Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Understanding the Hazards of Hexane and Short-Term Exposure to Air Contaminated with Hexane Affects the Nervous System and can Cause Dizziness
Authors: Dr. Chilakalapudi Meher Babu
DOI Link: https://doi.org/10.22214/ijraset.2024.63792
Certificate: View Certificate
Abstract
The term ‘hydrocarbon’ is self-explanatory which means compounds of carbon and hydrogen only. Hydrocarbons play a key role in our daily life. You must be familiar with the terms ‘LPG’ and ‘CNG’ used as fuels. LPG is the abbreviated form of liquified petroleum gas whereas CNG stands for compressed natural gas. Another term ‘LNG’ (liquified natural gas) is also in news these days. This is also a fuel and is obtained by liquifaction of natural gas. Petrol, diesel and kerosene oil are obtained by the fractional distillation of petroleum found under the earth’s crust. Coal gas is obtained by the destructive distillation of coal. Natural gas is found in upper strata during drilling of oil wells. The gas after compression is known as compressed natural gas. LPG is used as a domestic fuel with the least pollution. Kerosene oil is also used as a domestic fuel but it causes some pollution. Automobiles need fuels like petrol, diesel and CNG. Petrol and CNG operated automobiles cause less pollution. All these fuels contain mixture of hydrocarbons, which are sources of energy. Hydrocarbons are also used for the manufacture of polymers like polythene, polypropene, polystyrene etc. Higher hydrocarbons are used as solvents for paints. They are also used as the starting materials for manufacture of many dyes and drugs. Thus, you can well understand the importance of hydrocarbons in your daily life. In this unit, you will learn more about hydrocarbons.
Introduction
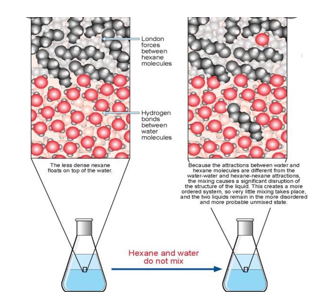
I. INTRODUCTION
The term hydrocarbon refers to an organic chemical compound that is composed exclusively of hydrogen and carbon atoms. Hydrocarbons are naturally-occurring and form the basis of crude oil, natural gas, coal, and other important energy sources. There are four main different types of hydrocarbons, which are classified as alkanes, alkenes, alkynes and aromatic hydrocarbons. The formula for acyclic saturated hydrocarbons (i.e., alkanes) is CnH2n+2. The bulk of a typical gasoline consists of a homogeneous mixture of small, relatively lightweight hydrocarbons with between 4 and 12 carbon atoms per molecule (commonly referred to as C4–C12). It is a mixture of paraffins (alkanes), olefins (alkenes), and napthenes (cycloalkanes). The most general form of saturated hydrocarbons, (whether linear or branched species, and whether with without one or more rings) is CnH2n+2(1-r), where r is the number of rings.)
A. Types of Hydrocarbons
- Saturated hydrocarbons.
- Unsaturated hydrocarbons.
- Cycloalkanes.
- Aromatic hydrocarbons.
- Aliphatic hydrocarbons.
- Alicyclic hydrocarbons.
Homogeneous Mixture is a substance composed of two or more components that are uniformly distributed at the molecular or microscopic level, creating a uniform appearance and consistent properties throughout the entire mixture. A mixture is nothing but a combination of two or more substances in which each substance maintains its chemical properties.
a. There are two types of mixtures i.e. homogenous mixture and heterogeneous mixture. In this particular article, we are going to learn about homogenous mixtures their types, properties, examples, and even how we can identify homogenous mixtures. We have to study Homogeneous Mixture
b. Soil. It is a heterogeneous mixture due to the presence of different-sized particles and variations in composition across different regions. On the other hand, options B, C, and D (sugar-water solution, gasoline, and salt-water solution) are considered homogeneous mixtures.
II. TABLE OF ALKANE HYDROCARBONS
The below lists the first 20 alkanes, although there are many more. Each type of fuel will have mixtures of different alkane chains, and these mixtures will give the fuel its desired properties.
