Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Hippocampus Prosthesis for Memory Impairment
Authors: Sawarya Chandra
DOI Link: https://doi.org/10.22214/ijraset.2023.54431
Certificate: View Certificate
Abstract
Hippocampus is the area of the brain that is vital for functions as learning, memory and mood setting. It also reacts to injury or neurodegeneration with robust plasticity. Damage to the hippocampus leads to loss of ability to convert short-term memory to new long-term memories. This can happen due to epilepsy, stroke, dimentia or head injuries. Hippocampal injuries typically lead to cognitive dysfunction, depression, and/or epilepsy. Neural stem cell grafting early after injury has promise for preventing neurological deficits. It modulates aberrant hippocampal post-injury plasticity.and adds new inhibitory GABA-ergic interneurons into the hippocampus. Hippocampal memory prosthesis is a Brain Machine Interface (BMI) device developed for restoring or enhancing memory functions. The objective is to restore the long-term memory function in a stimulus-specific manner by using a multi-input, multi-output (MIMO) nonlinear dynamical model which might serve as a boon for patients suffering from Alzheimer’s disease.
Introduction
I. INTRODUCTION
Hippocampus is an integral component of brain playing vital functions as learning, memory and mood. It also reacts to injury or neurodegeneration with robust plasticity. Hippocampal injury can occur due to head trauma, ischemia, hemorrhagic stroke, acute seizures, status epilepticus, encephalitis, brain tumors, drug withdrawal, and exposure to chronic unpredictable stress, and Alzheimer’s disease (AD) [1]. Hippocampus damage can lead to several neurophysiological diseases, such as epilepsy, depressive disorder and AD. AD could be considered as a severe problem as no effective medication is available. Hence, it can be a great alternative to explore hippocampal memory prosthesis [2].

II. HISTORICAL PROGRESS ON ROLE OF HIPPOCAMPUS
Hippocampus anatomy is well described in a review published by [3] in 2012. The role of hippocampus plays a key role in several brain functions. Memory, perception of space, and time. Between the late 19th century and early 20th century, scientists started to localize memory in the brain. In particular, growing evidence showed a correlation between memory and temporal lobe, where the hippocampus is located.
The first turning point happened with the work of Scoville and Milner published in 1957, and the famous patient Henry Molasion, who for decades, was known as the patient H.M. to protect his privacy. H.M. was severely epileptic and his seizure were resistant to anti epileptic drugs.
In an attempt to alleviate the seizures the patient underwent a bilateral medial temporal lobe resection. The operation did reduce the seizure, but left the patient with a profound amnesia. H.M. could not remember episodes after the operation, anterogade amnesia, and just before the surgery, retrograde amnesia. During the 60’s, the case of H.M. promoted a quest for animal model of amnesia, but the first attempts were not completely successful. Around 1970, several important developments took place that improve the situation. Researchers developed behavioral tests that were more selective of the hippocampus. In parallel, it became clear that there were several forms of memory, and only a subset of them depends on the hippocampus.
In fact, we can distinguish a declarative memory, which is conscious recollection of concept and episodes can be described in words, and our procedural memory, which includes motor skills, and dosen’t require a conscious control and attention.
Declarative memory can be further divided in episodic memory, which stores specific personal experiences, and semantic memory, which store factual information.
We now know that the hippocampus is involved mostly in episodic memory. Episodic memory is the memory or episodes, which occur in time and space. Does the hippocampus specifically encode space and time? In 1971, O’Keefe and Dostrovsky published a short communication documenting the first clear evidence, where now hippocampus cells represent space. In this classical experiment, rats had microelectrodes implanted in their brains, which recording the activity of several hippocampal neurons while the animal could move freely in an environment.
O’Keefe and Dotrovsky observed that 8 electrodes over 76 correlated with the position and direction of the animal, that first describe what we now call place cells. This discovery simulated O’Keefe and Nadel to formulate the first of many spatial theories of the hippocampus. In the following years, researchers discovered other cells that encode the relationship between the animal and its location in space.
