E. Lobel, A. Trevgoda, and Y. Peled, “A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye,” Journal of Controlled Release, vol. 44, no. 2–3, pp. 201–208, Feb. 1997, doi: 10.1016/S0168-3659(96)01523-4. [26] S. Srivastava, “Role Of In Situ Gel in Drug Delivery System,” International Journal of Pharmaceutical Research and Applications, vol. 5, no. 2, p. 449, 2008, doi: 10.35629/7781-0502449461. [27] G. T. Grant, E. R. Morris, D. A. Rees, P. J. C. Smith, and D. Thom, “Biological interactions between polysaccharides and divalent cations: The egg?box model,” FEBS Lett, vol. 32, no. 1, pp. 195–198, May 1973, doi: 10.1016/0014-5793(73)80770-7. [28] S. Y. Satav, S. D. Navale, and M. M. Thakare, “An Overview on In-situ Gel Introduction,” Int J Pharmtech Res, vol. 2022, no. 1, pp. 7–17, doi: 10.20902/IJPTR.2022.150102. [29] B. K. Nanjawade, F. V. Manvi, and A. S. Manjappa, “RETRACTED: In situ-forming hydrogels for sustained ophthalmic drug delivery,” Journal of Controlled Release, vol. 122, no. 2, pp. 119–134, Sep. 2007, doi: 10.1016/j.jconrel.2007.07.009. [30] Y. Wu et al., “Research progress of in-situ gelling ophthalmic drug delivery system,” Asian J Pharm Sci, vol. 14, no. 1, pp. 1–15, Jan. 2019, doi: 10.1016/j.ajps.2018.04.008. [31] A. A. Hajare, “A rational approach to ocular drug delivery systems: a overview.” [Online]. Available: www.wjpps.com [32] Y. Ramesh, C. B. Kothapalli, and J. R. P. Reddigari, “A NOVEL APPROACHES ON OCULAR DRUG DELIVERY SYSTEM,” Journal of Drug Delivery and Therapeutics, vol. 7, no. 6, Nov. 2017, doi: 10.22270/jddt.v7i6.1512. [33] “25”. [34] C. Goda, “OCULAR DRUG DELIVERY: A REVIEW,” 2010. [Online]. Available: https://www.researchgate.net/publication/235924442 [35] T. R. Hoare and D. S. Kohane, “Hydrogels in drug delivery: Progress and challenges,” Polymer (Guildf), vol. 49, no. 8, pp. 1993–2007, Apr. 2008, doi: 10.1016/j.polymer.2008.01.027. [36] T. P. Pandya, M. K. Modasiya, and V. M. Patel, “INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES Opthalmic In-Situ Gelling System,” Int. J. of Pharm. & Life Sci. (IJPLS), vol. 2, no. 5, pp. 730–738, 2011. [37] J. T. Orasugh, D. Chattopadhayay, S. S. Ray, and A. Adhikari, “Commercially available in situ gelling hydrogels for biomedical applications,” in Stimuli-Responsive Hydrogels for Ophthalmic Drug Delivery, Elsevier, 2024, pp. 589–602. doi: 10.1016/B978-0-323-99156-8.00021-6. [38] S. S. Das, P. Bharadwaj, S. K. Singh, P. R. P. Verma, and S. Beg, “In situ gelling systems of chitosan for potential drug delivery applications,” in Chitosan in Drug Delivery, Elsevier, 2022, pp. 195–224. doi: 10.1016/B978-0-12-819336-5.00010-8. [39] K. A. Soliman, K. Ullah, A. Shah, D. S. Jones, and T. R. R. Singh, “Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications,” Drug Discov Today, vol. 24, no. 8, pp. 1575–1586, Aug. 2019, doi: 10.1016/j.drudis.2019.05.036. [40] A. K. Nayak and H. Bera, “In situ polysaccharide-based gels for topical drug delivery applications,” in Polysaccharide Carriers for Drug Delivery, Elsevier, 2019, pp. 615–638. doi: 10.1016/B978-0-08-102553-6.00021-0. [41] S. Qian, Y. C. Wong, and Z. Zuo, “Development, characterization and application of in situ gel systems for intranasal delivery of tacrine,” Int J Pharm, vol. 468, no. 1–2, pp. 