Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Fuel Extraction from Plastic Waste
Authors: Harsh Panchal, Shreyas Baraskar, Prof. Sandeep Sabnis
DOI Link: https://doi.org/10.22214/ijraset.2025.66489
Certificate: View Certificate
Abstract
The extraction of fuel from plastic waste through the pyrolysis process offers a promising solution to address both environmental concerns and energy needs. In this project, a horizontal furnace setup is employed to conduct pyrolysis, utilizing a quartz tube containing small plastic pieces. The process involves raising the temperature within the furnace to initiate pyrolysis, leading to the breakdown of plastic polymers into valuable fuel components. The extracted fuel is subsequently subjected to testing to evaluate its properties. Through this innovative approach, we aim to demonstrate the feasibility and effectiveness of converting plastic waste into a valuable energy resource, contributing to sustainable waste management and reducing reliance on fossil fuels. This project is driven by the urgent need to reduce the harm that plastic waste is causing to marine life and ecosystems. Millions of tonnes of plastic are produced each year, making the disposal of plastic waste an urgent global concern. Using pyrolysis to turn plastic trash into useful fuel resources, this project aims to reduce environmental damage and promote sustainable energy sources.
Introduction
I. NOMENCLATURE
- Pyrolysis - the degradation of organic compounds in the absence of oxygen at temperatures ranging from 500?C to 800?C, with vapor residence durations ranging from 3 to 1500s
- Polytetrafluoroethylene (FYFFE) - a fluoropolymer and is commonly known by its trade name, Teflon®. Unique properties of PTFE include nonreactivity, hydrophobicity, a low coefficient of friction, and good insulating properties
- Polyethylene (PE)- lightweight thermoplastics that are resistant to chemicals and moisture and are used especially in packaging, insulation, surgical implants, prostheses, and tubing.
- Polyethylene terephthalate (PET or PETE) - a strong, stiff synthetic liber and resin and a member of the polyester family of polymers.
- Benzoic acid - an organic compound which is described by the chemical formula C6H5COOH.
- Zinc oxide - The most appropriate catalyst for diminishing the time and temperature requirement for biodiesel synthesis.
- Fourier-transform infrared spectroscopy (FTIR) - a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas.
II. INTRODUCTION
Plastics, as organic compounds composed of long-chained hydrocarbons derived from petroleum products, have experienced a rapid surge in popularity due to their unique properties. The widespread adoption of plastics can be attributed to factors such as their affordability, non-degradable nature, easy accessibility, versatile applications, and overall convenience. This has led to a significant increase in both plastic production and consumption rates globally.
According to the information provided by the book on Swachh Bharat Mission SBM Urban published by the Government of India, approximately 9.4 million Tonnes per annum of plastic waste is generated in India, which amounts to 26,000 Tonnes Per Day. of this, about 60% is recycled, most of it by the informal sector. While the recycling rate in India is considerably higher than the global average of 20%, there is still over 9,400 tonnes of plastic waste which is either landfilled or ends up polluting streams or groundwater resources.
To overcome these problems the project titled "Extraction of Fuel from Plastic Waste through Pyrolysis" presents an innovative approach to addressing two pressing global challenges: plastic pollution and dependence on fossil fuels. With the increasing production of plastic waste and its detrimental impact on the environment, there is an urgent need for sustainable solutions. Simultaneously, the depletion of fossil fuel reserves underscores the importance of exploring alternative sources of energy. This project proposes a method wherein plastic waste is converted into fuel through the process of pyrolysis.
Pyrolysis is a chemical process that involves the decomposition of organic materials at elevated temperatures in the absence of oxygen.
During pyrolysis, the organic material is heated to a high temperature, typically ranging from to 900 degrees Celsius (572 to 1652 degrees Fahrenheit), causing it to break down into simpler molecules. In the absence of oxygen, the organic material does not combust but instead undergoes thermal decomposition. As a result, the original material is converted into a mixture of gases, liquids, and solid residues. These products can vary depending on factors such as the temperature, heating rate, and composition of the material being pyrolyzed. Pyrolysis is used in various applications, including the production of biochar from biomass, the conversion of organic waste into biofuels; and the decomposition of plastic waste to produce fuel or feedstock for Other chemical processes. It is considered an important process for waste management and the production of renewable energy resources.
III. MATERIALS AND METHODS
A. Materials
1) Horizontal furnace: material used in furnace is stainless steel which has an ability to sustain high temperature.

Fig. 1 Horizontal furnace
2) Quartz tube: A quartz tube is made from the quartz glass, which is known for its high temperature resistance (1200?C) & chemical inertness.

