Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Gauging Expert Perspectives: A Survey on AI Integration in Pharmacovigilance
Authors: Humase Nazir
DOI Link: https://doi.org/10.22214/ijraset.2024.64304
Certificate: View Certificate
Abstract
The ever-growing landscape of drug safety monitoring demands innovative solutions. This paper explores how Artificial Intelligence (AI) can revolutionize pharmacovigilance by enhancing patient safety and enabling personalized medicine. A survey conducted by the author indicates a positive outlook on AI\'s potential in this field. AI excels at analysing vast data sources, leading to improved detection of subtle safety signals and emerging drug safety concerns. Additionally, AI-powered risk prediction allows for personalized treatment strategies, minimizing adverse drug reactions for individual patients. Furthermore, AI facilitates efficient literature mining and drug repurposing, accelerating drug discovery processes. While the potential benefits are significant, responsible implementation is crucial. Addressing potential biases in algorithms and ensuring data privacy and security remain critical considerations. Overall, AI holds immense promise for the future of pharmacovigilance, paving the way for a more efficient, accurate, and proactive system for safeguarding patient well-being.
Introduction
I. INTRODUCTION
Artificial intelligence often referred to as AI is a subject of discussion, in today’s world. In the field of computer science AI focuses on developing systems that can demonstrate intelligence to that of humans. Although AI has demonstrated promise and has proven advantageous in domains its influence on daily activities remains somewhat restricted. AI possesses capabilities; it can manage tasks that exceed capabilities due to cognitive or temporal limitations, venture, into unexplored realms of task fulfillment and mechanize tedious and repetitive tasks. AI is frequently featured in the media. There are still important questions left unanswered. These include inquiries, about how the technology works, evidence of its successful experiments and most importantly the real world benefits it could bring to people on a daily basis.
Machine learning (ML) – ‘the field of study that gives computers the ability to learn without being explicitly programmed’ – can be considered a branch of AI. It seems intuitive that a field as complex as medicine would benefit from processes that learn from data rather than relying solely on pre-defined rules[1]. Within the domain of medicine, pharmacovigilance (PV) is dedicated to helping physicians ‘do no harm.’ PV requires monitoring the effects of pharmaceutical products after licensure and includes ongoing surveillance of known side effects of medicines, as well as sifting through large volumes of data to identify and act on emerging, previously unknown side effects. For this reason, AI/ML seems, intuitively, to be well suited to perform PV tasks given the large volume of data, high degree of uncertainty and need to learn from data. One important caveat is heterogeneity: PV data are derived from a multitude of medicines, including vaccines, and, as a result, describing very different types of adverse drug reactions (ADRs) and adverse effects of vaccines, that can manifest differently, and have different, often unknown, mechanisms, and the data are captured and shared imperfectly. This makes analytics of any type challenging.
A. AI in healthcare and PV
In pharmacovigilance (PV), artificial intelligence and machine learning (AI/ML) have the capacity to influence various stages of the entire process and are currently under investigation Machine learning in pharmacovigilance can generally be categorized into three main areas: tasks related to data intake, activities involved in signal detection, and other miscellaneous applications. Artificial Intelligence and Machine Learning have demonstrated utility across various facets of data assimilation, including but not limited to identifying duplicate entries, detecting anomalies as an alternative method for ensuring quality and evaluating the reported causal relationships in individual safety reports.
At a subsequent phase in the lifecycle of Pharmacovigilance (PV), Machine Learning (ML) has been investigated and implemented for the identification and examination of signals.
Basic ML methods, exemplified by association rule analysis (known as disproportionality analysis in PV), have been regularly utilized since the late 1990s to quantitatively alert specific drug/vaccine-adverse event associations for clinical assessment[2]. Advanced ML techniques like Lasso shrinkage regression, Bayesian borrowing algorithms, and temporal scan statistics have also been assessed for signal detection. However, the overall performance of these sophisticated algorithms does not significantly outperform simpler methods in signal detection and lacks the transparency and user-friendliness of simpler approaches.
Rapid evolution of AI/ML has other implications. A recent systematic review suggested that only about 10% (42/393) of studies of AI/ML in PV published between 2000 and 2021 conformed to current best practices in ML[3]. This arguably suggests sub-optimal use of ML in PV, and that routine use of ML could have provided more practical benefit to date. While this is impossible to assess, it at least provides impetus to a perspective that it is necessary to rethink how AI/ML is being applied in PV to avoid overlooking the potential opportunities and failing to optimize impact[4].
B. What could happen?
With the improvements, in methods there is an expectation that the performance of Machine Learning (ML) tasks will improve and could eventually become a practice in Pharmacovigilance (PV). One might wonder if ML should adapt to meet PV requirements and current practices or if PV will adjust to make the most of ML or maybe a combination of both scenarios is possible. Artificial Intelligence (AI) and ML are intriguing technologies often linked to innovation and advancement. The idea of incorporating this state-of-the-art technology into existing PV systems without changes, to processes and regulations is appealing. While this approach could speed up processes and boost efficiency it may not lead to overall improvements. Nevertheless, the potential enhancement of patient safety through the integration of AI into current Pharmacovigilance (PV) systems may not be as significant as anticipated. The prevailing efforts in this field often fall short of adhering to optimal practices. Moreover, while the integration of AI may seem to introduce innovation on the surface, in reality, it could escalate the volume and intricacy of tasks. The deployment of AI/ML in PV operations should be strategic, focusing on delivering solutions that streamline work processes, reduce complexities, and optimize resource allocation. This approach aims to prioritize activities critical for ensuring patient safety efficiently. For example, intensifying efforts to identify potential ADRs and subsequently create, store, and analyze reports, and communicate results, could ultimately produce even more reports, but would not necessarily result in more rapid or accurate identification or understanding of true safety issues – the very objectives of PV systems! Automation may facilitate such endeavors but magnifying and replicating the volume of ADR reports does not make them more beneficial[5].
C. What should happen?
The preceding segment may lead one to consider that AI/ML and big-scale automation modifications are not appropriate for chemical tracking (PV). However, this is far from the truth. Of route, those technological advances are crucial to improving affected person protection. The mission of enforcing AI/ML for PV is hampered by way of regulatory framework and governance problems. AI/ML gives top-notch capability for PV, and the key question is how to first-class practice this technology for the improvement of a future that meets industry wishes. The ability of AI/ML systems not only to learn from past data analysis but also to identify unexpected outcomes is crucial. Insights and requirements should be strategically integrated seamlessly and efficiently throughout the pharmacovigilance lifecycle—from signal detection and data processing to knowledge sharing within the field of pharmacovigilance. This calls for cautious consideration to make sure that AI/ML technology gain in a manner that maximizes their value for each affected person safety and PV techniques.
We need a system where both inputs and outputs are seamlessly connected across different data systems. Such a system could ultimately inform an interactive continual learning solution that promotes understanding of the benefit–risk of medicines and vaccines and allows prescribers, patients, and other stakeholders to obtain information and pose questions as needed[6].
When patients and their healthcare providers believe that sharing population or sub-population data can help improve their own health outcomes, they are more likely to share their own healthcare data. This can provide valuable insights for themselves, their loved ones, and others. As the benefits of data sharing become more apparent, such as improving patient safety, the quality of data available in pharmacovigilance (PV) systems will also improve. This, in turn, can lead to even greater improvements in patient safety, creating a positive cycle of improvement.
The dictum, 'first do no harm,' which arguably is better expressed as 'first do no net harm'[7], remains a central tenet of medicine and is highly relevant to benefit–risk assessments of medicines and vaccines. However, it is unclear to what extent this dictum is being applied in the context of AI/ML in healthcare.
It is certainly true in the context of the safe use of AI algorithms and ensuring the algorithms learn appropriately. However, in the data-centric world in which we live, we often hear about misuse of data and the dangers of sharing data. But making fewer data available to an AI algorithm implies suboptimal algorithmic performance with potentially great implications for insights and, therefore, impact. So, what about the converse? Can we, as a PV community, say hand on heart that we do all we can to maximize the accessibility and, therefore, utility of healthcare data for patient safety? If we do not harness the properties of data to allow us to leverage AI optimally, we will fail to do all we can for patient safety
II. SCOPE AND PURPOSE OF THE RESEARCH
The integration of artificial intelligence (AI) dramatically changes the shaming of drugs. AI technologies, especially machine learning algorithms, offer excellent potential to improve drug safety monitoring and adverse event detection. These systems are able to analyze large amounts of drug recognition data with exceptional performance, enabling faster detection of potential safety concerns compared to traditional methods.
However, successful integration of AI requires more training data. Effective training of AI algorithms relies heavily on large, accurate and valid datasets. Configurations with limited resources often face challenges in accessing such data, highlighting the critical importance of improving data quality and accessibility for seamless AI integration.
Furthermore, several technical challenges may impede the successful implementation of AI based drug monitoring systems. These challenges include barriers to data quality and availability, insufficient human resources to monitor and interpret AI products, limitations in current AI technology itself, and potentially limited government support for these projects this is especially true in ensuring effective use of AI in drug monitoring in resource-limited settings It is important to prevent.
Initiatives such as giving priority to data quality initiatives, improving education and training related to AI technology, and advocating for increased government support can clear the path to address these challenges. Additionally, collaborative studies networks, advancements in pharmacogenomic studies, and the development of more sophisticated machine gaining knowledge of algorithms are key elements that keep promise for the destiny of AI-based totally pharmacovigilance.
In conclusion, the mixing of AI in pharmacovigilance provides a substantial and exciting scope with the capacity to revolutionize drug safety monitoring and unfavorable event detection. By addressing modern-day demanding situations, imposing strategies to improve information satisfactory, and harnessing the strength of advanced generation, AI has the potential to transform pharmacovigilance practices, specifically in resource-confined settings, in the end leading to a safer and greater efficient method to making sure drug safety.
A. Turning to Expert Opinion
While the previous segment explored the potential benefits and challenges of integrating AI into pharmacovigilance, an important takeaway for experts in the field is that AI offers a promising future. Our research addresses a number of key areas related to professional understanding of the integration of AI into pharmacovigilance. First, we explore how experts see the pros and cons of adopting AI in the industry. This includes understanding their views on all the blessings that AI can bring and the talent-demanding situations that can arise during implementation. In addition, the research examines the ethical issues and talent issues that experts have raised regarding the use of AI in pharmacovigilance. This is essential to ensure responsible development and deployment that minimizes capability risks associated with the AI ??era.
Finally, the study analyses expert attitudes toward AI integration as a whole. This includes information on how they recognize AI in pharmacovigilance and their expectations for the future of this generation in shaping the industry. By analysing these many factors, we can take advantage of the valuable insights of the professionals themselves, which in the long run will lead to a more efficient and collaborative method of integrating AI into pharmacovigilance.
The role of AI in pharmacovigilance: Like many. other industries, pharmacovigilance processes ever-increasing amounts of data. The collection, evaluation and reporting of adverse effects, i.e., case management and continuous monitoring of adverse events and drug reactions, can benefit from AI.
Automating repetitive and routine tasks enables faster reporting and also facilitates seamless real-time communication. Emerging companies are developing AI-based solutions to improve the productivity of information reporting and the efficiency of drug development as a process.AI holds great promise to address critical challenges and provide new opportunities for drug innovation. What are the most important applications of artificial intelligence in drug safety?
III. THE ROLE OF ARTIFICIAL INTELLIGENCE IN DRUG SAFETY MONITORING
Like many other industries, the field of pharmacovigilance is dealing with ever-increasing volumes of data. The collection, evaluation, and reporting of adverse events, known as single case processing and continuous monitoring of adverse events and drug reactions, can benefit from artificial intelligence. Automation of repetitive and routine tasks enables quicker reporting and also helps in seamless real-time communication. Emerging companies develop AI-based solutions to improve the productivity of data reporting and the efficiency of drug development as a process.
Artificial intelligence holds great promise to address critical challenges and to provide new opportunities for the pharmacovigilance area. What are the major applications of artificial intelligence for drug safety?
