Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Exercise Mimetics: Harnessing the Therapeutic Effects of Physical Activity
Authors: Ms. Aarti Labhane, Sonal Nuche, Omkar Mohite, Ms. Reema Rani, Dr. Rupali Tasgaonkar, Laiba Momin, Raj Patil
DOI Link: https://doi.org/10.22214/ijraset.2024.59159
Certificate: View Certificate
Abstract
Over the past decade, there has been an increase in both scientific and commercial interest in finding bioactive oral substances, also known as \"exercise pills\" or \"exercise mimetics,\" that replicate or enhance the benefits of exercise. The benefits of an active lifestyle for both the body and brain are becoming more and more obvious. However not everyone can exercise because of illness, trauma, or age-related disease. Both artificial and natural substances stimulate genes involved in the metabolic remodelling of skeletal muscle, activate some of the major regulators, and simulate exercise. The numerous health advantages of physical activity are not being put into practice. Exercise-mimetic drugs are being developed in an attempt to bypass the necessity of physical activity for promoting health they replicate the effects of peptide molecules released by muscles that are produced when exercised. The goal is to replicate physiological reactions even in the absence of physical activity. Since physical activity is still the most effective treatment, the creation of \"exercise mimetics\" that target specific muscles .The review deals with the disorders that can treated with exercise mimetics.
Introduction
I. INTRODUCTION
“Me thinks that the moment my legs begin to move, my thoughts begin to flow”
- David Henry Thoreau -
‘Thoreau’s Journal’ August 19, 1851 [2]
Thoreau was one of the thinkers who believed that engaging in physical activity was essential to maintaining mental clarity and a creative flow. Both young and old creatures benefit from exercise, as it increases skeletal mass, improves the cardiovascular system, regulates metabolism, and enhances cognitive, memory, and mood-related brain functions. There is mounting scientific evidence to support the positive effects of an active lifestyle on the brain. [2]
The world's fourth greatest cause of death is physical inactivity, with about one-third of the population not meeting the recommended minimum level for health benefits. [3]
Exercise is known to be a very successful non-pharmaceutical intervention for a variety of human health issues. Firstly, a dose-response relationship has been shown between regular physical exercise and a lower risk of all-cause death in a systematic assessment of data involving millions of individuals [12]. It also provides significant advantages in the management and prevention of several chronic metabolic diseases [12], including cancer [13], diabetes [6], and cardiovascular disease [5].
It is important to note that the advantages of consistent physical activity extend beyond metabolic disorders.
Hepatic function, skeletal muscle, skin, the nervous system's central nervous system, and oxygen transport mechanisms are among the other parts of the body that are disrupted by exercise-induced stress [5]. Exercise can effectively combat a wide range of physiological ailments, age-related function decline, and metabolic difficulties. All things considered, exercise is essential to leading a healthy lifestyle. Unfortunately, people with diseases requiring better glucose metabolism and weight management frequently find it challenging to use exercise as an intervention. In addition to improving mood and cognition, an active lifestyle has been linked to a delayed development of neurodegenerative disorders. One medication probably won't be able to replace the positive impacts of physical activity on the human body and brain. Numerous options for pharmacological therapies for various diseases are provided by the intricate underlying molecular mechanisms and ways of action. [2]