For example, in the 80’s, Ranck discovered head direction cells, which encode where the animal is facing. At the beginning of the new century, Edward and May Britt Moser’s group discovered grid cells, which represent space in an hexagonal coordinated system. Few years later, Neil Burgess’ group discovered boundary cells, which are sensitive to boundaries. This line of research has been so important for modern neuroscience, that in 2014, John O’Keefe and May Britt and Edward Moser, received a Nobel Prize in Physiology and medicine.
The perception of time is a perception of an order or sequence in time, so if the animal experiences a sequence of stimuli, it perceives that a stimulus occurred before, after, while at the same time as another. To test if the animal perceives the order of a sequence, the animal is tested for the memory of disorder. Early evidence show how damage to the hippocampus impairs memory of the temporal order of events in both human and animals. Other early studies associated the activation of the human hippocampus with encoding a recall of a sequence of events. At the cellular level, initial studies showing an association between cell firings and time, could not disclose the spatial component.
The work of [4] in 2007, described some of the first convincing evidence for such an association. In the experiment and encoding phase, rats alternated between two sides of a testing enclosure, as they sampled a unique sequence of five odors. At the subsequent test phase, rats were presented with a randomly selected pairs of non sample odors, and were rewarded for choosing the odor that had appear early in that sample phase. The rat performed almost 80%. Correct showing they could track the order of stimuli. The alteration of the location of the odors allows the experimenters to distinguish spatial clues from temporal clues. In 2011, MacDonald et al characterized cells in CA1 and named them time cells, stressing an analogy with the place cells. In fact, as place cells defined sequences in a spatial dimension, time cells, they find sequences in a temporal dimension. Memory, space and time are probably three phases of the same phenomena. In other words, the hippocampus combines stimuli to the spatial temporal context, and form any methodic memory trace.
The hippocampus is an important region also when we talk about some types of pathologies. For example, in the Alzheimer’s disease, the hippocampus has received substantial attentions since initial alteration of the pathology seems to start here before spreading eventually to the rest of the brain.
Another important example is epilepsy. As in the case of patient H.M., the temporal lobe is often the focus of seizures, since the hippocampus formation needs considerably less current to elicit epileptiform activity compared to other cortical areas. Finally, the hippocampus, in particular, CA1, is highly vulnerable to ischemic hypoxic insults, making this region critical also in cerebrovascular diseases.
III. THE HIPPOCAMPAL NEURAL NETWORK MODEL
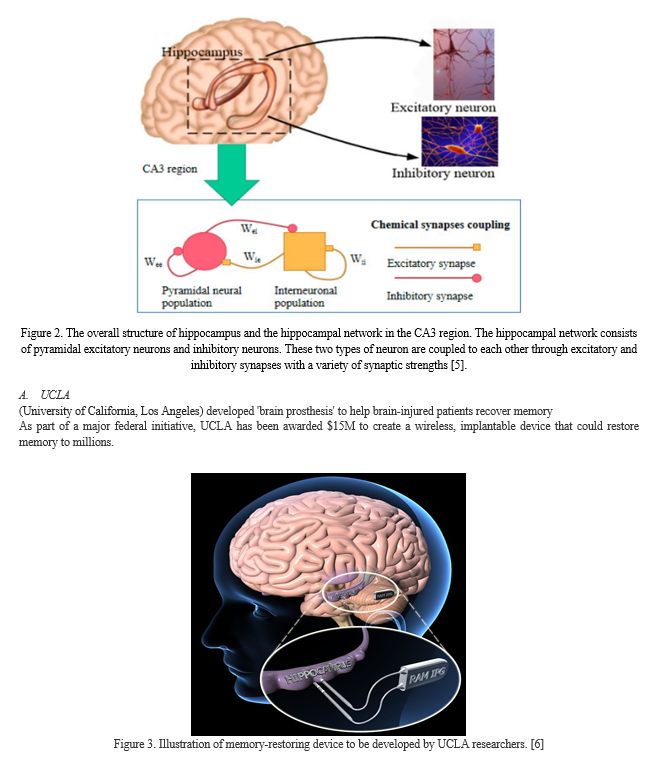
As shown in Figure 2, hippocampal neural coding is closely related to peridergic pyramidal neurons and inhibitory neurons, and the hippocampal neural network can contain both important types of neurons. Pyramidal neurons are asymmetrical in the narrow region between the dendrites and the cell body, whereas the structures of the genus rabbit on both sides of the cell body of the intermediate neuron are symmetrical.