A. Automation to improve case handling and reporting:
Much pharmacovigilance efforts are focused on identifying adverse reactions, collecting cases, evaluating them and turning relevant data into actionable information that can be processed by regulatory agencies and companies to address concerns about security and informing the public. Nowadays, solar companies manage increasing volumes of data by adding new ones to the solar team. But there is a limit to the sustainable growth of an organization without outsourcing to keep up with the exponentially increasing amount of data. In addition to volume and logistics, there are performance limits for human experts handling large amounts of data which affects the accuracy and consistency of data interpretation (often referred to as inter-rater agreement). As organizations grow and expand across geographies, these limitations increase even more. The good news is that almost all pharmacovigilance and pharmacovigilance processes can be automated. For example, automation supports functions such as data entry, triage, case handling, quality review, medical review and reporting. Supervised and unsupervised machine learning models can automate critical steps in the security process from intake to processing using existing security reports, continuous signal detection, and new unconventional sources to perform security-related adverse monitoring. AI-powered solutions provide better insights and capabilities to address the root causes of compliance metrics issues. Automation with artificial intelligence improves the quality of security data early in the process and helps streamline processing and improve signal detection and evaluation. Allowing AI to do the very repetitive manual work of extracting and reading incoming reports would free up time for solar experts to review and verify reports, ensuring that case descriptions are complete, accurate and of high quality. A simplified process would enable faster and more consistent security procedures than traditional manual procedures.
B. Cognitive case management using machine learning:
The number of adverse events from traditional and non-traditional sources is increasing, providing researchers with opportunities to understand product safety profiles. On the other hand, the cost of pharmacovigilance, both in terms of costs and resources, is constantly increasing, as more and more side effects are recorded in continuous post-marketing monitoring. A number of factors increase the number of reported side effects, such as an aging population, increased public awareness and the number of drugs on the market. At the same time, there are challenges in receiving and processing cases. The volume of cases is so high that PV organizations must transition from a manual process of managing all cases to cognitive automation and targeted expert assessment of selected complex cases. Machine learning has several tasks that can be applied to case management. it can bring value. ML algorithms are able to detect outliers. The model can identify unusual cases or data errors that require further explanation. Machine learning is also great for finding relationships between variables and can be applied to learning rules to relate security issues. Clustering models allow finding groups that are similar in some way and can be used to estimate detection, either by grouping similar safety reports, patients, facilities and other surrounding adverse events. Classification algorithms allow new cases to be divided into predefined groups. The most compelling case for AI case management solutions is its cognitive superpowers and ability to provide new insights or summaries that improve the quality and richness of case data coded for further investigation. and compliance efforts. A cognitive case processing application shifts the focus from manual data entry and analysis to controlled and review-based quality. The benefits mentioned above include lower per-care costs, increased case throughput and the need for expert manpower during the security review process. The combined strengths of pharmacovigilance experts and artificial intelligence systems can provide a robust solution that improves speed, scale, consistency and data quality.
C. Error-free reporting through natural language processing (NLP) and speech and text recognition:
Automating input and management significantly improves the efficiency and quality of the risk management cycle. How do you build a case reporting system that is scalable, usable and designed for large case volumes and large data sets?
How to automate incident report management and limit reporting time? Artificial intelligence is here to help with several technical solutions. With deep learning, pharmaceutical manufacturers can reduce the time it takes to report adverse drug reactions.[8]
NLP solutions help digitize and standardize incident reporting. Much of the process information is available only in unstructured and free text form, and NLP algorithms can immediately transform it into a standardized digital version. Medical content from other data sources such as patient and case records can be analysed using NLP to support signal verification. Thanks to speech and text recognition, companies can continue to deliver error-free reports on various clinical and official information. AI can reduce the cost of solving each case and free up valuable resources for more complex and additional tasks. Another AI solution, natural language generation (NLG), can be used for medical writing and to generate aggregate reports generated from unique case reports and a signal detection process[8].
These applications can free up resources to help you investigate anomalies. cases to better manage quality, improve our understanding of product safety profiles, prepare to respond to changing regulations and evaluate new safety options.
D. Mining literature and unusual data sources:
Another use of AI would be to apply NLP to big data such as free text in social media, news articles, literature or medical data to discover unexpected benefits of a pharmaceutical product. This approach uses artificial intelligence and trained analysts to observe signals that point to unlikely benefits or adverse effects[8]. These signals provide valuable real-world intelligence that cannot be found through data mining in controlled clinical situations. Automated mining of the literature and other non-traditional data sources can lead to expanded indications for a product already on the market, giving pharmacovigilance an opportunity to improve patient care while contributing to the company's bottom line. This automated initial search and analysis leaves room for further analysis and refinement by human experts[8].
E. From reactive to proactive pharmacovigilance:
A proactive, or proactive, approach in PV means that safety teams begin trying to identify potential drug reactions at each stage of the drug discovery and development process, opening up the opportunity to address a problem before it becomes one[8]. .AI, and especially deep learning, can be applied to drug development. early in the process. These techniques emphasize predictive accuracy over model interpretability. Enhanced learning through expanded datasets and cognitive innovations enables efficiency and insight at the decision-making stage, changing the way pharmacovigilance works. Incorporating knowledge, retrospective and prescriptive analysis, and decision recommendations at each stage of the process paves the way for predictive and proactive PV. Predictive analytics enables pharmaceutical vigilance teams to predict and correct safety issues before they occur to ensure patient safety and reduce operational costs. Predictive analytics can discover and evaluate insights and potential signals in data that were previously difficult or impossible to detect and solve critical business problems at scale[8].
IV. CURRENT STATE OF AI IN PHARMACOVIGILANCE PRACTICES
The current state of AI in pharmacovigilance is characterized with the aid of the development and integration of effective automation tools. These gear leverage machine getting to know algorithms and natural language processing (NLP) to research massive quantities of information from diverse sources, which include digital health facts, social media structures, and actual-world proof databases. This statistics analysis allows for earlier identity of capacity safety worries associated with medicinal drugs.
A. Pharmacovigilance Automation Tools
Medication Safety Automation Tools: AI-powered medication safety automation tools can transform the way ADRs are detected, reported and managed. These tools use machine learning algorithms to analyse large amounts of data from multiple sources, including electronic health records, social media, and other reliable and tangible evidence.
One example of such a tool is the FDA Sentinel System, which uses automated algorithms to analyse. large health databases to identify drugs and other medical products. related potential security signals. Another example is VigiLanz, a software platform that uses natural language processing and machine learning to identify potential side effects and other security issues in electronic health records[9].
US drug companies TransCelerate Biopharma Inc. according to the study, compliance improved by 45%. and 80% improvement in efficiency are among other beneficial developments in AI and the integration of pharmacovigilance automation tools[9].
Other pharmacovigilance automation tools using AI include IBM Watson for Drug Discovery, which uses machine learning and predictive analytics. to accelerate drug discovery and development, and Trifacta, a data preparation and cleaning tool to help identify and correct data quality issues in pharmacovigilance datasets[9].
- VigiFlow: VigiFlow is a cost-effective database control tool that helps the reporting of Individual Information Security Reports (ICSRs) according with worldwide requirements. It helps streamline the drug alert technique by offering a greater green data control and reporting mechanism, ensuring compliance with regulatory requirements.
- OHDSI: OHDSI (Observational Health Data Sciences and Informatics) affords terminology and software program tools for collaborative research, facts trade, and analysis within the healthcare industry. It allows healthcare providers, researchers, engineers, and industry companions to paintings collectively to improve fitness results through data-pushed methods. OHDSI plays a vital function in efforts to improve drug signals by using enabling standardized data analysis and sharing among health care structures.
- FDA Sentinel System: The FDA Sentinel System is a safety evaluation device for FDA-regulated pills. It uses real-international facts sources to conduct put up-marketplace analysis and capability safety problems related to prescribed drugs and clinical gadgets. Perceive worries. The system enhances drug surveillance activities via presenting a sturdy framework for non-stop product protection tracking and facilitating timely criminal action to shield public health.
1) Adverse Event Reporting Systems
FDA reporting of pharmaceuticals is generally carried out with the help of Adverse Event Reporting Systems (AERS) and with the integration of machine learning algorithms, the scope of drug data analysis can be scaled up with ease. AI/ML-driven solutions for AERS have helped develop clear explanations for timely FDA reporting of adverse events[9].
Back in 2020 when the pandemic arose, forcing drug safety departments to reassess the way they analyze data, several automation solutions were suggested by leading tech conglomerates. An important component that ensures timely reporting of adverse events is where consumers are empowered to report these events. And that is where Amazon's Interactive Voice Response (IVR) solution known as Amazon Connect came to the fore during the pandemic[9].
Throughout the pandemic, medical professionals were forced to provide quick solutions, and this resulted in ill-conceived prescriptions being written out on the fly. Not all medications suited every case of COVID and the IVR-driven reporting of adverse events helped narrow down the most viable drugs that could hold off the fatal impact of the pandemic to a large extent[9].
2) Individual Case Safety Reports
Pharmaceutical companies are required to adhere to certain legal regulations imposed by the US Food and Drug Administration (FDA). These statutes are governed under the Individual Case Safety Reports (ICSR) shared among key stakeholders such as pharmaceutical companies and regulatory bodies subordinated by the FDA. However, the existing process involves a human pharmacovigilance professional manually assessing the validity of each case related to ADRs or events of serious reactions to specific drugs[9].
A breakthrough tooling strategy known as cognitive computing combines NLP and Deep Learning (DL) to automate these pharmacovigilance reporting workflows. This methodology also helps reduce the cost of case reporting and improves data quality by automating formerly resource-intensive activities such as pharmacovigilance analytics and benefit-risk assessment. AI-powered ICSR allows safety departments, and pharmacovigilance executives to ensure better reporting outcomes with highly relevant data at their fingertips[9].
3) Sentiment Analysis and Social Media Monitoring
Social media platforms and online forums have become places where individuals often share their healthcare experiences, including adverse drug reactions (ADRs). AI-powered sentiment analysis can sift through these vast amounts of unstructured text data to identify and categorize mentions of drug-related experiences. It can determine whether the sentiment expressed is positive, negative, or neutral and understand the context in which the drug is discussed[9].
The article, shared after the Pacific Symposium on Biocomputing, reveals how using AI to analyze five million posts can provide valuable insights into the effectiveness of antidepressants. The study also emphasizes the importance of social listening in understanding safe drug combinations and adverse reactions[9].
4) Genomic Data Integration
Integrating genomic data with pharmacovigilance efforts allows for a more personalized approach to drug safety. AI can analyze a patient's genetic profile to identify genetic markers associated with drug metabolism and adverse reactions. By considering genetic variations, healthcare providers can make more informed decisions about which medications are most suitable for individual patients, reducing the risk of adverse events[9].
V. METHODOLOGY: EXPERT SURVEY DESIGN AND IMPLEMENTATION
To survey specialists within the location of drug safety worried with the incorporation of AI strategies to this discipline, a particular questionnaire with questions about this subject matter may be evolved. This examine will look to apprehend how those one-of-a-kind stakeholders understand this problem. The questions of the survey might be in particular designed to cope with the dangers, barriers, and ethical questions surrounding AI implementation in pharmacovigilance. Professionals might be endorsed to deliver their perspectives about the effectiveness of AI as regards to figuring out unfavorable activities, making hazard proactive, and drug protection supervision tracking within the destiny.
Secondly, the survey will attempt to collect expert critiques on the restrictions and possible deficiencies in AI-based totally methods used in pharmacovigilance. This consists of concerns related with the breach of privateness, extra reliance on algorithms, and finding an equilibrium between AI automation and human understanding.
- Sample Selection: A random sampling technique could be used to select experts from a huge spectrum of the drug protection subject. This guarantees that the records gathered represents the numerous perspectives in the field and avoids capability biases from precise subgroups. The target populace may want to include medical doctors, professors, nurses, surgeons, pharmacists, and other relevant healthcare specialists.
- Dissemination Strategy: To ensure data quality, the survey on AI in pharmacovigilance was disseminated exclusively through online channels frequented by relevant professionals, preventing participation from non-specialists.