The body adapts in several ways as a result of exercise training. The musculoskeletal and cardiovascular systems have well-documented effects, but in reality, few, if any, organs are likely unaffected. These adaptations have several positive health effects in addition to improving physical performance. Indeed, several lifestyle disorders like obesity, cardiovascular disease, and type 2 diabetes are at least partially attributed to physical inactivity, which is now acknowledged as a serious health risk (Lees and Booth, 2005). [8, 14] Drug therapy in mice can imitate several of the muscle phenotypic modifications known to be critical for human health, as demonstrated by Narkar et al. (2008). [8, 15] Western societies are witnessing an epidemic increase in many pathologies such as obesity, metabolic syndrome, cardiovascular problems, and other chronic diseases. This is mostly due to an increasingly sedentary lifestyle and historically unparalleled access to excess high-calorie food [10]. Remarkably, a lack of physical activity is also linked to an increased risk for other illnesses like mood disorders, neurodegeneration, and some forms of cancer that don't seem to have a direct connection to skeletal muscle [16,10] The significant rise in life expectancy in affluent nations has further aggravated the incidence rates of the majority of these long-term illnesses [17,10]
II. PATHWAYS ACTIVATED BY EXERCISE
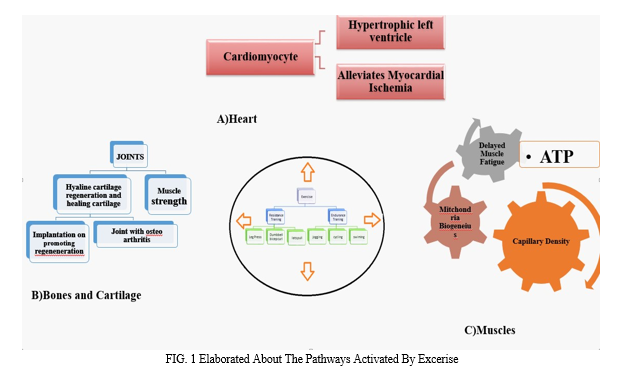
An overview of exercise-induced cellular effects and exercise-mimetics. In skeletal muscle, AMP-Kinase (AMPK) is stimulated by AICAR, Metformin, and Resveratrol. Positive regulation of signaling pathways related to fat metabolism, mitochondrial biogenesis, and endurance capacity is facilitated by activated AMPK. Also, by activating the transcription factor PPAR-δ specifically, the chemical GW501516 exerts impacts on metabolism. (-)Epicatechin and Resveratrol increase nitric oxide synthase (NOS), which influences angiogenesis and vasodilation in the brain and peripheral tissues. (-)Epicatechin also stimulates the TrkB-AKT-CREB pathway, which raises BDNF expression. These chemicals have certain effects on the brain and skeletal muscle, similar to those of exercise. [2]
A. AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide, or AICA-ribotide, is an intermediate metabolite in the purine production pathway and an analog of AMP. The active ingredient in 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside is AICAR, an endogenous molecule. Cellular adenosine kinase phosphorylates AICA-riboside, which then acts as AMP to activate AMPK by binding the AMPK γ subunit and triggering the phosphorylation of Thr172 [18].
AICAR's effects on peripheral organs:
AICAR mimics the effects of exercise in both in vitro and in vivo investigations, which impacts numerous organs and regulates a wide range of metabolic processes. For example, AICAR can replicate the effects of exercise by increasing the number of muscle mitochondria, hexokinase action, resting glycogen content, and the activity of glucose transporters type-4 (GLUT-4) [19, 20]. AICAR has also been shown to promote vascularization and angiogenesis by enhancing VEGFa's expression in a way close to physical activity [21, 22]. However, this mechanism is independent, distinct, and as unclear as AMPK activation.
AICAR is also known for its direct AMPK-dependent effects on the inflammatory process, resulting in a reduction of cytokine levels and inflammation. For example, in vitro incubation of human aortic smooth muscle cells with AICAR reduced vascular inflammation and pro-inflammatory cytokine levels in a dose-dependent manner [24]. In vivo, treatment of mice with AICAR decreased cytokine levels in the liver and improved glucose tolerance in obese diabetic mice in an insulin-independent manner by decreasing the rate of gluconeogenesis in the liver and increasing GLUT-4-mediated glucose uptake [23].
B. SIRT1
Primarily found in the nucleus, sirtuin 1 (SIRT1) functions as a major regulator of metabolic activities in response to energy availability [23]. Through AMPK's activation, it is sensitive to NAD+ and NADH concentrations, which in turn affects the availability of cellular energy [26]. It is also perceptive of alterations to intracellular redox conditions [25, 31]. Peroxisome promoter-activated gamma coactivator 1-alpha (PGC1-α) is activated and deacetylated with SIRT1 activation, which increases its specific activity as a transcription factor that acts on genes associated with fatty acid metabolism and mitochondrial respiration [25, 28]. However, data suggests that SIRT1 can also function as a PGC1-α inhibitor when overexpressed or knocked off, which would lower mitochondrial activity[25].