272–282, Jul. 2014, doi: 10.1016/j.ijpharm.2014.04.015. [42] A. K. Burkoth and K. S. Anseth, “A review of photocrosslinked polyanhydrides:,” Biomaterials, vol. 21, no. 23, pp. 2395–2404, Dec. 2000, doi: 10.1016/S0142-9612(00)00107-1. [43] S. Jacob and A. Mathew, “ORAL IN-SITU GELLING SYSTEM-A REVIEW.” [44] F. Ganji and E. Vasheghani-Farahani, “Hydrogels in Controlled Drug Delivery Systems,” 2009. [Online]. Available: www.SID.ir [45] A. Padhan, B. K. Nanda, B. C. Behera, and Y. Nath, “FLOATING ORAL IN-SITU GEL, A COMPREHENSIVE APPROACH OF GASTRO-RETENTIVE DRUG DELIVERY SYSTEM: A REVIEW,” Int J Pharm Sci Res, vol. 10, no. 9, p. 4026, 2019, doi: 10.13040/IJPSR.0975-8232.10(9).4026-39. [46] Y. Qiu and K. Park, “Environment-sensitive hydrogels for drug delivery,” Adv Drug Deliv Rev, vol. 53, no. 3, pp. 321–339, Dec. 2001, doi: 10.1016/S0169-409X(01)00203-4. [47] S. Miyazaki, N. Kawasaki, W. Kubo, K. Endo, and D. Attwood, “Comparison of in situ gelling formulations for the oral delivery of cimetidine,” Int J Pharm, vol. 220, no. 1–2, pp. 161–168, Jun. 2001, doi: 10.1016/S0378-5173(01)00669-X. [48] H.-R. Lin and K. C. Sung, “Carbopol/pluronic phase change solutions for ophthalmic drug delivery,” Journal of Controlled Release, vol. 69, no. 3, pp. 379–388, Dec. 2000, doi: 10.1016/S0168-3659(00)00329-1. [49] S. Gotmare, R. Khope, N. Padole, N. Dhoble, P. Dhapke, and J. Baheti, “IN-SITU GEL: A NOVEL APPROACH FOR CENTRAL NERVOUS SYSTEM DISORDERS,” Certified Journal ? 1834 Gotmare et al. World Journal of Pharmacy and Pharmaceutical Sciences WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor, vol. 12, pp. 1834–1870, 2015, doi: 10.20959/wjpps20234-24466. [50] B. Srividya, R. M. Cardoza, and P. D. Amin, “Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system,” Journal of Controlled Release, vol. 73, no. 2–3, pp. 205–211, Jun. 2001, doi: 10.1016/S0168-3659(01)00279-6. [51] S. Cohen, E. Lobel, A. Trevgoda, and Y. Peled, “A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye,” Journal of Controlled Release, vol. 44, no. 2–3, pp. 201–208, Feb. 1997, doi: 10.1016/S0168-3659(96)01523-4. [52] B. V. Aiwale, B. P. Chaudhari, and V. K. Redasani, “A Review on Polymers for In situ Drug Delivery System,” Asian Journal of Research in Pharmaceutical Sciences, pp. 77–80, Mar. 2024, doi: 10.52711/2231-5659.2024.00012. [53] S. Khatry, S. Vodithala, N. Shastri, and M. Sadanandam, “Formulation and evaluation of ion activated ocular gels of ketorolac tromethamine,” 2010. [Online]. Available: https://www.researchgate.net/publication/236170643 [54] W.-D. Ma, H. Xu, C. Wang, S.-F. Nie, and W.-S. Pan, “Pluronic F127-g-poly(acrylic acid) copolymers as in situ gelling vehicle for ophthalmic drug delivery system,” Int J Pharm, vol. 350, no. 1–2, pp. 247–256, Feb. 2008, doi: 10.1016/j.ijpharm.2007.09.005. [55] R. B. Saudagar and M. M. Kulkarni, “Review on in-situ nasal gel drug delivery system,” Res J Pharm Technol, vol. 10, no. 6, p. 1870, 2017, doi: 10.5958/0974-360X.2017.00328.6. [56] S. Gajanan Kadam, A. A. Harsulkar, V. P. Patange, R. R. Suryawanshi, S. D. Narwade, and A. R. Kadam, “Review Article SSN 2277-7105 www,” Certified Journal ? Kadam et al. World Journal of Pharmaceutical Research 253 World Journal of Pharmaceutical Research SJIF Impact Factor 8.084, vol. 