|
Alkane |
Molecular Formula |
Composition |
Carbon atoms in chain |
Hydrogen atoms in chain |
|
Methane |
CH4 |
CH4 |
1 |
4 |
|
Ethane |
C2H6 |
CH3-CH3 |
2 |
6 |
|
Propane |
C3H8 |
CH3-CH2-CH3 |
3 |
8 |
|
Butane |
C4H10 |
CH3-2(CH2)-CH3 |
4 |
10 |
|
Pentane |
C5H12 |
CH3-3(CH2)-CH3 |
5 |
12 |
|
Hexane |
C6H14 |
CH3-4(CH2)-CH3 |
6 |
14 |
|
Heptane |
C7H16 |
CH3-5(CH2)-CH3 |
7 |
16 |
|
Octane |
C8H18 |
CH3-6(CH2)-CH3 |
8 |
18 |
|
Nonane |
C9H20 |
CH3-7(CH2)-CH3 |
9 |
20 |
|
Decane |
C10H22 |
CH3-8(CH2)-CH3 |
10 |
22 |
|
Undecane |
C11H24 |
CH3-9(CH2)-CH3 |
11 |
24 |
|
Dodecane |
C12H26 |
CH3-10(CH2)-CH3 |
12 |
26 |
|
Tridecane |
C13H28 |
CH3-11(CH2)-CH3 |
13 |
28 |
|
Tetradecane |
C14H30 |
CH3-12(CH2)-CH3 |
14 |
30 |
|
Pentadecane |
C15H32 |
CH3-13(CH2)-CH3 |
15 |
32 |
|
Cetane (Hexadecatane) |
C16H34 |
CH3-14(CH2)-CH3 |
16 |
34 |
|
Heptadecane |
C17H36 |
CH3-15(CH2)-CH3 |
17 |
36 |
|
Octadecane |
C18H38 |
CH3-16(CH2)-CH3 |
18 |
38 |
|
Nonadecane |
C19H40 |
CH3-17(CH2)-CH3 |
19 |
40 |
|
Eicosane |
C20H42 |
CH3-18(CH2)-CH3 |
20 |
42 |
A. Types of Hydrocarbons
- Saturated Hydrocarbons: In these compounds, carbon-carbon atoms and carbon-hydrogen atoms are held together by single bonds. These single-bonded compounds are the simplest hydrocarbons. These types of hydrocarbons don’t have double or triple bonds. In terms of hybridization, they have Sp3 hybridised carbon atoms with no Sp2 or Sp hybridised carbon atoms. They are together called alkanes which have a general formula of CnH2n+2. For example, CH4C3H6.
- Unsaturated Hydrocarbons: These compounds consist of a single, double or triple bond between carbon-carbon atoms. The double-bonded compounds are called alkenes, and the triple-bonded compounds are called alkynes. The general formula for alkenes is CnH2n, and for alkynes, the general formula is CnH2n-2.
- Cycloalkanes: These hydrocarbons possess one or multiple carbon rings. The hydrogen atom is attached to the carbon ring.
- Aromatic Hydrocarbons: They are also called arenes. Arenes are compounds which consist of at least one aromatic ring.
- Aliphatic Hydrocarbons: They are straight chain structures having no rings in them.
- Alicyclic Hydrocarbons: They are hydrocarbons having a ring structure in them. The carbons atoms can be Sp, Sp2, or Sp3 hybridised.
III. HEXANE - ENVIRONMENTAL PROTECTION
IV. SOURCES AND POTENTIAL EXPOSURE
The most probable route of human exposure to hexane is by inhalation. Individuals are most likely to be exposed to hexane in the workplace. Monitoring data indicate that hexane is a widely occurring atmospheric pollutant. Assessing Personal Exposure Laboratory tests can detect a breakdown product of hexane in urine.
A. Health Hazard Information Acute Effects
Acute inhalation exposure of humans to high levels of hexane causes mild CNS depression. CNS effects include dizziness, giddiness, slight nausea, and headache in humans. Acute exposure to hexane vapors may cause dermatitis and irritation of the eyes and throat in humans. Acute animal tests in rats have demonstrated hexane to have low acute toxicity from inhalation and ingestion exposure.
B. Chronic Effects (Noncancer)
Chronic inhalation exposure to hexane is associated with sensorimotor polyneuropathy in humans, with Chronic inhalation exposure to hexane is associated with sensorimotor polyneuropathy in humans, with numbness in the extremities, muscular weakness, blurred vision, headache, and fatigue observed. Rats, chronically exposed by inhalation, have exhibited neurotoxic effects. Mild inflammatory, erosive, and degenerative lesions in the olfactory and respiratory epithelium of the nasal cavity have been observed in mice chronically exposed by inhalation. Pulmonary lesions have also been observed in chronically exposed rabbits. The Reference Concentration (RfC) for hexane is 0.2 milligrams per cubic meter (mg/m3) based on neurotoxicity in humans and epithelial lesions in the nasal cavity in mice. The RfC is an estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without appreciable risk of deleterious noncancer effects during a lifetime. It is not a direct estimator of risk but rather a reference point to gauge the potential effects. At exposures increasingly greater than the RfC, the potential for adverse health effects increases. Lifetime exposure above the RfC does not imply that an adverse health effect would necessarily occur. EPA has medium confidence in the epidemiological study on which the RfC was based because the lowest-observed-adverse-effect level (LOAEL) in this study was based on neurotoxicology, and this endpoint is supported by numerous other subchronic inhalation studies in animals and by human occupational studies; medium confidence in the database because of the lack of long-term inhalation studies and appropriate reproductive studies; and, consequently, medium confidence in the RfC. EPA has not established a Reference Dose (RfD) for hexane. EPA has calculated a provisional RfD of 0.06 milligrams per kilogram body weight per day (mg/kg/d) based on neurological and reproductive effects in rats. The provisional RfD is a value that has had some form of Agency review but is not on IRIS.
C. Reproductive/Developmental Effects
No information is available on the reproductive or developmental effects of hexane in humans.Testicular damage has been observed in male rats exposed to hexane via inhalation. Teratogenic effects were not observed in the offspring of rats chronically exposed via inhalation in several studies.