- Ethical Considerations:
- Informed Consent: Before taking part in the survey, all experts could be supplied with an informed consent shape that clearly explains the motive of the observe, how the data can be used, and their rights as individuals. Participation might be voluntary, and people can withdraw from the look at any point.
- Anonymity: The survey will be designed anonymously. No for my part identifiable statistics will be accrued from the members. Their responses might be coded and anonymized for the duration of evaluation and reporting.
- Data Privacy: All data accrued from the survey may be stored securely and confidentially. Measures could be taken to comply with relevant statistics privateness guidelines.
VI. KEY INSIGHTS FROM PHARMACOVIGILANCE PROFESSIONALS
In the dynamic area of pharmacovigilance, professionals are important for making sure drug protection. Analyzing survey facts from these professionals offers treasured insights into their perspectives on key subjects along with Artificial Intelligence integration, organizational affiliations, and familiarity with current practices. These insights provide valuable implications for advancing drug protection practices and patient care. The data presented herein was collected following the acquisition of 148 responses through an online survey platform. The survey was administered utilizing electronic means to reach a diverse respondent pool, ensuring comprehensive data collection.
A. General info
1) Age Group Analysis
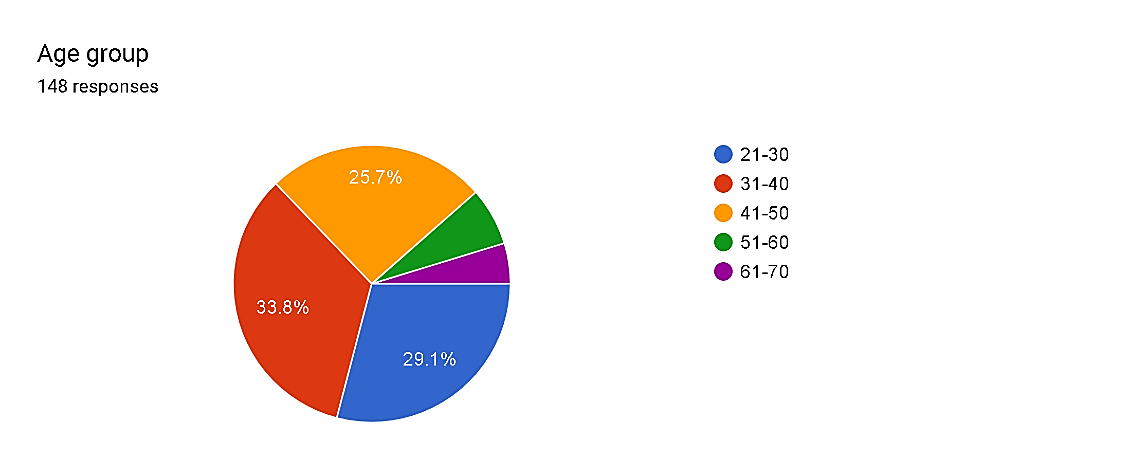
Fig. 1
The distribution of respondents throughout age businesses offers insights into the demographics of pharmacovigilance experts. The biggest percentage, comprising 33.8%, falls within the 31-40 age bracket, suggesting a great representation of mid-profession experts. Additionally, the 21-30 age group constitutes 29.1%, indicating a big presence of younger experts getting into the field. Notably, the 41-50 age range encompasses 25.7% of respondents, while older age agencies (51-60 and 61-70) make up smaller chances, at 6.8% and 4.7%, respectively. These findings suggest a diverse age distribution within the pharmacovigilance staff, highlighting opportunities for intergenerational understanding alternate and professional development initiatives tailor-made to distinct profession degrees.
2) Gender Analysis
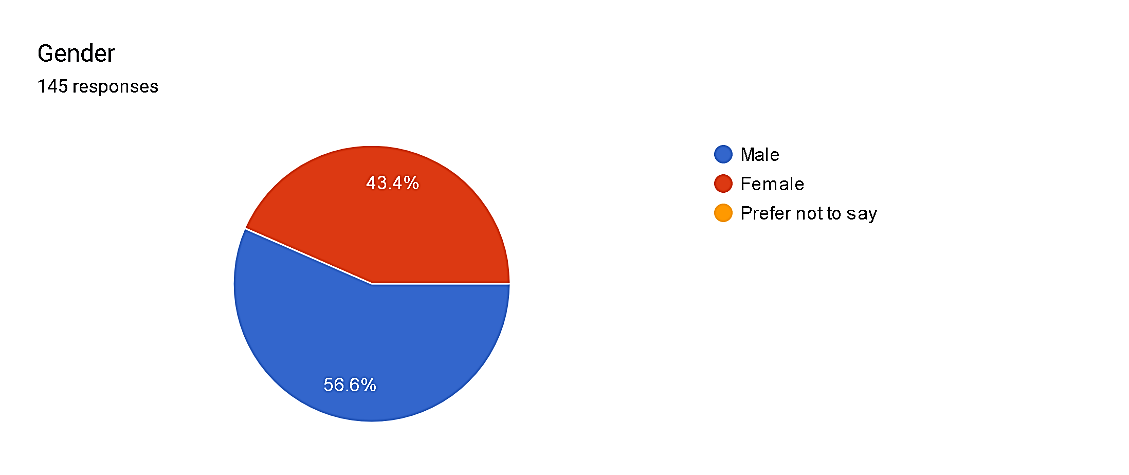 Fig. 2
Fig. 2
Among the respondents, gender distribution displays a mild predominance of males, constituting 56.6% of the pattern, whilst females account for 43.4%. Notably, there were no respondents who favored now not to disclose their gender. This gender distribution in the pharmacovigilance area shows a fantastically balanced representation, though men are slightly greater widespread. Such insights into gender demographics can tell strategies for selling gender range and inclusivity inside the profession, making sure equitable opportunities for all practitioners no matter gender identity.
3) Profession Distribution
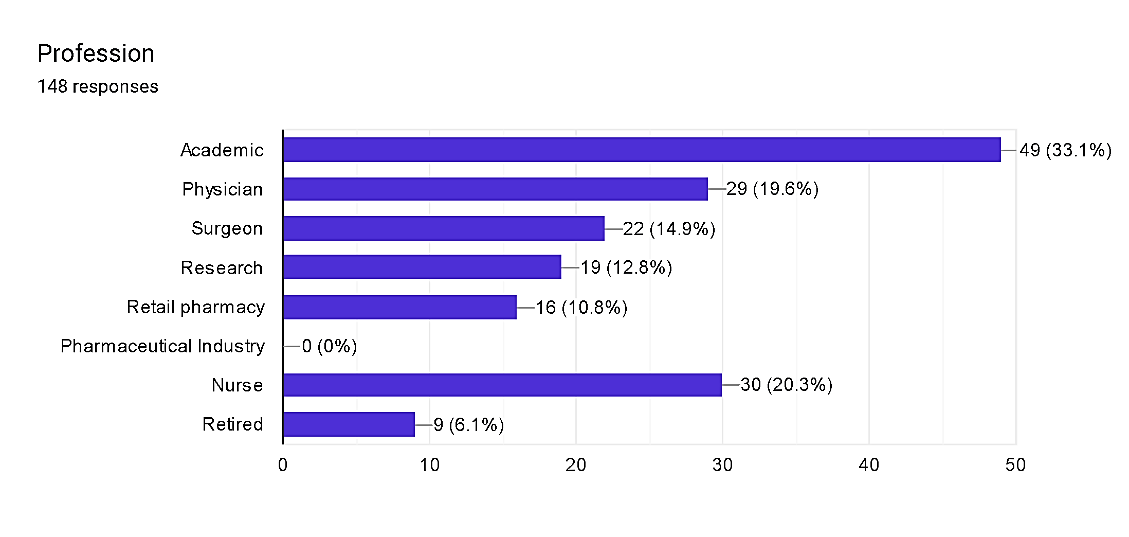 Fig. 3
Fig. 3
The distribution of respondents across various professions within pharmacovigilance sheds mild on the diverse expertise contributing to the sector. Academics and physicians come to be the maximum prominent companies, each comprising 33.1% and 19.6% of respondents, respectively. Surgeons and nurses also constitute sizeable cohorts, with proportions of 14.9% and 20.3%, respectively. Additionally, studies experts represent 12.8% of respondents, while the ones from retail pharmacy and retired people each make up smaller however extremely good percentages at 10.8% and 6.1%, respectively. These findings underscore the interdisciplinary nature of pharmacovigilance, drawing knowledge from academia, clinical exercise, research, and other sectors to ensure comprehensive oversight of drug protection and efficacy throughout the healthcare continuum.
4) Organization Affiliation
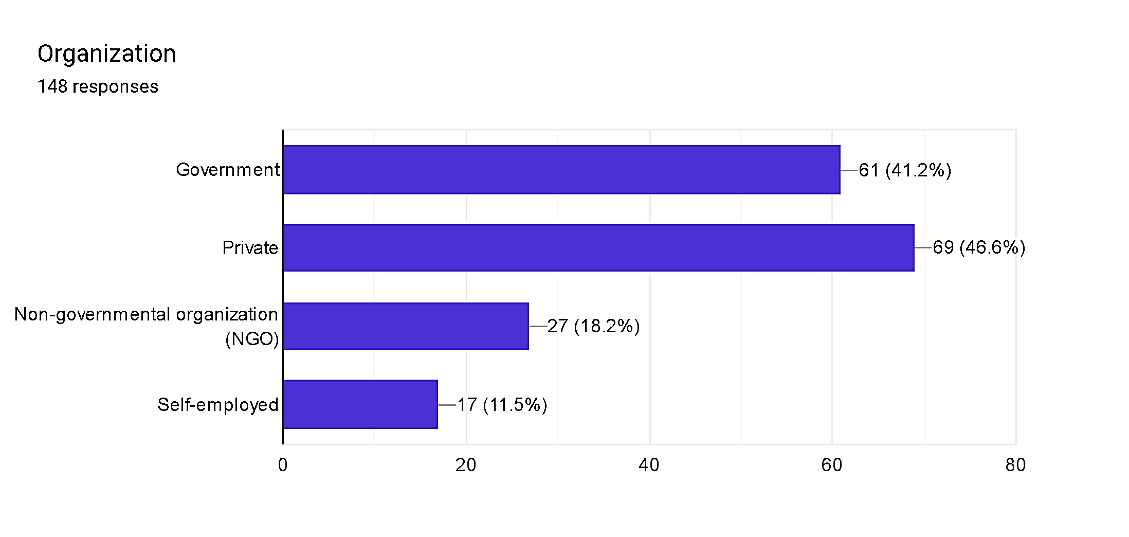 Fig. 4
Fig. 4
The distribution of respondents across specific organizational affiliations inside pharmacovigilance highlights the numerous landscapes of the field. Private region entities end up the maximum regular, constituting 46.6% of respondents, indicating a big industrial presence in pharmacovigilance endeavors. Government groups observe closely at the back of, representing 41.2% of respondents, underscoring the tremendous involvement of public zone entities in regulatory oversight and coverage implementation. Non-governmental businesses (NGOs) represent 18.2% of respondents, reflecting the contributions of civil society groups to drug protection initiatives. Self-hired professionals, comprising 11.5%, represent independent practitioners contributing to pharmacovigilance efforts. This distribution underscores the collaborative efforts across diverse sectors to make certain powerful pharmacovigilance practices and shield public fitness.
B. Questionnaire
1) How familiar are you with the current practices of pharmacovigilance?