As a result, the two types of neurons have different effects in the electrical field outside the cell. Besides, the network is coupled with various synaptic connections due to the relatively local dense connectivity
Hippocampal injuries typically evolve into cognitive dysfunction, depression, and/or epilepsy. Neural stem cell grafting early after injury has promise for preventing neurological deficits. It modulates aberrant hippocampal post-injury plasticity.and adds new inhibitory GABA-ergic interneurons into the hippocampus. Further, it adds new astrocytes secreting a variety of neurotrophic factors. However, source of neural stem cells needs to be resolved before going for clinical applications [1].
B. Neural Stem Cells (NSCs)
NSCs being self-renewing are multipotent cells capable of generating all three central nervous system (CNS) phenotypes as neurons, astrocytes and oligodendrocytes. Currently, Cell therapy using NSCs as donor cells has received great interest as one of the promising therapeutic approach for restoring hippocampus injury-induced memory and mood dysfunction [7].
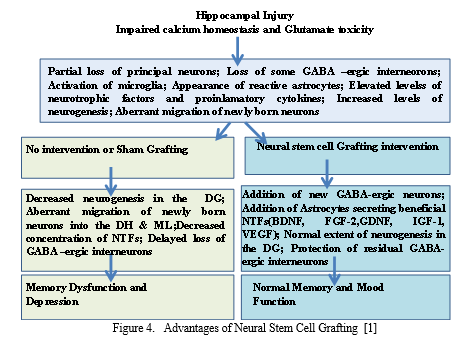
(GABA) Gamma-amino butyric acid; BDNF, brain derived neurotrophic factor; FGF-2, fibroblast growth factor-2; IGF-1, insulin-like growth factor-1; VEGF, vascular endothelial growth factor, spontaneous recurrent seizures (SRS);(DG) dDentate gyrus; (DH) Dentate hilus ; (ML) Molecular layer.
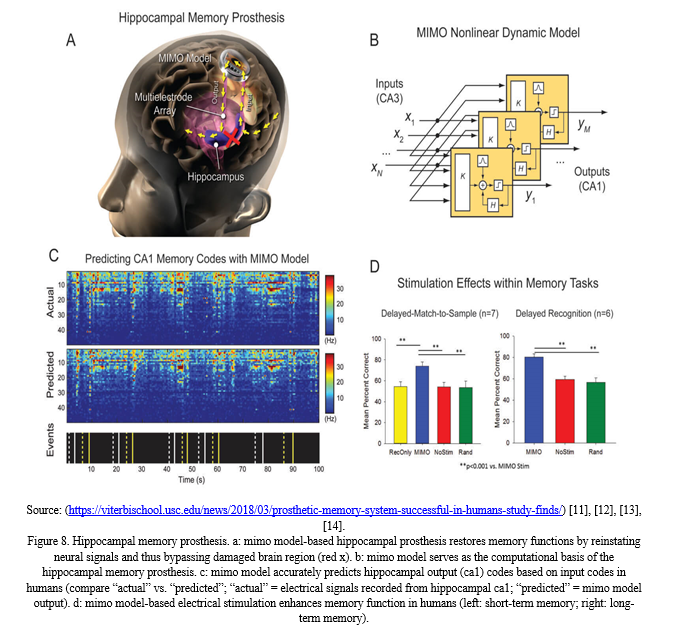
IV. NEURAL PROSTHESIS FOR HIPPOCAMPAL MEMORY FUNCTION
Hippocampal memory prosthesis is a Brain Machine Interface (BMI) device developed for restoring or enhancing memory functions. The objective is to restore the long-term memory function in a stimulus-specific manner by using a multi-input, multi-output (MIMO) nonlinear dynamical model.