Also,because there is less glucose available when nutrients are scarce, SIRT1 causes a change in the metabolism of cells toward fatty acid oxidation [27]. Through its ability to stimulate catabolic processes and inhibit anabolic ones, SIRT1 contributes to the maintenance of energy homeostasis inside cells and supports cellular energy balance [27].SIRT1 activity has been demonstrated to increase in human skeletal muscle with physical exercise, particularly high-intensity interval training, and this has been linked to mitochondrial biogenesis [29]. Chronic exercise also induces systemic adaptations that normalize cellular processes and lessen the severity of neurodegenerative illnesses in patients by increasing the amount of SIRT1 transcription in the kidney, liver, and brain [30]. Numerous metabolic illnesses have been linked to defects in pathways that are controlled in part by SIRT1. As a result, pharmaceutical modification of this target continues to be a focus of attention due to the potential benefits of exercise-associated SIRT1 activation for health and disease. [4]
III. MECHANISMS
AMPK and SIRT1 are vivacious sensors and transduce work-out actuated signs to the record co-activator PGC1a by post-translational modifications. Because of the raised movement of PGC1α, PPARS, and ERRa/y straightforwardly control the declaration of downstream objective qualities engaged with FA oxidation, mitochondrial biogenesis, and angiogenesis, which ultimately lead to work-out-instigated medical advantages. Moreover, the co-repressors NCOR1 and Tear 140 are smothered by practice through obscure systems, which de-stifle PPARδ and ERRa/y and initiate their objective qualities.
IV. MOLECULAR TARGETS OF EXERCISE MIMETICS
1-AMP-activated protein kinase (AMPK)
AMPK is the expert controller of digestion-detecting energy supplies. AMPK is actuated in skeletal muscle during practice in response to increased binding of AMP and decreased binding of ATP. Transgenic mice conveying idle muscle-explicit AMPK showed decreased practice limit and weakened glucose resistance and insulin reaction
AMPK performance is expected for work-out-initiated mitochondrial biogenesis utilizing PGC-1α. Many investigations showed that the AMPK activator, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) mirrors the impacts of activity. AICAR utilization alone upgraded showing perseverance to 44% and metabolic qualities in stationary mice. AICAR builds the degrees of glucose carrier type 4 (GLUT4) and mitochondrial catalyst in skeletal muscle [32]. AICAR likewise expands angiogenesis and vascularization by prompting VEGF-An articulation, which thus works with a stable stock of oxygen and supplements-like activity.
AICAR was utilized as a "future" execution upgrading drug in the Olympic Spanish Cycling Crew, and a games specialist was captured for doping
V. DISORDERS THAT CAN BE TREATED WITH EXERCISE MIMETICS
Exercise mimetics are substances designed to mimic the physiological effects of physical activity. It's important to remember that not all problems can be healed by exercise mimetics, even if they are being explored for their potential in treating a variety of disorders. , exercise, and these mimetics may offer significant benefits in managing certain conditions. Some disorders that may benefit from exercise mimetics or exercise itself include:-
- Type 2 Diabetes: Regular exercise can improve insulin sensitivity and glucose uptake in cells, helping to manage and sometimes even reverse type 2 diabetes. Exercise mimetics targeting similar pathways could potentially offer similar benefits.
- Obesity: Exercise helps burn calories and build muscle, contributing to weight loss and management. Exercise mimetics that enhance metabolism or mimic exercise-induced metabolic changes may aid in managing obesity.
- Cardiovascular Diseases: Regular exercise is known to improve cardiovascular health by reducing blood pressure, improving blood lipid profiles, and enhancing heart function. Exercise mimetics that target cardiovascular health pathways may offer similar benefits.
- Metabolic Syndrome: Exercise is beneficial in managing metabolic syndrome by improving insulin sensitivity, reducing abdominal fat, and lowering blood pressure. Mimetics that replicate these effects could be helpful.
- Depression And Anxiety: Exercise has been shown to have antidepressant and anxiolytic effects by promoting the release of endorphins and neurotransmitters like serotonin and dopamine. Exercise mimetics targeting these pathways may have similar mood-enhancing effects.
- Age-Related Muscle Loss (Sarcopenia): Regular exercise, particularly resistance training, helps maintain muscle mass and strength. Mimetics that mimic exercise-induced muscle protein synthesis may help counteract age-related muscle loss.
- Neurodegenerative Disorders: Exercise has been shown to have neuroprotective effects and promote neurogenesis. Exercise mimetics that target brain-derived neurotrophic factor (BDNF) or other neuroprotective pathways may have potential in managing neurodegenerative disorders like Alzheimer's and Parkinson's disease.
- Osteoporosis: Weight-bearing and resistance exercises can improve bone density and strength. Mimetics that mimic exercise-induced bone remodelling processes may be beneficial in managing osteoporosis.