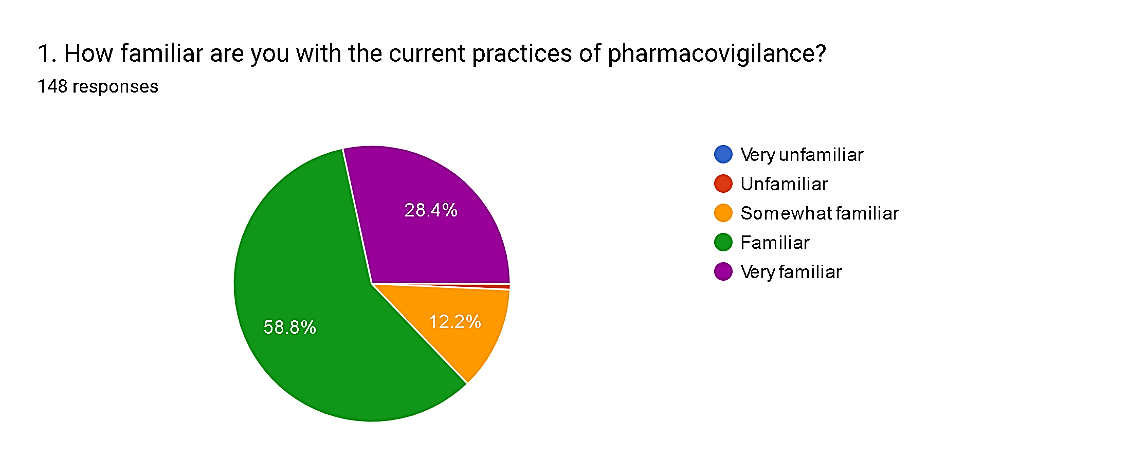 Fig. 5
Fig. 5
The survey results illustrate a spectrum of familiarity levels among respondents regarding pharmacovigilance practices. A notable portion, 28.4%, express being very familiar, indicating a high level of expertise in the field. Additionally, 58.8% consider themselves familiar, while 12.2% feel somewhat familiar, suggesting a broad understanding among the majority of participants. However, 7% admit to feeling unfamiliar, and there is a negligible percentage (0.7%) reporting feeling very unfamiliar. These findings emphasize the importance of continuous education and training to bridge knowledge gaps and ensure a more comprehensive understanding of pharmacovigilance practices across all professional cohorts.
2) How familiar are you with Artificial Intelligence (AI)?
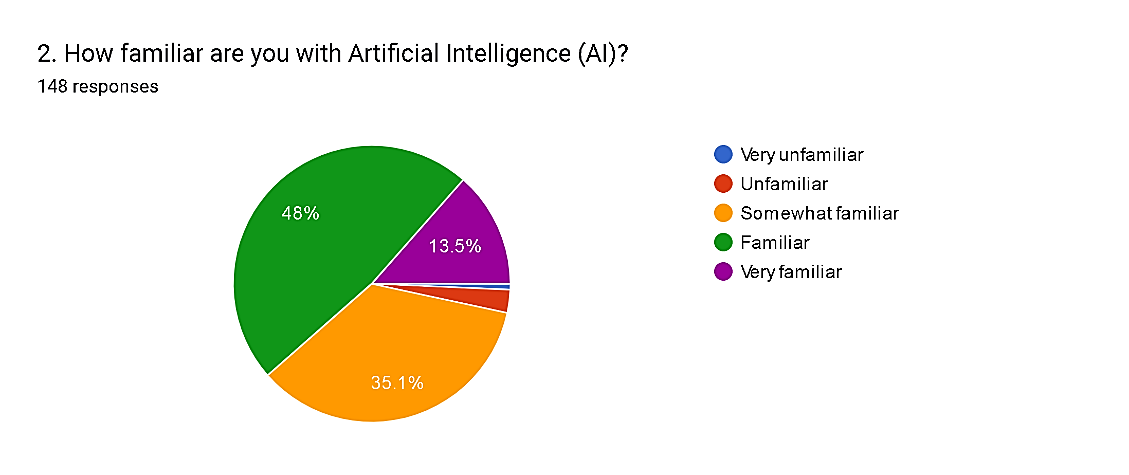 Fig. 6
Fig. 6
The survey results regarding familiarity with Artificial Intelligence (AI) among respondents demonstrate varying levels of understanding and exposure within the pharmacovigilance community. A significant proportion, 48%, feel familiar with AI, indicating a substantial level of knowledge and experience with AI technologies. Additionally, 35.1% consider themselves somewhat familiar, suggesting a broad awareness among the majority of participants. However, a notable percentage, comprising 13.5%, feel very familiar, suggesting a high degree of expertise in AI applications. Conversely, 2.7% of respondents feel unfamiliar, and a negligible percentage (0.7%) feel very unfamiliar. These findings underscore the increasing relevance of AI in pharmacovigilance and the importance of continuous education to ensure professionals remain abreast of technological advancements shaping the field.
3) In your opinion, the overall impact of Artificial Intelligence (AI) integration on pharmacovigilance will be:
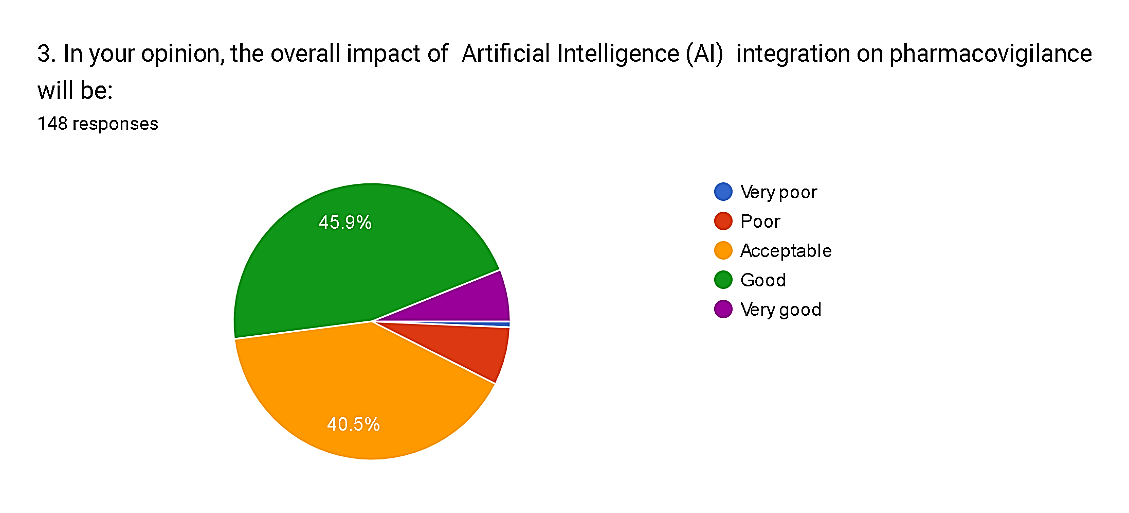 Fig. 7
Fig. 7
The survey results provide insights into the perceived impact of Artificial Intelligence (AI) integration on pharmacovigilance among respondents. Notably, a significant majority, comprising 45.9%, anticipate a positive impact, rating it as "good," while 40.5% consider it to be "acceptable." Conversely, only a small percentage, totaling 7.5%, foresee negative impacts, with 6.8% rating it as "poor" and 0.7% as "very poor." Moreover, 6.1% of respondents express optimism, envisioning a "very good" impact from AI integration. These findings underscore the overall optimism surrounding AI's potential to enhance pharmacovigilance practices, with the majority anticipating beneficial outcomes that can improve drug safety and public health.
4) Do you believe Artificial intelligence (AI) will play a significant role in revolutionizing the field of pharmacovigilance in the future?
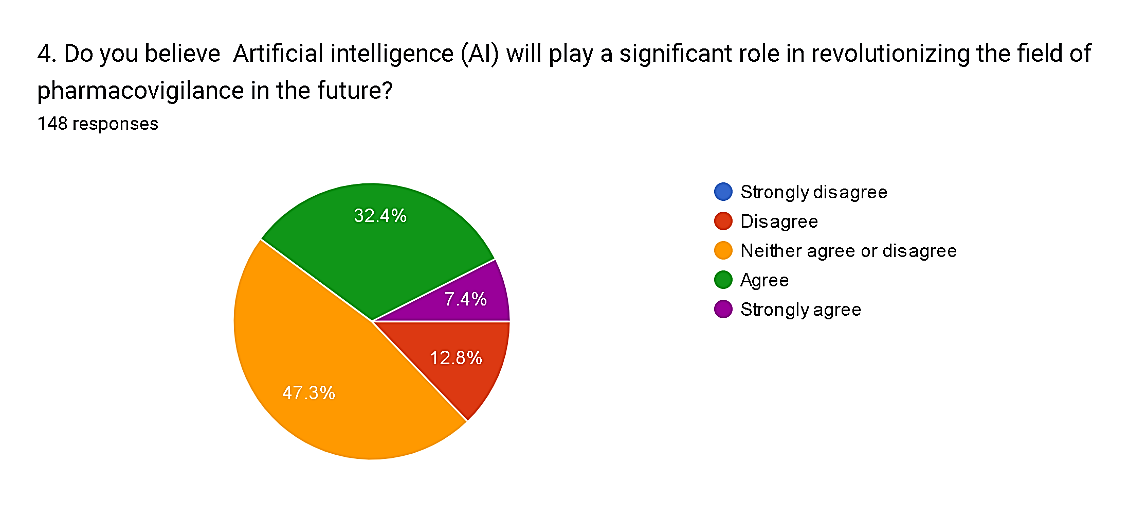 Fig. 8
Fig. 8
The survey responses regarding the anticipated role of Artificial Intelligence (AI) in revolutionizing pharmacovigilance reveal a spectrum of perspectives among respondents. While a notable minority, comprising 20.2%, express skepticism or opposition towards AI's significance, with 12.8% disagreeing and 7.4% strongly disagreeing, a larger proportion, totaling 39.8%, express uncertainty, neither agreeing nor disagreeing. Conversely, 39.8% of respondents anticipate AI playing a significant role, with 32.4% agreeing and 7.4% strongly agreeing. These results indicate a diverse range of opinions within the pharmacovigilance community regarding the potential impact of AI, highlighting the need for further dialogue and research to address concerns and leverage opportunities for AI integration effectively.
5) Which aspect of pharmacovigilance do you think will benefit most from Artificial intelligence (AI) integration: Signal detection or risk prediction?
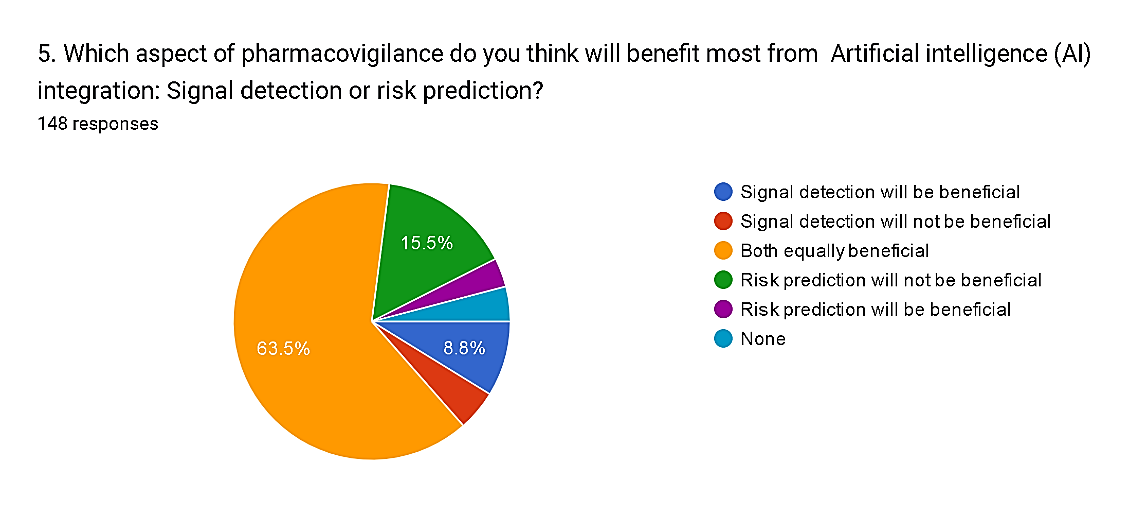 Fig. 9
Fig. 9
The survey results reveal a prevailing belief among respondents regarding the potential benefits of Artificial Intelligence (AI) integration in different aspects of pharmacovigilance. A significant majority, comprising 63.5%, perceive both signal detection and risk prediction as equally beneficial with AI integration, highlighting the perceived versatility of AI technologies in enhancing various aspects of drug safety monitoring. Conversely, a smaller percentage, totaling 20.2%, express opinions on the specific benefits of each aspect: 8.8% believe that signal detection will be more beneficial, while 3.4% anticipate greater benefits from risk prediction. However, a minority of respondent’s express reservations, with 4.7% believing that signal detection will not be beneficial and 15.5% expressing similar doubts regarding risk prediction. Additionally, 4.1% of respondents indicate a belief that neither aspect will benefit from AI integration. These findings underscore the complexity of integrating AI into pharmacovigilance practices and the need for tailored approaches to leverage AI's potential effectively across different domains within the field.