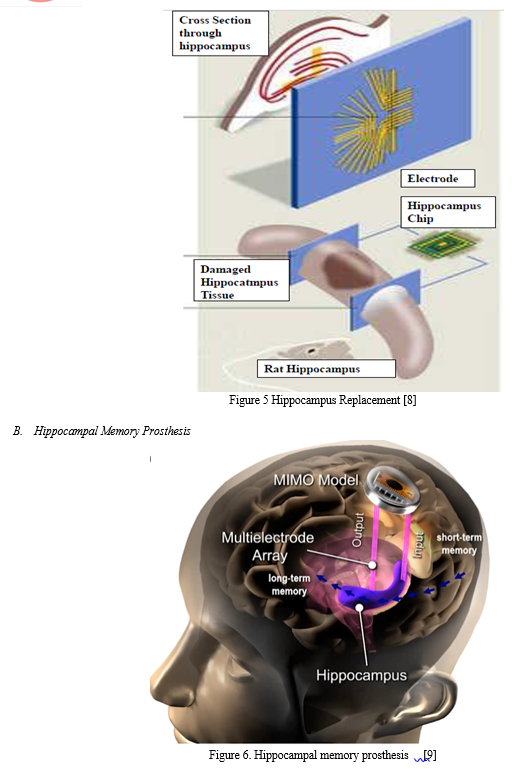
A. Hippocampus Replacement
The world’s first brain prosthesis – an artificial hippocampus
Chip takes over the processing of nervous signals normally performed by the hippocampus
- Multiple electrodes are placed on each array. They are positioned to mimic the structure of nerve tissue within a slice of the hippocampus, and make contact with other parts of the brain.
- Recording electrode array “listens” to neuron activity coming into the hippocampus and feeds it to the chip.
- Stimulating electrode array delivers the appropriate electrical output to the rest of the brain.

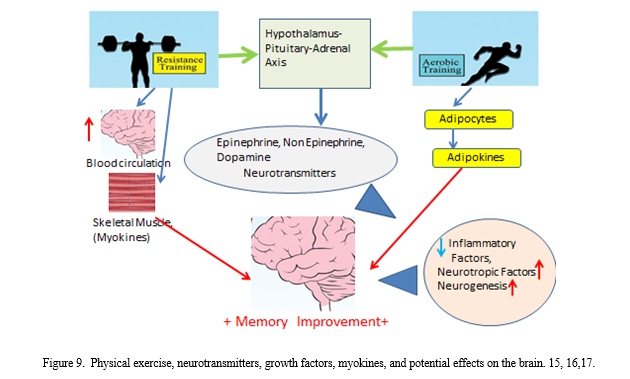
C. Effect of Physical Exercise on Increase in Cognitive Functions with Age
Recent reviews [15],[16], [17] evaluated the effects of exercise on reducing the rate of cognitive decline and aging. Studies concluded that physical exercise could be effective at increasing and preserving hippocampal volume, improving the blood circulation in hippocampus and preventing the loss of memory functions. Besides, it might strengthen the brain’s ability to generate new nerve cells and helping in removing the amyloid plaque, that contributes to the progression of Alzheimer’s disease. Hippocampus might correlate functionally with other parts of the brain, such that it might also get involved in controlling other activities as vision, hearing, and touch. So, hippocampus could be described as the “heart of the brain.”
 \
\
V. ACKNOWLEDGEMENT
The author is thankful to the BIT Mesra, Ranchi for providing the support and infrastructural facilities.