Fig. 2 Quartz tube
3) Flanges: materials used for flanges is MS (mild steel). Teflon is a material that sustain high temperature.

Fig. 3 flanges and Teflon rings
4) Gasket: The material used in the gasket is Polytetrafluoroethylene (PTFE) which can sustain a temperature of 315?C.

Fig. 4 Fig. Gasket
5) Water Tube condenser: Glass tube condensers efficiently transfer heat by using water as a coolant which is supplied through it

Fig. 5 Water tube condenser
6) Submersible pump: For circulation, purposes a submersible pump is sussed which has a maximum lift of 6 feet (approximately 1.85 m).

Fig. 6 Submersible Pump
B. Experimental Setup
The main Components Consist are:
- Horizontal Furnace with Quartz tube
- Submersible pump
- Glass tube Condenser

Fig. 7 Experimental setup

Fig. 8 Practical Setup with Solidworks model of Furnace with tube & flanges
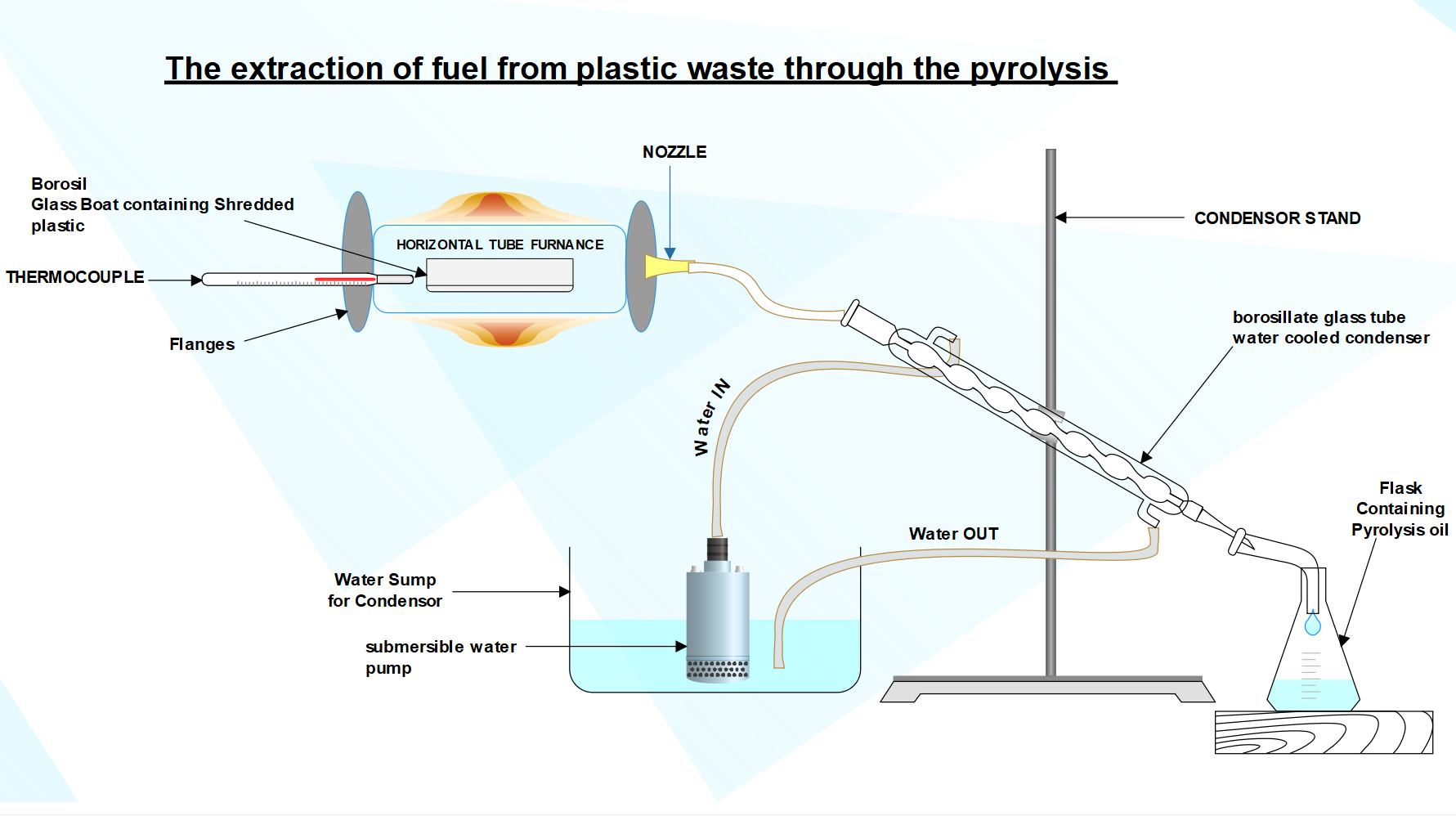
Fig. 9 Schematic diagram of pyrolysis setup
C. Procedure
The Experimental setup describes a process for pyrolyzing plastic material (Polyethylene Terephthalate & Polyethylene to convert it into gas and condensing that gas back into liquid form for collection and analysis of various properties like calorific value & flash point.
The Process consist of various Step which are as follows:
- Shredded plastic materials of 3-5 mm in size are firstly placed in the glass boat. This step ensures uniform heating and conversion of plastic into gas.
- Further the glass boat containing the shredded plastic is placed in the quartz tube. The quartz tube will provide a controlled environment for heating of plastic samples in absence of oxygen.
- Both the ends of the quartz tube are sealed by using gasket & flanges and tightened by nuts & bolts. This sealing will make sure that the process will occur in the closed system, preventing escape of gas & maintaining constant temperature.
- The supply is turned ON and the system is heated around 470?C and using a thermocouple which will measure the
 temperature inside the tube. At this temperature the plastic will undergo Pyrolysis, converting plastic into the gas.
temperature inside the tube. At this temperature the plastic will undergo Pyrolysis, converting plastic into the gas. - The gas produced during the pyrolysis process is further passed through the nozzle which is provided on the other side of the flange. This nozzle will direct the gas into the condenser. In the condenser the gas is cooled and condensed back into the liquid form.
- The condensed liquid is collected in the beaker kept on the other end of the condenser. The liquid collected in the beaker represents the condensed form of the gas produced during plastic pyrolysis and the carbon residue form remains inside the boat.