- Cancer Risk: No information is available on the carcinogenic effects of hexane in humans or animals. EPA has classified hexane as a Group D, not classifiable as to human carcinogenicity, based on a lack of data concerning carcinogenicity in humans and animals.
- Physical Properties: The chemical formula for hexane is C6H14, and its molecular weight is 86.17 g/mol. Hexane is a colorless volatile liquid that is insoluble in water and highly flammable. The odor threshold for hexane is 130 parts per million (ppm), with a faint peculiar odor reported. The vapor pressure for hexane is 150 mm Hg at 25 °C.
- Conversion Factors: To convert concentrations in air (at 25 °C) from ppm to mg/m3 : mg/m3= (ppm) × (molecular weight of the compound)/(24.45). For hexane: 1 ppm = 3.53 mg/m3.
V. HEALTH DATA FROM INHALATION EXPOSURE
Hexane evaporates easily, so breathing contaminated air during the manufacture and use of the chemical is the most common form of exposure.
- Please Note: The main source of information for this fact sheet is EPA's Integrated Risk Information System (IRIS), which contains information on inhalation chronic toxicity of hexane and the Reference Concentration (RfC). Another secondary source used is the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Hexane.
- Uses: The main use of hexane is as a solvent to extract edible oils from seed and vegetable crops (e.g., soybeans,peanuts, corn). Commercial grades of hexane are used as solvents for glues (rubber cement, adhesives), varnishes, and inks. Hexane is also used as a cleaning agent (degreaser) in the printing industry. Hexane is used as the liquid in low temperature thermometers.
Conclusion
When happens hexane burns in air? in chemical reaction, we can see that two molecules of hexane react with thirteen molecules of oxygen and produce twelve molecules of carbon monoxide and fourteen molecules of water. A. The hexane flammable in air? Fire: Flash point: -23C (-9F) CC Autoignition temperature: 224C (435F) Flammable limits in air % by volume: lel: 1.2; uel: 7.7 Extremely Flammable Liquid and Vapor! Vapor may cause flash fire. Dangerous fire hazard when exposed to heat or flame. B. Can hexane an Air pollutant? Hexane is a federal hazardous air pollutant and was identified as a toxic air contaminant in April 1993 under AB 2728. Hexane is a colorless, volatile liquid with a faint odor. It is soluble in alcohol, ether, chloroform, and acetone, and insoluble in water. C. hexane catch fire? n-Hexane is a FLAMMABLE LIQUID. DO NOT attempt to extinguish fire unless flow can be stopped. Shut off supply or let burn. Use dry chemical, CO2, water spray or alcohol-resistant foam as extinguishing agents. D. How Explosive is Hexane? Like gasoline, hexane is highly volatile and is an explosion risk. The 1981 Louisville sewer explosions, which destroyed over 13 mi (21 km) of sewer lines and streets in Kentucky, were caused by ignition of hexane vapors which had been illegally discharged from a soybean processing plant owned by Ralston-Purina. E. Is hexane harmful to humans? Chronic (long- term) exposure to hexane in air is associated with polyneuropathy in humans, with numbness in the extremities, muscular weakness, blurred vision, headache, and fatigue observed. Neurotoxic effects have also been exhibited in rats. F. Does hexane smell? Pure n-hexane is a colorless, volatile liquid with a slightly disagreeable, gasoline-like odor. It evaporates very easily in air and dissolves only slightly in water. Exposure. The most probable route of human exposure to hexane is by inhalation. Individuals are most likely to be exposed to hexane in the workplace. Monitoring data indicate that hexane is a widely occurring atmospheric pollutant.
References
[1] U.S. Department of Health and Human Services. Hazardous Substances Data Bank (HSDB, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993. [2] M. Sittig. Handbook of Toxic and Hazardous Chemicals and Carcinogens. 2nd ed. Noyes Publications, Park Ridge, NJ. 1985. [3] U.S. Environmental Protection Agency. n-Hexane Health Advisory. Office of Drinking Water, Washington, DC. 1987. [4] U.S. Department of Health and Human Services. Registry of Toxic Effects of Chemical Substances (RTECS, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993. [5] U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on n-Hexane. National Center for Environmental Assessment, Office of Research and Development, Washington, DC. 1999. [6] Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Hexane. Draft for Public Comment. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. 1997. [7] E.J. Calabrese and E.M. Kenyon. Air Toxics and Risk Assessment. Lewis Publishers, Chelsea, MI. 1991. 8. The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals. 11th ed. Ed. S. Budavari. Merck and Co. Inc., Rahway, NJ. 1989. [8] J.E. Amoore and E. Hautala. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. Journal of Applied Toxicology, 3(6):272-290. 1983. [9] U.S. Environmental Protection Agency. Health Effects Assessment Summary Tables. FY 1997 Update. Solid Waste and Emergency Response, Office of Emergency and Remedial Response, Cincinnati, OH. EPA/540/R-97-036. 1997.
Copyright
Copyright © 2024 Dr. Chilakalapudi Meher Babu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63792
Publish Date : 2024-07-28
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