Conclusion
The hippocampus part of brain plays a key role in learning and memory activities. Alzheimer’s disease, depression, stress, and epilepsy could affect the hippocampus’ function. It has been found that physical exercise, interaction, social activities, music, dance might help preserve brain function and protect the health of the hippocampus. Damage in Hippocampus evolves into cognitive dysfunction, depression, and severe or mild epilepsy. Neural stem cell grafting early after injury holds promise for preventing neurological deficits. It modulates aberrant hippocampal post-injury plasticity and adds new inhibitory GABA-ergic interneurons into the hippocampus. Progress towards clinical application of NSC therapy for neurological conditions has been moving slowly but steadily. However there is a lot of optimism for such therapies to take up this to clinical stage in the coming years.
References
[1] Shetty A. Hippocampal Injury Induced Cognitive and Mood Dysfunction, Altered Neurogenesis and Epilepsy: Can Early Neural Stem Cell Grafting Intervention Provide Protection? Epilepsy Behav 2014; 38: 117–124. [2] Xuhai Liu, Fengyun Wang, Ramakrishna S. Hippocampus-guided engineering of memory prosthesis. Current Opinion in Biomed Engg 2022; 24: 100415. [3] Anand KS, Dhikav V. Hippocampus in health and disease: An overview. Ann Indian Acad Neurol 2012; 15:239-46. [4] Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci 2007; 30: 343-349. [5] Sun W, Wang J, Zhang N & Yang S. Scalable Implementation of Hippocampal Network on Digital Neuromorphic System towards Brain-Inspired Intelligence. Appl. Sci ; 2020, 10, 2857. [6] https://newsroom.ucla.edu/releases/ucla-to-develop-brain-prosthesis-to-help-brain-injured-patients-recover-memory [7] Shetty AK. Neural Stem Cell Therapy for Temporal Lobe Epilepsy. In: Noebels, JL.; Avoli, M.; Rogawski, MA.; Olsen, RW.; Delgado-Escueta, AV., editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet]. 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [8] URL:https://www.newscientist.com/article/dn3488-worlds-first-brain-prosthesis-revealed/ [9] (https://www.freethink.com/health/memory-prosthesis) (An illustration of the memory prosthesis. Credit: USC / Wake Forest University) [10] Hampson, R E, Song, D, Robinson, BS, Fetterhoff, D, Dakos, AS, Roeder, BM, Wicks, RT, et al. A hippocampal neural prosthetic for restoration of human memory function. J Neural Engg 2018; 15: 036014. [11] https://viterbischool.usc.edu/news/2018/03/prosthetic-memory-system-successful-in-humans-study-finds/ . [12] Song D, Robinson BS, Hampson RE, Marmarelis VZ, Deadwyler SA, & Berger TW. Sparse large-scale nonlinear dynamical modeling of human hippocampus for memory prostheses. IEEE Transactions Neural Systems Rehab Engg 2018; 26(2): 272-280. [13] Song D, Chan R H M, Robinson B S, Marmarelis VZ, Opris I, Hampson R E, Deadwyler S A & Berger TW. Identification of functional synaptic plasticity from spiking activities using nonlinear dynamical modeling. j Neurosci Method 2015; 244: 123-135. [14] Robinson BS, Berger TW, & Song D. Identification of stable spike-timing-dependent plasticity from spiking activity with generalized multilinear modeling. Neural Comput 2016; 28:2320-2351. [15] Wilckens K A, Stillman M, Aashna M, Waiwood, Chaeryon Kang, Regina L, Leckie, Jamie C, et al. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus 2021; 31(3): 335-347. [16] Babaei P & Azari HB. Exercise Training Improves Memory Performance in Older Adults: A Narrative Review of Evidence and Possible Mechanisms. Front. Hum. Neurosci. 2021; 15:771553. [17] Erickson K I, Voss W M. Exercise training increases size of hippocampus and improves memory. PNAS 2011; 108 (7): 3017-3022.
Copyright
Copyright © 2023 Sawarya Chandra. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54431
Publish Date : 2023-06-26
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