- The collected liquid samples represent the pyrolyzed products of Polyethylene Terephthalate (PET) & Polyethylene (PE) plastics, and then are subjected to various tests to determine their properties. The test may consist of measuring calorific value, density, flash point, composition analysis and other properties.
- To facilitate the condensation, process a pump recirculates the water through the condenser. This water flow helps efficiently cool the gas and further convert the gas into liquid form.
In short, our setup allows for controlled pyrolysis of the plastic material, followed by its collection and analysis of the resulting liquid products. so, by comparing those liquid Form from the different types of plastic, we can gain insights into its chemical composition & their applications.
IV. RESULTS AND DISCUSSION
A series of experiments conducted to evaluate the influence of catalysts and plastic types on pyrolysis for polyfuel production. Four distinct experiments were performed, encompassing two plastic types (Polyethylene and Polyethylene Terephthalate) With  and Without the addition of a Zinc Oxide catalyst. The outcomes are detailed for the quantity of polyfuel generated, and the temperature profiles over time for each experiment. These results are subsequently interpreted to reveal the effects of catalysts and plastic types on the pyrofuel production process and temperature control within the reactor. By Conducting the test various result has been achieved.
and Without the addition of a Zinc Oxide catalyst. The outcomes are detailed for the quantity of polyfuel generated, and the temperature profiles over time for each experiment. These results are subsequently interpreted to reveal the effects of catalysts and plastic types on the pyrofuel production process and temperature control within the reactor. By Conducting the test various result has been achieved.
A. From the Test 1
Plastic Used: Polyethylene Terephthalate (PET)
Temperature: 420? C
Duration: 30 minutes
Quantity of Plastic: 88.8 Grams
Weight or Boat: 692 Grams
Products Formed:
- Yellow Powder (Benzoic Acid): PET plastic Will undergo a pyrolysis process at 470?C resulting in the formation of benzoic acid. This powder is likely a by- product of the oxidation of evaporated matter during the pyrolysis process. This Acid can be used in the food, chemicals & pharmaceuticals etc.
- Carbon residue: Along With the yellow powder, a carbon residue is also formed. this residue is the solid carbonaceous material which is left behind after the volatile components of the plastic has been driven off.