6) How important do you consider moral principles in the development and implementation of AI-powered pharmacovigilance solutions?
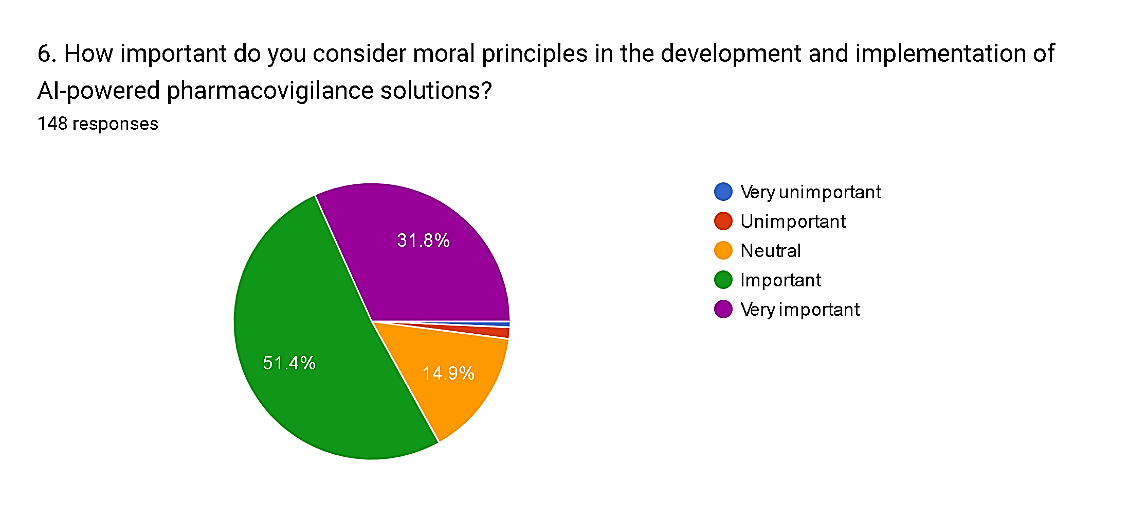 Fig. 10
Fig. 10
The survey results demonstrate a consensus among respondents regarding the significance of moral principles in the development and implementation of AI-powered pharmacovigilance solutions. A majority, comprising 83.2% of respondents, consider moral principles to be important or very important. Specifically, 51.4% of respondents rate moral principles as important, while 31.8% regard them as very important. This reflects a strong emphasis on ethical considerations and values in the adoption of AI technologies within healthcare. Conversely, only a small minority, totaling 1.4%, perceive moral principles as unimportant. Additionally, 14.9% of respondents express a neutral stance, suggesting a need for further exploration and clarification of ethical frameworks in the context of AI-powered pharmacovigilance solutions. These findings underscore the imperative of integrating ethical considerations into the development and deployment of AI technologies to ensure patient safety, privacy, and trust within the pharmacovigilance domain.
7) In the field of pharmacovigilance, do you agree that AI-powered signal detection methods have the potential to offer more value than traditional approaches?
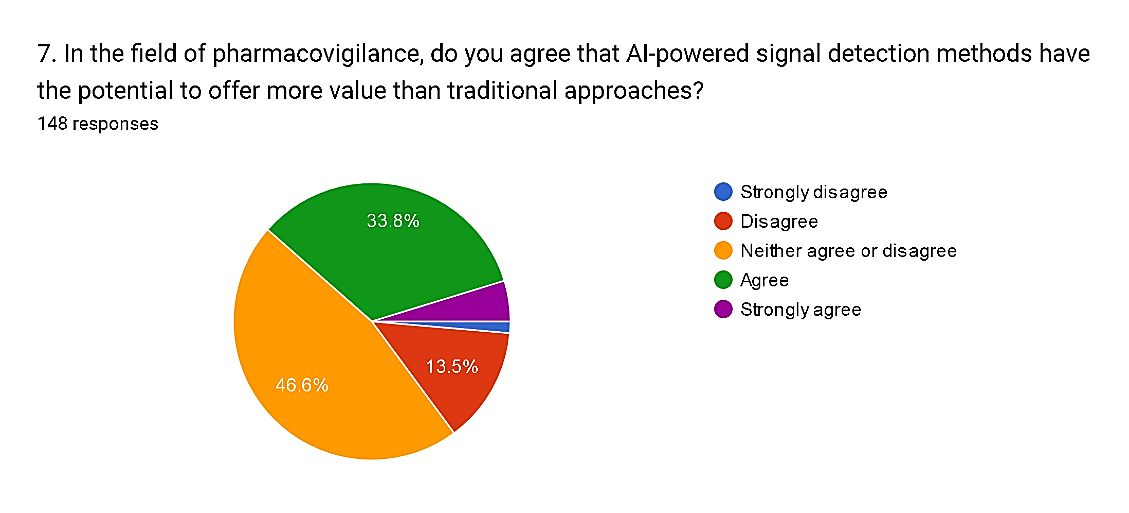 Fig. 11
Fig. 11
The survey results reflect diverse perspectives on the potential value of AI-powered signal detection methods compared to traditional approaches in pharmacovigilance. While a significant proportion, totaling 38.5%, express agreement or strong agreement with the statement, with 33.8% agreeing and 4.7% strongly agreeing, there is also notable skepticism among respondents. Specifically, 13.5% disagree and 1.4% strongly disagree with the notion that AI-powered signal detection methods offer more value. Moreover, a substantial portion, comprising 46.6%, neither agree nor disagree, indicating uncertainty or a need for further evidence to form a conclusive opinion. These findings underscore the complexity of assessing the comparative efficacy of AI-powered approaches versus traditional methods in pharmacovigilance, reflecting the ongoing debate within the field regarding the adoption and optimization of AI technologies to enhance drug safety monitoring.
8) Do you believe AI-driven personalized risk prediction has the potential to significantly improve patient safety in pharmacovigilance?
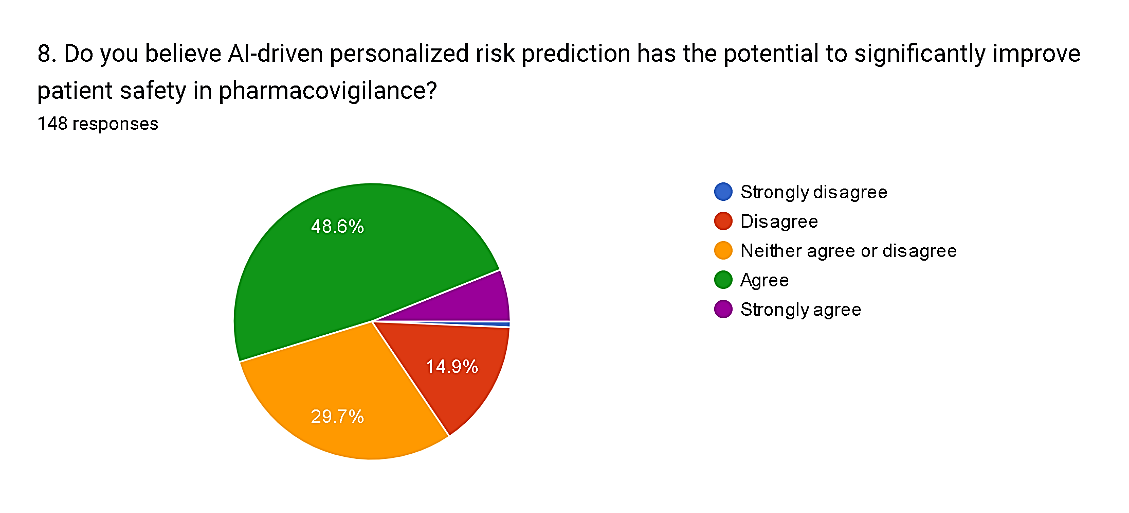 Fig. 12
Fig. 12
The survey findings suggest a nuanced perspective regarding the potential of AI-driven personalized risk prediction to improve patient safety in pharmacovigilance. While a notable proportion, totaling 54.7%, express agreement or strong agreement with the statement, with 48.6% agreeing and 6.1% strongly agreeing, there are also reservations among respondents. Specifically, 14.9% disagree and 0.7% strongly disagree with the notion, indicating skepticism or concerns about the efficacy of AI-driven risk prediction. Additionally, 29.7% neither agree nor disagree, suggesting a need for further evidence or clarification regarding the capabilities and limitations of AI in this context. These findings highlight the importance of ongoing research and validation studies to assess the real-world impact of AI-driven personalized risk prediction on patient safety outcomes in pharmacovigilance.
9) Do you believe the increased efficiency offered by Artificial intelligence (AI) outweighs potential concerns about algorithmic bias in pharmacovigilance?
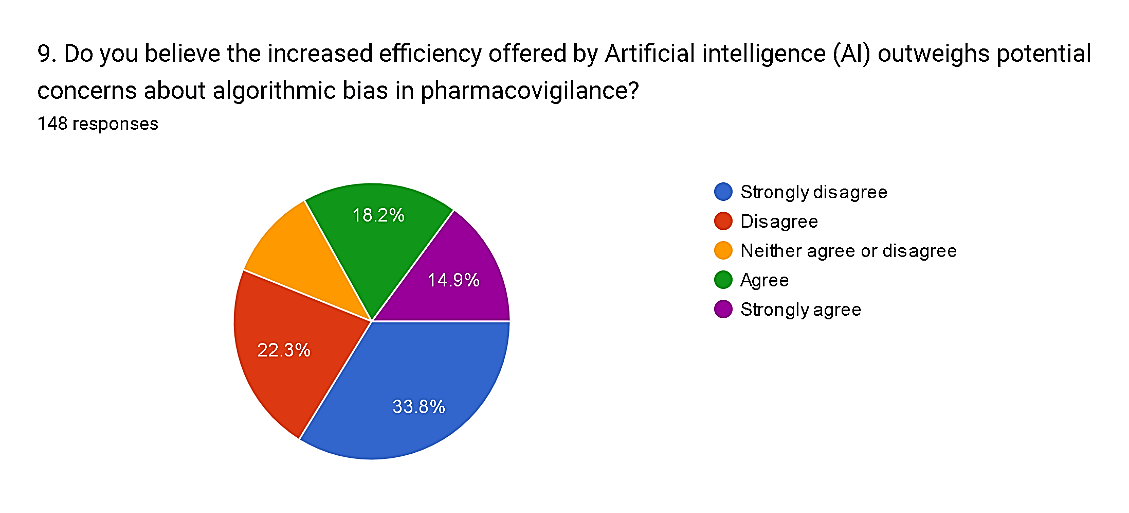 Fig. 13
Fig. 13
The survey responses indicate a mixed perspective on whether the increased efficiency offered by Artificial Intelligence (AI) outweighs potential concerns about algorithmic bias in pharmacovigilance. While a notable proportion, totaling 33.8% and 22.3%, respectively, express disagreement or strong disagreement with the notion, reflecting significant apprehension about the trade-off between efficiency and bias. Conversely, a smaller but still substantial portion, comprising 33.1% of respondents, agree or strongly agree with the statement, suggesting confidence in the potential benefits of AI-driven efficiency despite concerns about bias. Moreover, 10.8% neither agree nor disagree, indicating a need for further examination and mitigation strategies to address algorithmic bias in AI-powered pharmacovigilance. These findings underscore the importance of implementing robust governance frameworks and transparency measures to ensure that AI technologies enhance, rather than compromise, patient safety and equity in pharmacovigilance practices.