The obstacles and controversies behind the creation of an "exercise pill"
Certainly, a pharmaceutical approach that could mimic the varied and multifaceted impacts of physical exercise would be beneficial for populations like those with impairments, illnesses, injuries, or elderly who are unable to engage in physical activity. For example, it could be a "stepping stone" for people who are extremely obese or a way to resume exercise after suffering a serious accident. Nonetheless, several significant issues must be taken into account. [4]
Conclusion
Research on exercise mimetics is still in its infancy. Exercise mimetics are chemicals or live organisms that mimic the beneficial effects of exercise-induced cytokines and microbiota on the body. New compounds synthesized by biotechnology are included in this phrase. Small compounds, peptides, antibodies, non-coding RNA, gut microbiota, and epigenetic editing structures are examples of exercise mimetics. Furthermore, studies on exercise mimetics are complementary to studies on exercise itself, to develop novel therapeutic approaches by using the characteristics and functional attributes of the health advantages brought about by simulated exercise. Exercise mimetics currently target the neurological system, circulatory system, and exercise system. It has also been observed that there is probably a biological improvement effect in the immune and endocrine metabolism systems. In the future, bioinformatics and artificial intelligence technologies should help advance the field of exercise mimetics.
References
[1] Weiwei Fan1 and Ronald M. Evans1,2, http://dx.doi.org/10.1016/j.cmet.2016.10.022 [2] doi: 10.3233/BPL-160043 David Guerrieria Hyo Youl Moon, b and Henriette van Praagaga https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5928571/ [3] John A. Hawley, Michael J. Joyner, Daniel J. Green https://doi.org/10.1113/JP278761 [4] Mohamed Magdy Aly Hassan ElMeligie Submitted: 01 October 2021 Reviewed: 07 January 2022 Published: 12 February 2022 DOI: 10.5772/intechopen.102533 https://www.intechopen.com/chapters/80310 [5] Warburton DER, Bredin SSD. Health benefits of physical activity: A systematic review of current systematic reviews. Current Opinion in Cardiology. 2017; 32:541-556 [6] Fong DYT, Ho JWC, Hui BPH, et al. Physical activity for cancer survivors: Meta-analysis of randomized controlled trials. BMJ. 2012; 344:17 [7] Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2016; 39:2065-2079 [8] Can Exercise Mimetics Substitute for Exercise? Erik A. Richter Bente Kiens Jørgen F.P. Wojtaszewski https://doi.org/10.1016/j.cmet.2008.07.004 [9] Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab2015;22:4-11 https://doi.org/10.1016/j.cmet.2015.05.011 [10] Christoph Handschin https://doi.org/10.1016/j.phrs.2015.11.009 [11] Narkar V.A. Downes M.Yu R.T. Embler E. Wang Y.X. Banayo E. Mihaylova M.M. Nelson M.C.Zou Y. Juguilon H.et al.Cell. 2008; [12] Tian D, Meng J. Exercise for prevention and relief of cardiovascular disease: Prognoses, mechanisms, and approaches. Oxidative Medicine and Cellular Longevity. 2019;2019: 3756750. Hindawi Oxidative Medicine and Cellular Longevity Volume 2019, Article ID 3756750, 11 pages https://doi.org/10.1155/2019/3756750 [13] Hawley JA, Hargreaves M, Joyner MJ, et al. Integrative biology of exercise. Cell. 2014; 159:738-749 [14] Lees S.J.Booth F.W.World Rev. Nutr. Diet. 2005; 95: 73-79 [15] Narkar V.A.Downes M.Yu R.T.Embler E.Wang Y.X.Banayo E.Mihaylova M.M.Nelson M.C.Zou Y.Juguilon H.etal.Cell. 2008 [16] Handschin C, Spiegelman BM. The role of exercise and pgc1alpha in inflammation and 494 chronic disease. Nature 2008; 454 463?469. 495 [17] Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, Pawelec G. Aging, frailty 496 and age?related diseases. Biogerontology 2010;11:547?563. [18] Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558-65. [19] Sanchez J, Nozhenko Y, Palou A, Rodriguez AM. Free fatty acid effects on myokine production in combination with exercise mimetics. Molecular Nutrition & Food Research. 