Fig.9 Products obtained from test 1.
B. From The Test 2
Plastic Used: Polyethylene Terephthalate (PET) With addition of Zinc Oxide catalyst
Temperature: 570? C
Duration: 1 hour
Quantity of Plastic: 88.8 Grams
Weight of Boat: 69.2
Grams Products Formed:
1) Molten Gel (WAX)
- The Combination of PET plastic With Zinc Oxide Catalyst under high temperature (570?C) & duration (l Hour) leads to formation of molten gel.
- The high temperature & long exposure time cause PET molecules to undergo extensive thermal degradation resulting in formation of viscous, molten wax.
- The presence of Zinc Oxide catalyst facilitates chemical reaction during the pyrolysis process, contributing to formation of molten gel.
2) Residue Of Zinc Oxide Catalyst
- Along with the formation of molten gel, a residue of zinc oxide catalyst is also present. The residue of this catalyst may be observed as solid deposits after the pyrolysis process is completed
- This residue indicates that while some of the catalyst precipitated in facilitating the pyrolysis reactions, the remaining unchanged portion is partially reacted leading to its presence in the final product mixture.
Fig. 10 Products got from test 2
C. From The Test 3
Plastic Used: Polyethylene Terephthalate (PET) plastic (Oil Cans) With addition of Zinc Oxide catalyst
Temperature: 420? C
Duration: 45 minutes
Quantity of Plastic: 88.8 Grams
Weight of Boat: 69.2 Grams
Products Formed:
1) Liquid Wax
- In Test 3, PET plastic undergoes Pyrolysis leading to the formation of a liquid substance that resembles wax.
- The liquid wax formed in this process is likely to have similar properties to the molten gel, which was observed in Test 2, but its consistency Will vary depending on the temperature & duration of the exposure.
- After some time, the liquid wax produced during test 3 undergoes solidification. the solidification occurs as the liquid wax cools down to room temperature, causing the wax molecules to arrange themselves as solid structure forms