10) Do you believe widespread adoption of Artificial intelligence (AI) in mainstream pharmacovigilance practices will occur within the next five years?
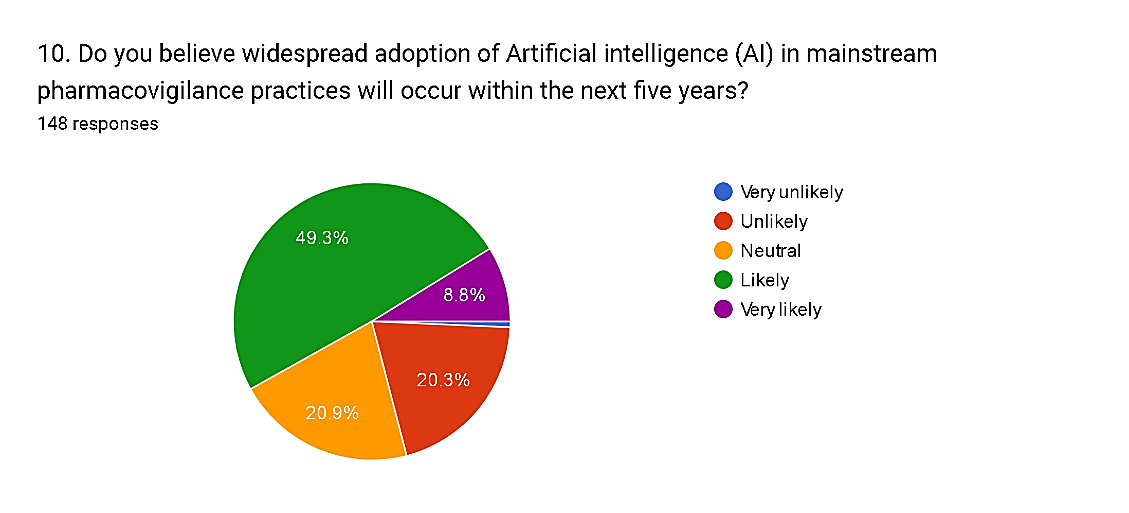 Fig. 14
Fig. 14
The survey responses reflect a diverse range of perspectives on the timeline for the widespread adoption of Artificial Intelligence (AI) in mainstream pharmacovigilance practices. While a majority, comprising 58.1% of respondents, express confidence in the likelihood of widespread adoption, with 49.3% rating it as likely and 8.8% as very likely, there are also notable reservations among some respondents. Specifically, 20.3% consider it unlikely, with 0.7% rating it as very unlikely. Additionally, 20.9% express a neutral stance, suggesting uncertainty or a lack of consensus regarding the pace of AI adoption in pharmacovigilance. These findings underscore the complexity of factors influencing the adoption of AI technologies in healthcare and the need for careful consideration of regulatory, technical, and organizational challenges to facilitate successful integration into mainstream pharmacovigilance practices.
11) Artificial intelligence (AI) can positively impact pharmacovigilance by automating tasks, identifying hidden patterns, and predicting potential drug risks. How much do you agree with this statement?
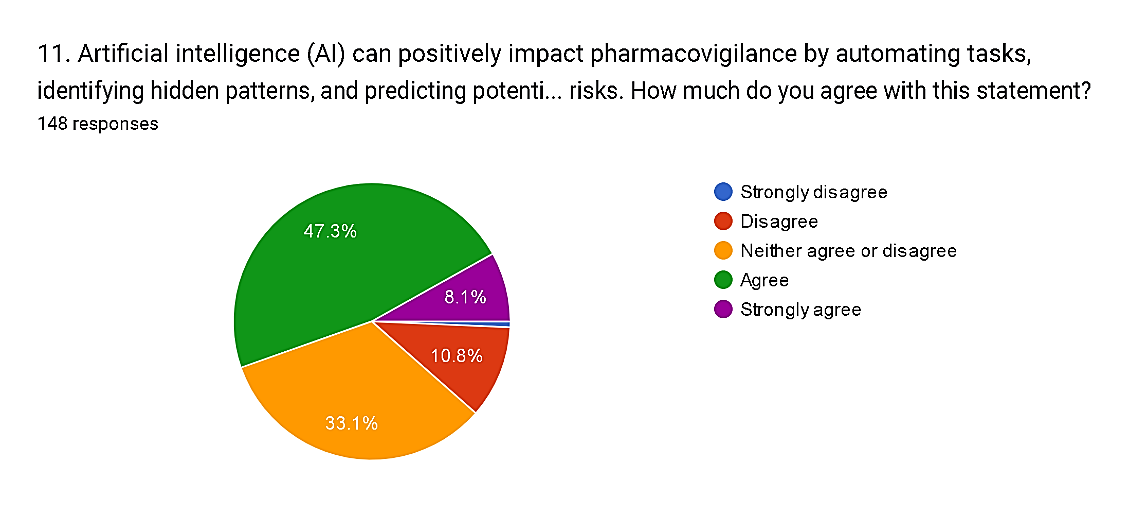 Fig. 15
Fig. 15
The survey results indicate a general consensus among respondents regarding the positive impact of Artificial Intelligence (AI) on pharmacovigilance. A majority, comprising 55.4% of respondents, express agreement, or strong agreement with the statement, with 47.3% agreeing and 8.1% strongly agreeing. Conversely, a smaller but still notable proportion, totaling 11.5%, disagree or strongly disagree with the notion, reflecting some skepticism about the efficacy of AI in enhancing pharmacovigilance practices. Additionally, 33.1% neither agree nor disagree, suggesting a need for further evidence or clarification regarding the specific benefits and limitations of AI technologies in this context.
These findings highlight the importance of continued research and validation studies to assess the real-world impact of AI-driven automation, pattern recognition, and risk prediction on improving drug safety and public health outcomes in pharmacovigilance.
VII. CHALLENGES AND RECOMMENDATIONS FOR AI IN PHARMACOVIGILANCE: ADDRESSING RESOURCE LIMITATIONS
A. Challenges
1) AI-Assisted Reporting for Pharmacovigilance (PV) Database Establishment
Data is the key to AI technology. Therefore, it is important to establish a comprehensive PV database. Every country is in a unique situation when establishing their PV database. Generally, PV is often initiated by HCPs, starting with spontaneous reporting of ICSRs. Several large-scale databases for PV or PV systems have been building up in both developed and developing countries, such as the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS)[10] and the Vaccine Adverse Event Reporting System (VAERS)[11] in the United States, the pharmacovigilance database in France, China’s pharmacovigilance system[12] and VigiBase[13]. VigiBase is maintained by the Uppsala Monitoring Center and contains data contributed from more than 150 countries around the world. However, for LMICs, the main issue when establishing a PV database is underreporting. This is due to a combination of multiple factors, including the poor infrastructure of reporting systems, low financial support, and the lack of human resources and relative policies. A group of data reflects the situation: Western countries (the United States, countries in the European Union, etc.) have contributed approximately 70% of the data in VigiBase, whereas only 0.9% of VigiBase ICSRs were contributed by Africa in 2019. The number of ICSRs received by the National Medicines Regulatory Authorities in Kenya, Ethiopia, and Tanzania (mainland) were 35.0, 6.7, and 4.1 per million inhabitants, respectively[14]. Alongside underreporting by HCPs, self-medication accounts for a large proportion of medicine use in LMICs, particularly in rural and remote areas, which may not be recorded by any medical records. Hence, how to use AI to assist in increasing the reporting rate of ADEs would be the main priority in resource-limited settings; for example, by extracting unreported ADEs recorded in the EHR [15].
2) Human Resources Challenges: Training and Education
Inadequate training opportunities and education leads to a shortage of HCPs for PV in LMICs. A traditional PV system utilizes professionals in four areas: operations, surveillance, systems, and Qualified Person for Pharmacovigilance (QPPV). In addition to drug safety supervisors, drug safety physicians, data/system security administrators and QPPVs necessary for traditional PV, AI-based PV also requires experts in various fields of AI, such as engineers for developing NLP and machine learning algorithms. This is another huge challenge for LMICs. Training for AI-based PV is cross-specialty, even cross-language, and is offered in only a small number of countries, with few LMICs on the list. Although AI technologies have been put into use for PV[10], there are currently few specific courses for AI-based PV, even on a global scale, and opportunities to attend such courses may be limited by insufficient financial support for HCPs from LMICs. Despite this, training on AI technologies, such as deep learning, NLP, and data mining, could be carried out for HCPs in developed countries. However, AI training opportunities in LMICs are in short supply. This type of training is beneficial for AI-based PV since the use of these advanced techniques helps data processing as well as data analysis.
A shortage of professionals for AI-based PV is common in LMICs. Education would be a way to increase the number of experts in both PV and AI. The biggest step for AI-based PV in education would be to introduce courses to undergraduates in teaching institutions for students in related majors. Education on AI-based PV could not only cultivate more interdisciplinary professionals but also improve their awareness of PV data. An example of insufficient education can be found in China; although it has established an online spontaneous self-reporting ADR monitoring system in recent years[16], a study of PV in Western China showed that the ADR reporting rate was extremely low because of unclear feedback pathways and a lack of understanding about the seriousness of ADRs[17]. For AI education in LMICs, increasing the rate of higher education is the main challenge. Also, the interdisciplinary nature of AI-based PV would be a problem for AI education, as it requires AI engineers to be familiar with medical knowledge.
3) Technological Challenges
There are two technological challenges for AI-based PV in resource-limited settings—data integration and data annotation.
Each medical institution supports its own data model, which may differ from those of others, and each type of data model may be unique, with differing terminologies and value representations[18].
Data from different sources needs to be integrated and converted into a common data model (CDM)-compatible format among multiple institutions. This requires both HCPs and engineers to spend time and effort transforming the data. In LMICs, however, language might be a barrier to data transformation, because it needs to be managed in several local languages or expression habits.
Data annotation is important for AI-based PV because the algorithms and training models rely on annotated data to make predictions on those that are not annotated. The lack of high-quality data for training the model and the high cost of annotation restricts research on AI-based PV. In a structured database, the potential PV signals are not annotated even though a large number of ICSRs exist in these databases. Additionally, unstructured data such as clinical notes, medical records, biomedical literature, and social media posts enrich PV information more than structured data. A study found that only 28.6% of adverse reactions to statins were recorded in a structured format in the hospital information system (HIS), while the rest were recorded in unstructured clinical narratives[19].
Nevertheless, the biggest technological challenge for AI-based PV is term variation. The terms used (e.g., the names of drugs and diseases) are less formal and highly variable, the description of side effects may not be clear, and poor grammar and spelling mistakes may be common[20,21]. Besides, the diversity of local languages makes it difficult for data processing, integration, and normalization. Concept normalization helps to solve this issue and improve data quality, reduce dimensions, increase retrieval recall, and integrate various data sources. However, concept normalization is an intensive and tedious task. Sources from social media[22], medical records[23], and biological literature[24] contain a large number of aliases, abbreviations, and informal terms that require concept normalization to achieve entity alignment. Nonetheless, the diversity of language in LMICs and lack of mapping between local languages and standard vocabularies are major gaps. This weakens the technology migration of mature technology platforms in developed countries.
4) Regulations and Funding for Pharmacovigilance
In AI-based PV, government plays a critical role in decision making. For most LMICs, the PV systems in their countries are young and are not underpinned by strong legal or regulatory provisions, and thus require stronger regulations to engage local industry and HCPs[25]. Although many of these countries have developed PV guidelines, the regulatory basis for the PV system is sometimes too weak for the authority to enforce regulatory actions, even in resource-limited countries with well-functioning PV systems[25]. Other regulations related to AI-based PV are those around data safety and privacy protection. This is vital as AI technologies need to access a large amount of personal information, and the leak of this information may result in severe consequences.