12, no. 7, pp. 253–275, 2015, doi: 10.20959/wjpr20237-27959. [57] R. Bashir, A. Majeed, T. Ali, S. Farooq, and N. A. Khan, “Floating Oral In-Situ Gel: A Review,” Journal of Drug Delivery and Therapeutics, vol. 9, no. 2, pp. 442–448, Mar. 2019, doi: 10.22270/jddt.v9i2.2372. [58] C. M. Caramella, S. Rossi, F. Ferrari, M. C. Bonferoni, and G. Sandri, “Mucoadhesive and thermogelling systems for vaginal drug delivery,” Adv Drug Deliv Rev, vol. 92, pp. 39–52, Sep. 2015, doi: 10.1016/j.addr.2015.02.001. [59] A. K. Agrawal, M. Das, and S. Jain, “In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery,” Expert Opin Drug Deliv, vol. 9, no. 4, pp. 383–402, Apr. 2012, doi: 10.1517/17425247.2012.665367. [60] B. Vigani et al., “Development of a Mucoadhesive and an in Situ Gelling Formulation Based on ?-Carrageenan for Application on Oral Mucosa and Esophagus Walls. II. Loading of a Bioactive Hydroalcoholic Extract,” Mar Drugs, vol. 17, no. 3, p. 153, Mar. 2019, doi: 10.3390/md17030153. [61] A. A. Kassem, F. A. Ismail, V. F. Naggar, and E. Aboulmagd, “Comparative Study to Investigate the Effect of Meloxicam or Minocycline HCl In Situ Gel System on Local Treatment of Periodontal Pockets,” AAPS PharmSciTech, vol. 15, no. 4, pp. 1021–1028, Aug. 2014, doi: 10.1208/s12249-014-0118-7. [62] Y. Wen et al., “A potential nanoparticle-loaded in situ gel for enhanced and sustained ophthalmic delivery of dexamethasone,” Nanotechnology, vol. 29, no. 42, p. 425101, Oct. 2018, doi: 10.1088/1361-6528/aad7da. [63] J.-Y. Lai, “Biodegradable in situ gelling delivery systems containing pilocarpine as new antiglaucoma formulations: effect of a mercaptoacetic acid/N-isopropylacrylamide molar ratio,” Drug Des Devel Ther, p. 1273, Oct. 2013, doi: 10.2147/DDDT.S53759. [64] L. Niži? et al., “Innovative sprayable in situ gelling fluticasone suspension: Development and optimization of nasal deposition,” Int J Pharm, vol. 563, pp. 445–456, May 2019, doi: 10.1016/j.ijpharm.2019.04.015. [65] H. Gholizadeh et al., “Application of a Thermosensitive In Situ Gel of Chitosan-Based Nasal Spray Loaded with Tranexamic Acid for Localised Treatment of Nasal Wounds,” AAPS PharmSciTech, vol. 20, no. 7, p. 299, Oct. 2019, doi: 10.1208/s12249-019-1517-6. [66] H. Zhang, Y. Jiang, Y. Liu, and Q. Li, “Chromatic aberration and decay resistance performance of bamboo and bamboo scrimber decayed by white-rot and brown-rot fungi,” Wood Mater Sci Eng, vol. 18, no. 6, pp. 1841–1847, Nov. 2023, doi: 10.1080/17480272.2023.2193822. [67] E.-S. Ibrahim, S. Ismail, G. Fetih, O. Shaaban, K. Hassanein, and N. Abdellah, “Development and characterization of thermosensitive pluronic-based metronidazole in situ gelling formulations for vaginal application,” Acta Pharmaceutica, vol. 62, no. 1, pp. 59–70, Mar. 2012, doi: 10.2478/v10007-012-0009-y. [68] S. Rossi et al., “Comparison of poloxamer- and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis,” Drug Dev Ind Pharm, vol. 40, no. 3, pp. 52–360, Mar. 2014, doi: 10.3109/03639045.2012.762654
Copyright
Copyright © 2024 Maithili Brahmavale, Pooja Bhopi, Shraddha Bhavsar, Sakshi Bramhane, Murshid Bubere, Nilesh Bonde. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET62471
Publish Date : 2024-05-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