Fig. 11 Products got from test 3.
D. From The Test 4
Plastic Used: Polyethylene (PE) With the addition of Zinc Oxide catalyst
Temperature: 350? C
Duration: 30 min
Quantity of Plastic: 14 Grams
Weight of Boat: 55.8 Grams
Product formed:
1) Wax Formation
The PE plastic undergoes pyrolysis in the presence of a zinc oxide catalyst at a temperature of around 350?C for a duration of 30 minutes. Here the long-chain hydrocarbon molecules break down into smaller hydrocarbons.
E. FTIR Analysis Pyrolysis-Derived Wax: Characterizing Chemical Composition and Structure
Fourier Transform Infrared Spectroscopy (FTIR) operates based on the principle of molecular interaction with infrared radiation. When a material is subjected to infrared light, its chemical bonds absorb frequencies of light, resulting in characteristic patterns of absorption. These absorption patterns are unique to different functional groups and Chemical bonds present in the material.
For the wax obtained from pyrolysis Oil, FTIR analysis provides us with valuable insights into its chemical composition and structure. Specifically, FTIR helps identify the types of chemical bonds present in the wax, such as hydrocarbons, esters, and oxygen-containing functional groups. This information is crucial for understanding the properties and potential applications of the wax. During FTIR analysis of the wax sample, obtain a spectrum showing absorption peaks at wavelengths. Each peak corresponds to the absorption of infrared light by particular chemical bonds within the sample. By comparing these absorption peaks to reference spectra or databases, researchers can identify the functional groups and chemical bonds present in the wax.
Ultimately, the F-TIR Spectrum of the wax from pyrolysis Oil Will provide valuable information about its chemical composition, allowing researchers to better understand its properties and potential uses. This knowledge can inform decisions regarding further processing, refining, or application of the wax in various industries, such as energy, materials, or chemicals.
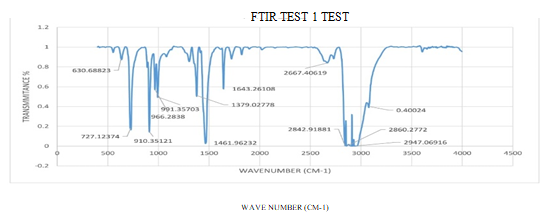
Fig. 12 FTIR Test readings
TABLE I
TYPES OF CHEMICAL BONDS PRESENT IN THE WAX
|
FTIR of wax from HDPE |
||
|
Peak |
Wave No. (cm-1) |
Functional Groups |
|
1 |
630.68823 |
CH=CH (cis) |
|
2 |
727.12374 |
CH=CH (cis) |
|
3 |
910.35121 |
CH=CH(Trans) |
|
4 |
966.2838 |
CH=CH(Trans) |
|
5 |
1379.02778 |
CH3 (Methyl) |
|
6 |
1461.96232 |
CH3 (Methyl) |
|
7 |
1643.26108 |
Amines |
|
8 |
2860.28772 |
C-CH3 |
|
9 |
2947.06916 |
C-CH |
Conclusion
The experiment\'s results demonstrate the efficiency of Zinc Oxide as a catalyst in the process. It is evident that Zinc Oxide is a highly effective catalyst, as indicated by several key observations. Firstly, its use leads to a notable increase in the heating rate, facilitating the more rapid breakdown of plastic. This enhanced breakdown results in a substantial rise in the quantity of fuel produced When compared to experiments Without the catalyst. Furthermore, the addition of Zinc Oxide significantly reduces the reaction time by approximately 30 minutes in each experiment, underscoring its role in expediting the process. Additionally, the presence of the catalyst contributes to a reduction in the amount of residue left inside the reactor, further highlighting its beneficial effects. In the investigation of different plastics, it is evident that the choice of plastic has a substantial impact on the experimental outcomes. Polyethylene (PE) outperforms Polyethylene Terephthalate (PET) in several aspects. PE yields a larger quantity of fuel, making it a more efficient choice for fuel production. PET, on the other hand, requires more time to break down within the reactor, resulting in an extended reaction time. In contrast, PE undergoes a quicker breakdown, leading to a shorter reaction time. Additionally, the residue left inside the reactor is considerably less When using PE in comparison to PET. Furthermore, while PET produces less fuel in terms of quantity, the fuel it generates is denser. However, this denser fuel burns at a slower rate than the fuel produced from PE. In conclusion, Zinc Oxide proves to be an effective catalyst for the experiment, enhancing the heating rate, fuel quantity, and reaction time while reducing residue. The choice of plastic, With PE outperforming PET in terms of fuel quantity and efficiency, is a crucial factor influencing the experimental outcomes. These findings have significant implications for processes involving plastic conversion to fuel, offering insights into catalyst selection and deselection
References
[1] Ministry of Housing & Urban Affairs Government of India, March 2019, \"Plastic Waste: A Global Concern\", \"Plastic Waste Management Issues, Solutions & Case Studies\" PP 1-2. [2] Chittaranjan Tembhekar, June 16, 2023, The Times of India, \"Plastic use: Mumbai casts a tight net, yet half of its waste slips through\" [3] Arvind Kumar, 2016, \"PREPARATION OF WAX FROM WASTE PLASTIC”, Department of Chemical Engineering, National Institute of Technology Rourkela [4] \"Wastetireoil.com\", \"What is pyrolysis process, Pyrolysis Plant\", last modified November 14, 2017, https://www.wastetireoil.com/pyrolysis_faq/pyrolysis_ PlantJpyrolysis-products-compostion-284.html [5] \"Maria do Carmo Rangel, Francieli Martins Mayer, Mateus da Silva Carvalho, Giovanni Saboia, Arthur Motta de Andrade\", 10 January, 2023, \"Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass\", pp. 36-45 [6] Vijaykumar B. Chanashetty and B.M. Patil, \"Fuel from Plastic Waste\", International Journal on Emerging Technologies (Special Issue on NCRIET 2015). [7] Chythenyen devika Kulasekaran, “how plastic caused fire in affected air, water, Soil life in Brahmapuram”, 28 March, 2023. [8] Yelizabeth Royte, Is burning plastic a good idea?, National Geographic, March 12, 2019. [9] Kundan Kumar Jha, T. T. M. Kannan, and Ashutosh Das, “Fuel from Plastic Waste: A Review”, Research Gate Publication, October 2020. [10] Kundan Kumar Jha, T.T.M. Kannan “Recycling of plastic waste into fuel by pyrolysis - a review”, Research Gate Publication, November 2020. [11] Sanjay Singh, “KINETICS OF PYROLYSIS OF AGRICULTURAL CROP RESIDUE AND CO-PYROLYSIS OF AGRICULTURAL CROP RESIDUE AND PLASTIC WASTE VIATHERMOGRAVIMETRIC ANALYSIS”, DEPARTMENT OF CHEMICAL ENGINEERING”, Motilal Nehru National Institute of Technology Allahabad, Prayagraj, Uttar Pradesh, INDIA, JULY 2021. [12] (Dr.) R.K. Singh, “PREPARATION OF WAX FROM WASTE PLASTIC”, Department of Chemical Engineering, National Institute of Technology Rourkela, 2016. [13] Johan E. Hustad “Review and Design Overview of Plastic Waste-to-Pyrolysis Oil Conversion with Implications on the Energy Transition”, Hindawi Journal of Energy, Volume 2023, Article ID 1821129, 25 pages, https://doi.org/10.1155/2023/1821129, 22 May 2023.
Copyright
Copyright © 2025 Harsh Panchal, Shreyas Baraskar, Prof. Sandeep Sabnis. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66489
Publish Date : 2025-01-12
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