Moreover, the lack of financial support is another challenge. This includes the support of existing systems, improvement of information infrastructure, remuneration of HCPs and AI engineers, and education. Many countries may have set up their PV system, however, without a sustainable budget to support it, the system will not work well in the long term. In this regard, China and India provide good examples whereby financial support has led to these countries establishing sustainable PV systems[25]. In addition, the development of AI demands financial support for both hardware and software. Hardware is the foundation that provides a platform for software to run, while the application of AI software realizes the operation of the system. This can be a huge expense as the equipment in LMICs is often lacking. Governments therefore need to issue relevant policies not only to guarantee the budget but also for the education of HCPs and AI engineers.
B. Recommendations
1) Electronic Health Record (EHR) Improvement and Establishing a Database for an AI-Based Pharmacovigilance System
EHR improvement helps to store and manage clinical data. Integration of EHRs into the health care systems of LMICs is still in the nascent stage[26]. In addition to releasing relative policies to ensure the implementation of EHRs in the health care system, the improvements should focus on workforce training, system quality and integrity. Critical factors include identification of the patient, standards for data exchange, education and training, storage of EHRs and quality assurance. Important information such as laboratory results, medical records, and medication orders in EHRs can be integrated and used for AI-based PV modeling. Liu et al. reported that medication orders and inpatient laboratory test results helped to validate and identify ADRs[27]. In MADE1.0[28], researchers developed algorithms for drug safety surveillance in unstructured clinical notes and achieved micro F1 scores from 0.826 to 0.868[29,30]. The performance of AI-based PV detection can be further improved by integrating with external databases[31]. Embedding AI algorithms into the EHR system can monitor PV signals actively, send reminders, and fill in forms automatically.
In addition to EHR, biomedical literature and the social network are other approaches for collecting PV information. As complementary data, these PV signals proved to be valuable information in public health[20], and such enrichment of the database benefits AI-based PV by minimizing the biases caused by data incompleteness. Compared with countries with complete PV systems, this multi-sourced information might be more important in LMICs because it could compensate for their incomplete PV systems and provides reasonable information to local HCPs for prescriptions. While the literature is written in natural language and published in the database for retrieval and acquisition, integrating this data requires NLP-related technologies such as named entity recognition, named entity relationship extraction, and concept normalization[32]. Similarly, PV information shared through social media, such as Facebook, Twitter, and websites, is also unstructured text and needs to be processed as a complementary source of formal ADE reporting for LMICs. With the application of NLP, this information could also be collected for the database and used for AI-based PV.
2) Offering Training Opportunities and Courses in Education Institutions
The early development of national PV projects largely depends on a small number of trained professionals and their ability to inspire and train others. It is important to integrate PV within the curricula for health training institutions. India has mandated that all medical schools include PV training in undergraduate courses[25]. AI-based PV classes are suggested to be set up at graduate schools of computational medicine or biomedical informatics. This is worthy of consideration by all LMICs as this will raise the capability of informaticians or programmers to utilize AI in the PV domain and improve the practice of data reporting, which is an ongoing process for many countries or regions. Furthermore, related training in PV practices based on machine learning, information retrieval, knowledge organization, etc., can be beneficial for resource-limited countries. In addition, as AI-based PV is interdisciplinary, it is necessary to train multidisciplinary professionals. However, this is still in the exploratory stage.
3) Implementation of AI Technologies
As computers and network technologies are rapidly becoming available in healthcare systems, medical data is expanding. Although it requires greater investment and infrastructure to develop AI technologies for LMICs, it is and will be a great opportunity to catch up with the big data era and leverage the latest technology. For data processing, with the development of medical vocabularies that are interoperable among countries, such as SNOMED CT, LOINC, RxNorm and MeDRA, concept standardization becomes feasible in the pharmacovigilance area, which will then help improve the accuracy and recall of side-effect signaling systems. In resource-limited settings, mapping local nonstandard terms to standard vocabularies will greatly improve data quality and promote international PV research and practices.
4) Government Supports
Governments can guide and regulate the development of AI-based PV in terms of funds, talent policies, laws, and regulations, etc. In LMICs, there are a lot of problems in establishing AI-based PV systems, such as a lack of financial, material, and human resources. However, it is important to make the government aware of the benefits and cost effectiveness of such systems. For instance, AI can minimize human workload in data reporting and mining, and help in analyzing vast medical data, potentiating predictive and preventive healthcare. With knowledge of the benefits comes the need for regulations to be issued to (i) increase efforts for AI-based PV development, including funding, training and education, infrastructure and technology; (ii) ensure the operation of the system; (iii) supervise the enforcement of the regulations; and (iv) protect data security and personal information security.
VIII. FUTURE DIRECTIONS FOR AI-ENHANCED PHARMACOVIGILANCE SYSTEMS
In standard, AI-pushed technology hold the capacity to streamline nearly every issue of pharmacovigilance (PV), along with case processing, reporting, and risk tracking, thereby decreasing general processing time. Four capability destiny directions for AI-driven PV in aid-restricted settings are proposed.
A. Multinational Collaboration for Data Sharing and Validation
Multinational collaboration is a feasible mechanism to collect data to build up an AI-based PV platform for LMICs. Under this mechanism, LMICs share and verify data with each other. The data becomes rich and diverse, covering multiple regions and remaining highly representative across geographies and subpopulations.
Based on this, the AI-based PV platform can analyze, discover, and validate PV signals across countries.
In some LMICs, such as those in the Economic Community of West African States, PV plays a part in regional or subregional initiatives involving economic communities. PV centers are established in one or two countries at first and are used by the others [25]. In this situation, a CDM is an important part of the multinational collaboration. Based on the CDM, countries can integrate data from different sources, share standardized data, and analyze data with the same tools. Countries should choose or design a CDM according to their own circumstances. Rivera et al.[33] proposed five key areas and 12 consensus recommendations for designing sustainable linked data resources to generate actionable evidence in healthcare research. VigiFlow allows LMICs to maintain a database at low cost and helps them report ICSRs in compliance with international standards so that new safety signals can be identified early [25]. There are various tools or systems for data exchange, such as OHDSI [34], The FDA’s Sentinel System [35], CNDOES and ADVANCE [36]. Of these, OHDSI provides vocabularies and software for the full process of collaborative research, from data conversions to data analysis, and maintains an active community involving a large number of HCPs, researchers, engineers, and enterprises. These experiences are beneficial to multinational collaboration and are worth learning from.
Additionally, databases from developed countries, such as FAERS and VAERS, may be leveraged to help inform PV in limited resource settings. On one hand, LMICs are still accumulating post-market evaluation data for drugs, biologics, vaccines, etc. Being informed of PV signals from these databases is therefore beneficial to improve the safety of drugs and protect public health. On the other hand, the geographic correlations between races and ADR reporting rates in developed countries can be analyzed by integrating these databases and the population data. This may uncover potential racial differences in ADRs and provide some useful information for LMICs with common races [37].
B. Integration of Multiple Data Sources to Discover and Verify PV Signals
Spontaneous reports from HCPs and consumers have some limitations, such as bias and under-reporting. At present, there have been studies using AI algorithms to detect PV signals from other data sources, such as biomedical literature [38,39], knowledge graphs [40] and genetic data [41]. These data sources are complementary, observing drug safety from different perspectives.
Mower et al. [42] pointed out that using biomedical literature and spontaneous reports together performed better than using either source alone for drug side-effect prediction. Zheng and Xu [43] constructed a disease comorbidity network from FAERS and found that it correlated with the human genetic network and the disease treatment network. Some ADRs are related to inherited characteristics of the genome. With the integration of pharmacogenomic tests and the decision support system based on these data, PV signal triggering will become more accurate on the personal risk of potential drug side effects [44]. In the case of limited resources, if it is known that some biomarkers are especially prevalent, ADRs can be anticipated and prevented in advance.
Two typical examples are HLA-B*1502 and HLA-B*5801. Non-carriers of HLA-B*1502 and HLA-B*5801 have reduced risk of two severe cutaneous adverse reactions, Stevens-Johnson syndrome, and toxic epidermal necrolysis, caused by carbamazepine and allopurinol, respectively, whereas these two biomarkers are widely prevalent in Asia [45,46].
Moreover, Internet of Medical Things (IoMT) devices are capable of measuring the heart rate, blood pressure, temperature, blood glucose etc. of a patient [47]. This allows researchers to analyze changes in health parameters during ADRs and helps to determine the cause [48].
C. Expansion to Special Populations and the Dynamically Evolving Landscape
An important issue in resource-limited settings is that special populations, women, children, indigenous populations, etc., have low representativeness in previous data sources or evidence systems. Furthermore, due to the development of new drugs and shifts in population characteristics, limited resource settings and their associated populations may evolve.
In general, the above issues can be generalized as continuously increasing population coverage. Reactive learning algorithms can handle the dynamically evolving landscape. The algorithms sample the most valuable data from a large number of new data by similarity measurement and sampling strategy. Such algorithms can cover more diverse populations with fewer samples, which also means lower costs, and can continue to adapt to the dynamically evolving landscape [49]. For example, during the COVID-19 pandemic, investigators in Greece used a reinforcement-learning system for target data collection to maximize the detection of infected asymptomatic travelers. Compared with random sampling, the reinforcement-learning system improved testing efficiency up to 2–4 times during peak travel [50].
D. Routine, Affordable, and Sustainable Regulatory Monitoring
In resource-limited settings, without sufficient self-driven effort from multinational companies, which are mainly based in developed countries, a routine regulatory requirement enforced by the government is an essential way to promote effective monitoring of drug side effects. Cost-effectiveness analysis of the pilot projects mentioned above will help evaluate the affordability of such technologies [51]. Multi-stakeholder involvement will be the feasible solution to maintain a sustainable AI-based PV system on a national level. Government, pharmaceutical companies, high-tech IT companies, hospitals and patient advocacy groups are all important stakeholders, and they can contribute different perspectives.
It is important to consider particular contexts in resource-limited settings when developing and implementing AI-based technologies for pharmacovigilance. For instance, (i) government-driven regulatory monitoring is essential since there is not sufficient self-driven effort from multinational companies; (ii) the required computational resource needs enough financial support, which might be complemented through strategic collaboration; (iii) the need for data collection to be expanded to special populations (women, children, indigenous populations, etc.) is noteworthy because there was low representativeness in previous data sources or evidence systems; (iv) the AI-based PV system should cover popular drugs that the local people accept, such as Western drugs and herbal medicine; (v) manpower is key to the sustainable development of AI-based PV, thus education and training in resource-limited settings will play a crucial role.
Conclusion
Drug safety monitoring landscape is persistently changing, requiring applied solutions to protect the health of the patients. Among those leading approaches, AI undoubtedly is the one that can transform pharmacovigilance to a whole new level, switching it to a completely different quality with a very different paradigm than the old one. The article draws heavily on the survey conducted by the author to discuss the different intersections of Artificial Intelligence in pharmacovigilance underscoring a positive tendency of medical professionals to see the breakthrough technology come about as a beneficial development to the entrenched practice. An important feature of AI is its capacity to systematically analyse datasets from a number of resources, which would allow for the detection of the possible valuable safety signals and for the accurate assessment of the emerging drug safety issues, that might remain unnoticed under the traditional methods. The Machine learning AI can achieve this by examining massive, complex datasets, identifying the patterns and the associations behind the scenes. This leads to early detection and intervention. This ability is very notable and significant in pharmacovigilance, when the dissemination of adverse drug reactions recognition is critical in ensuring patient safety. Moreover, emerging risk prediction technology is a cutting-edge advancement in personalized medicine. It may help develop optimal treatment strategies tailored to the needs and characteristics of individual patients. By analysing patient data and predicting potential risks, AI enables healthcare professionals to make data-driven decisions, ultimately reducing severe side effects and improving treatment outcomes. AI takes part in both signal detection and risk prediction and at the same time provides advantages in liquid screening and drug repurposing. Performing a comprehensive review of millions of records and pieces of scientific literature and clinical data, AI systems can identify medications that could be repurposed, thus speeding up the drug discovery process and supplying more alternatives for cure. However, while AI can offer significant advantages to pharmacovigilance monitoring, exceptional care and deliberation should be applied. The success and sustainability of AI necessitate a responsible development and implementation, which act as a push factor to make the AI as an extension of the human intellect, not a replacement for it. Addressing AI algorithms bias, data privacy, and security and having human oversight are the clear decision space in AI pharmacovigilance. In the final point, AI fascinating possibilities for drug safety future of pharmacovigilance reveals, which can empower to strengthen operational efficiency, accuracy and proactivity of monitoring drug safety. With wise use of AI, we can enable the patient to feel safe and, thus, together, we can create a sound health system. Although AI has the potential to radically transform pharmacovigilance, more extensive research and improving systems is absolutely essential to achieve the maximum results of AI in this field, in addition to overcoming side effects. At the same time, the utilization of AI provides a rather substantial leap forward in the process of drug safety monitoring, representing a category of transformative actions which change the field in order to leads to improvements in patient care.