2013;57(8):1456-67. [20] Lauritzen HP, Brandauer J, Schjerling P, Koh HJ, Treebak JT, Hirshman MF, et al. Contraction and AICAR stimulateIL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes. 2013;62(9):3081-92. [21] Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circulation Research.2005;96(8):838-46. [22] Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression but is not necessary for the [23] Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, Mounier R, et al. Targeting the AMPK pathway forthe treatment of Type 2 diabetes. Frontiers in Bioscience. 2009;14:3380-400. [24] He G, Zhang YW, Lee JH, Zeng SX, Wang YV, Luo Z,et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Molecularand Cellular Biology. 2014;34(2):148-57. [25] Guerrieri D, Moon HY, van Praag H. Exercise in a pill: The latest on exercise mimetics. Brain Plasticity. 2017;2:153 [26] Matsakas A, Narkar VA. Endurance exercise mimetics in skeletal muscle. Current Sports Medicine Reports. 2010;9:227-232 [27] Gurd BJ, Perry CGR, Heigenhauser GJF, et al. High-intensity interval training increases SIRT1 activity in human skeletal muscle. Applied Physiology, Nutrition, and Metabolism. 2010;35:350-357 [28] Radak Z, Suzuki K, Posa A, et al. The systemic role of SIRT1 in exercise-mediated adaptation. Redox Biology. 2020;35:101467 [29] Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiological Reviews. 2012;92:1479-1514 [30] Qi Z, Zhai X, Ding S. How to explain exercise-induced phenotype from molecular data: Rethink and reconstruction based on AMPK and mTOR signaling. Springerplus. 2013;2:1 [31] Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. JAm Med Dir Assoc. 2018;14(6):392–7. https://doi.org/10.1016/j.jamda.2013.03.022 [32] Briggs AM, Cross MJ, Hoy DG, Sànchez-Riera L, Blyth FM, Woolf AD, March L. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist. 2016;56(Suppl2):S243–55. https://doi.org/10.1093/geront/gnw002. [33] McGuigan FE, Bartosch P, Åkesson KE. Musculoskeletal healthand frailty. Best Pract Res Clin Rheumatol. 2017;31(2):145–59.https://doi.org/10.1016/j.berh.2017.11.002. [34] Crow RS, Lohman MC, Titus AJ, Cook SB, Bruce ML, MackenzieTA, Bartels SJ, Batsis JA. Association of obesity and frailty in older adults: NHANES 1999-2004. J Nutr Health Aging. 2019;23(2):138–44. https://doi.org/10.1007/s12603-018-1138-x. [35] Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: aging-related loss of musclemass and function. Physiol Rev. 2019;99(1):427–511. https://doi.org/10.1152/physrev.00061.2017. [36] Hambright WS, Niedernhofer LJ, Huard J, Robbins PD. Murine models of accelerated aging and musculoskeletal disease. Bone. 2019;125:122–7. https://doi.org/10.1016/j.bone.2019.03.002. [37] Davies B, García F, Ara I, Artalejo FR, Rodriguez-Mañas L, Walter S. Relationship between sarcopenia and frailty in the Toledo study of healthy aging: a population-based cross-sectional study. J Am Med Dir Assoc. 2018;19(4):282–6 https://doi.org/10.1016/j.jamda.2017.09.014 [38] Pin F, Bonewald LF, Bonetto A. Role of myokines and osteogenesis cancer cachexia. Exp Biol Med (Maywood). 2021;246(19): 2118–27. https://doi.org/10.1177/15353702211009213. [39] Atella V, Piano Mortari A, Kopinska J, Belotti F, Lapi F, Cricelli C, Fontana L. Trends in age-related disease burden and healthcare utilization. Aging Cell. 2019;18: e12861. [40] Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Aging as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–81. [41] Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer’sdisease. Lancet. 44. 2021;397:1577–90. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer\'s Dement. 2021;17:1966–75. [42] Gubert C, Hannan AJ. Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov. 2021;20:862–79. [43] Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–94. [44] Liu H-W, Chang S-J. Moderate exercise suppresses NF-?B signaling and activates the SIRT1-AMPK-PGC1? axis to attenuate muscle loss in diabetic db/db mice. Front Physiol. 2018; 9:636.molecular) https://doi.org/10.1016/j.jesf.2023.12.002 [45] Yuping Zhu, Gang Song https://doi.org/10.1016/j.jesf.2023.12.002
Copyright
Copyright © 2024 Ms. Aarti Labhane, Sonal Nuche, Omkar Mohite, Ms. Reema Rani, Dr. Rupali Tasgaonkar, Laiba Momin, Raj Patil. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59159
Publish Date : 2024-03-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