References
[1] Schwartz WB, Patil RS, Szolovits P, et al. Artificial intelligence in medicine. Where do we stand? New England Journal of Medicine. 1987;316:685-688. [2] Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM, et al. A Bayesian neural network method for adverse drug reaction signal generation. European Journal of Clinical Pharmacology. 1998;54:315-321. [3] Kompa B, Hakim JB, Palepu A, Kompa KG, Smith M, Bain PA, Woloszynek S, Painter JL, Bate A, Beam AL, et al. Artificial intelligence based on machine learning in pharmacovigilance: a scoping review. Drug Safety. 2022;45:477-491. [4] Bate A, Stegmann JU. Safety of medicines and vaccines - building next generation capability. Trends in Pharmacological Sciences. 2021;42:1051-1063. [5] van Stekelenborg J, Kara V, Haack R, Vogel U, Garg A, Krupp M, Gofman K, Dreyfus B, Hauben M, Bate A, et al. Individual case safety report replication: an analysis of case reporting transmission networks. Drug Safety. 2023;46:39-52. [6] Chan JTH, Liew DFL, Stojanova J, McMaster C, et al. Better pharmacovigilance through artificial intelligence: what is needed to make this a reality? Health Policy and Technology. 2022;11:Article 100638. [7] Sokol DK. \"First do no harm\" revisited. BMJ. 2013;347:Article f6426. [8] Owczarek D. Augmenting Drug Safety and Pharmacovigilance Services with Artificial Intelligence (AI). April 27, 2021. [9] Sachdeva N. What is the Role of AI in Pharmacovigilance? October 4, 2023. [10] Luo Y, Thompson WK, Herr TM, Zeng Z, Berendsen MA, Jonnalagadda SR, et al. Natural language processing for EHR-based pharmacovigilance: a structured review. Drug Saf. 2017;40(11):1075–1089. doi: 10.1007/s40264-017-0558-6. [11] Jeetu G, Anusha G. Pharmacovigilance: a worldwide master key for drug safety monitoring. J Young Pharm. 2010;2(3):315–320. doi: 10.4103/0975-1483.66802. [12] Li Q, Xie P. Outpatient workload in China. Lancet. 2013;381(9882):1983–1984. doi: 10.1016/S0140-6736(13)61198-8. [13] Linder JA, Haas JS, Iyer A, Labuzetta MA, Ibara M, Celeste M, et al. Secondary use of electronic health record data: spontaneous triggered adverse drug event reporting. Pharmacoepidemiol Drug Saf. 2010;19(12):1211–5. doi: 10.1002/pds.2027. [14] Barry A, Olsson S, Minzi O, Bienvenu E, Makonnen E, Kamuhabwa A, et al. Comparative assessment of the national pharmacovigilance systems in East Africa: Ethiopia, Kenya, Rwanda and Tanzania. Drug Saf. 2020;43(4):339–350. doi: 10.1007/s40264-019-00898-z. [15] Boland MR, Tatonetti NP. Are all vaccines created equal? using electronic health records to discover vaccines associated with clinician-coded adverse events. AMIA Jt Summits Transl Sci Proc. 2015;2015:196–200. [16] Zhao Y, Wang T, Li G, Sun S. Pharmacovigilance in China: development and challenges. Int J Clin Pharm. 2018;40(4):823–831. doi: 10.1007/s11096-018-0693-x. [17] Chen Y, Wang Y, Wang N, Xiang Y, Zhang R, Xiao J, et al. Knowledge, attitude, and practice regarding pharmacovigilance among the general public in Western China: a cross-sectional study. Curr Med Res Opin. 2021;37(1):101–108. doi: 10.1080/03007995.2020.1846171. [18] Chen R, Zhang Y, Dou Z, Chen F, Xie K, Wang S. Data sharing and privacy in pharmaceutical studies. Curr Pharm Des. 2021;27(7):911–918. doi: 10.2174/1381612827999210112204732. [19] Skentzos S, Shubina M, Plutzky J, Turchin A. Structured vs. unstructured: factors affecting adverse drug reaction documentation in an EMR repository. AMIA Annu Symp Proc. 2011;2011:1270–9. [20] Sloane R, Osanlou O, Lewis D, Bollegala D, Maskell S, Pirmohamed M. Social media and pharmacovigilance: a review of the opportunities and challenges. Br J Clin Pharmacol. 2015;80(4):910–920. doi: 10.1111/bcp.12717. [21] Li Z, Yang Z, Wang L, Zhang Y, Lin H, Wang J. Lexicon knowledge boosted interaction graph network for adverse drug reaction recognition from social media. IEEE J Biomed Health Inform. 2021;25(7):2777–86. [22] Sarker A, Belousov M, Friedrichs J, Hakala K, Kiritchenko S, Mehryary F, et al. Data and systems for medication-related text classification and concept normalization from Twitter: insights from the Social Media Mining for Health (SMM4H)-2017 shared task. J Am Med Inform Assoc. 2018;25(10):1274–1283. doi: 10.1093/jamia/ocy114. [23] Luo Y-F, Sun W, Rumshisky A. MCN: a comprehensive corpus for medical concept normalization. J Biomed Inform. 2019;92:103132. doi: 10.1016/j.jbi.2019.103132. [24] Do?an RI, Leaman R, Lu Z. NCBI disease corpus: a resource for disease name recognition and concept normalization. J Biomed Inform. 2014;47:1–10. doi: 10.1016/j.jbi.2013.12.006. [25] Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015;8(4):449–460. doi: 10.1586/17512433.2015.1053391. [26] Kumar M, Mostafa J. Research evidence on strategies enabling integration of electronic health records in the health care systems of low-and middle-income countries: a literature review. Int J Health Plan Manag. 2019;34(2):e1016–e1025. doi: 10.1002/hpm.2754. [27] Liu M, McPeek Hinz ER, Matheny ME, Denny JC, Schildcrout JS, Miller RA, et al. Comparative analysis of pharmacovigilance methods in the detection of adverse drug reactions using electronic medical records. J Am Med Inform Assoc. 2013;20(3):420–426. doi: 10.1136/amiajnl-2012-001119. [28] Jagannatha A, Liu F, Liu W, Yu H. Overview of the first natural language processing challenge for extracting medication, indication, and adverse drug events from electronic health record notes (MADE 1.0) Drug Saf. 2019;42(1):99–111. doi: 10.1007/s40264-018-0762-z. [29] Wunnava S, Qin X, Kakar T, Sen C, Rundensteiner EA, Kong X. Adverse drug event detection from electronic health records using hierarchical recurrent neural networks with dual-level embedding. Drug Saf. 2019;42(1):113–122. doi: 10.1007/s40264-018-0765-9. [30] Chapman AB, Peterson KS, Alba PR, DuVall SL, Patterson OV. Detecting adverse drug events with rapidly trained classification models. Drug Saf. 2019;42(1):147–156. doi: 10.1007/s40264-018-0763-y. [31] Wang L, Rastegar-Mojarad M, Ji Z, Liu S, Liu K, Moon S, et al. Detecting pharmacovigilance signals combining electronic medical records with spontaneous reports: a case study of conventional disease-modifying antirheumatic drugs for rheumatoid arthritis. Front Pharmacol. 2018;9:875. doi: 10.3389/fphar.2018.00875. [32] Zhao S, Su C, Lu Z, Wang F. Recent advances in biomedical literature mining. Brief Bioinform. 2021;22(3):bbaa057. doi: 10.1093/bib/bbaa057. [33] Rivera DR, Gokhale MN, Reynolds MW, Andrews EB, Chun D, Haynes K, et al. Linking electronic health data in pharmacoepidemiology: appropriateness and feasibility. Pharmacoepidemiol Drug Saf. 2020;29(1):18–29. doi: 10.1002/pds.4918. [34] Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574. [35] Cocoros NM, Fuller CC, Adimadhyam S, Ball R, Brown JS, Dal Pan GJ, et al. A COVID-19-ready public health surveillance system: The Food and Drug Administration’s Sentinel System. Pharmacoepidemiol Drug Saf. 2021;30(7):827–837. doi: 10.1002/pds.5240. [36] Willame C, Dodd C, van der Aa L, Picelli G, Emborg HD, Kahlert J, et al. Incidence rates of autoimmune diseases in European Healthcare Databases: a contribution of the ADVANCE project. Drug Saf. 2021;44(3):383–395. doi: 10.1007/s40264-020-01031-1. [37] Huang J, Du JC, Duan R, Zhang XY, Tao C, Chen Y. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:8. [38] Zhang Y, Wu H-Y, Xu J, Wang J, Soysal E, Li L, et al. Leveraging syntactic and semantic graph kernels to extract pharmacokinetic drug interactions from biomedical literature. BMC Syst Biol. 2016;26:10. [39] Zhang Y, Wu HY, Du J, Xu J, Wang J, Tao C, et al. Extracting drug-enzyme relation from literature as evidence for drug drug interaction. J Biomed Semantics. 2016;7:11. [40] Dasgupta S, Jayagopal A, Hong ALJ, Mariappan R, Rajan V. Adverse drug event prediction using noisy literature-derived knowledge graphs: algorithm development and validation. JMIR Med Inform. 2021;9(10):e32730. doi: 10.2196/32730. [41] Choi YH, Han CY, Kim KS, Kim SG. Future directions of pharmacovigilance studies using electronic medical recording and human genetic databases. Toxicol Res. 2019;35(4):319–330. doi: 10.5487/TR.2019.35.4.319. [42] Mower J, Cohen T, Subramanian D. Complementing observational signals with literature-derived distributed representations for post-marketing drug surveillance. Drug Saf. 2020;43(1):67–77. doi: 10.1007/s40264-019-00872-9. [43] Zheng C, Xu R. Large-scale mining disease comorbidity relationships from post-market drug adverse events surveillance data. BMC Bioinformatics. 2018;19(Suppl 17):500. [44] Yue Q-Y. The International Society of Pharmacovigilance (ISoP) Pharmacogenomic Special Interest Group: pharmacogenomics in pharmacovigilance. Drug Saf. 2021;44(6):615–617. doi: 10.1007/s40264-021-01068-w. [45] Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126–1133. doi: 10.1056/NEJMoa1009717. [46] Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015;351:h4848. doi: 10.1136/bmj.h4848. [47] Chatterjee P, Tesis A, Cymberknop LJ, Armentano RL. Internet of things and artificial intelligence in healthcare during COVID-19 pandemic-A south american perspective. Front Public Health. 2020;8:600213. [48] Dwivedi R, Mehrotra D, Chandra S. Potential of internet of medical things (IoMT) applications in building a smart healthcare system: a systematic review. J Oral Biol Craniofac Res. 2021. 10.1016/j.jobcr.2021.11.010. [49] Luo Y, Wunderink RG, Lloyd-Jones D. Proactive vs reactive machine learning in health care. JAMA. 2022;327(7):623. doi: 10.1001/jama.2021.24935. [50] REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–502. [51] Schmider J, Kumar K, LaForest C, Swankoski B, Naim K, Caubel PM. Innovation in pharmacovigilance: use of artificial intelligence in adverse event case processing. Clin Pharmacol Ther. 2019;105(4):954–961. doi: 10.1002/cpt.1255.
Copyright
Copyright © 2024 Humase Nazir. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET64304
Publish Date : 2024-09-22
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

