Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Huntington’s Disease-Emerging Therapeutic Methods and Potential Utilisation of Bacteriophage to Overcome Current Challenges
Authors: Justin Zhang
DOI Link: https://doi.org/10.22214/ijraset.2024.58581
Certificate: View Certificate
Abstract
Huntington’s disease (HD) is a neurodegenerative trinucleotide repeat disorder resulting from abnormal CAG repeat length within Huntingtin gene (Htt). Onset of the disease usually starts in middle age, with symptoms including chorea, depression, and irritability. There are currently no cure for HD. Current treatments have limited effectiveness and does not target the pathology of the disease, only alleviating symptoms. New therapeutic methods such as CRISPR, antisense oligonucleotides (ASO) and stem cell therapies all offer potential curative and disease-modifying therapies for Huntington’s Disease. However, a major problem for all these new therapeutic methods is their route of administration. Here, I present a brief overview of the recent advancements in bacteriophage engineering and propose a novel solution of using bacteriophages to treat HD.
Introduction
I. INTRODUCTION
Huntington’s disease (HD) is a neurodegenerative disorder caused by a mutation in the HTT gene, with symptoms characterised by chorea, behavioural and psychiatric symptoms such as Depression and other motor symptoms. HD is caused by excessive trinucleotide repeats of CAG (>36). CAG represents the codon for the amino acid Glutamine and HD is characterised as one of the 10 polyglutamine diseases (polyQ). There is currently no cure for HD. HD affects males and females at the same frequency however early onset HD, where symptoms develop before the age of 20 (also called Juvenile Huntington’s disease), correlates significant to paternal transmission; in 75% of the juveniles the father is the affected parent (Trottier et al., 1994). Studies have shown that in the US, there are around 30,000 HD patients and that there are about 150,000 people at risk of developing the disease (Margolis & Ross, 2003).
II. PATHOLOGY OF HD
The wild type Huntingtin gene (wtHTT) codes for the protein huntingtin, which plays roles in regulation of neuronal and glial function, as well as apoptotic signaling and axonal transport. Moreover, wtHTT is essential during embryogenesis being critical in processes such as gastrulation and formation of neurons (neurogenesis). Accumulation of mutant Huntingtin protein causes neurodegeneration and forms protein aggregates within the neurons (Scherzinger et al., 1999). Capase (the protease that cleaves HTT) activity positively correlates with CAG repeat length, forming fragments which may contribute to the increase in toxicity of HD (Sari, 2011). In a study conducted by Bonin and colleagues to understand effects of RNA toxicity using a Drosophilia model, it demonstrated that excess CAG folding into long hairpins cause neurodegeneration (Li et al., 2008). Furthermore, a feature of HD suggests it causes abnormal activation of NMDA receptors which are receptors that induce a calcium ion influx, causing a calcium overload leading to apoptosis and activating degradation pathways (Sari, 2011). Due to neuronal death caused by induced mutant huntingtin (mHTT) toxicity, some studies have suggested that this activates microglial cells and astrocytes, which drives even more neuronal cell death and inflammation in HD (Möller, 2010). Thus, the pathology of HD shows how the degradation of neurons and dysfunction of the immune system causes the motor (e.g. Chorea) and behavioural (irritability) symptoms displayed by HD patients.
III. CURRENT TREATMENT METHODS FOR HD
A. Chorea Treatment
Although there is currently no cure for HD, many different medications are used to reduce the symptoms present in HD. For example, in 2008 tetrabenazine was the first US Food and Drug Administration (FDA) approved drug to treat chorea induced by HD (Hayden et al., 2009).
Tetrabenazine (TBZ) acts on the CNS by reducing the levels of dopamine in the patient, helping to reduce chorea as a result. However, TBZ has significant adverse side effects, as shown in a 15 year longitudinal study examining 526 patients taking TBZ. 36.5% experienced drowsiness, 15% experienced depression and many other serious psychiatric effects (Jankovic & Beach, 1997). Deutetrabenazine (DBZ), was approved in 2017, almost 10 years later since the approval of TBZ by the FDA and is a modified version of TBZ with reduced side effects. DBZ improves the half life of the compound, allowing for less frequent administration (Ferguson et al., 2022). However, current treatment methods require very frequent administration, with the objective of symptomatic treatment instead of eliminating the illness.
B. Antidepressants and Antipsychotic Mediation
HD also presents behaviour and psychotic symptoms, with depression being one of the most common experienced in HD. Furthermore, studies have shown suicide to be a significant cause of mortality in individuals with HD, estimating between 5-10% (Epping & Paulsen, 2011).Therefore, antidepressant drugs are often prescribed to HD patients suffering from depression. Other psychotic symptoms such as irritability, aggression and psychosis are treated with antipsychotic medications, such as Olanzapine. Olanzapine is the second most prescribed drug for HD, prescribed to treat many HD patients experiencing behaviour and psychiatric symptoms (Ferguson et al., 2022). It is important to note that both Olanzapine and TBZ reduce the levels of dopamine in HD patients, further confirming the important role of Dopamine in the symptoms of HD. However, the effect of these drugs has not been studied in HD patients specifically, and further research is required to discover the optimal medication to treat HD induced depression and high rates of suicide.
IV. NEW APPROACHES TO TREATING HD
In recent years, the advancements in technology have allowed new potential approaches and methodology to treat HD. Previously, treatment of HD targeted the symptoms, with high frequency administration and subjective outcome measures with little evidence based efficacy. The new approaches’ objective is to tackle the disease; by either slowing its progression or eliminating HD itself. These new methods will provide objective outcomes, reduced frequency of administration and targeting not only patients with symptomatic disease, but also those at risk of HD who have not developed symptoms yet. The effectiveness of reducing mHTT has been demonstrated using murine models. In one study reduction of mHTT mRNA (-75%) and protein (-65%) achieved partial reversal of disease progression and delayed motor dysfunction(Rodriguez-Lebron et al., 2005).
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/ CRISPR-
A. Associated System (Cas) Therapies
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR- associated proteins (Cas-9) are a naturally occurring genome editing system that prokaryotes use as immune defense (Mengstie & Wondimu, 2021). The mechanism of CRISPR Cas-9 starts by using a guide RNA (gRNA) molecule which directs Cas-9 to the target sequence allowing Cas-9 to bind to the protospacer adjacent motif (PAM), a short sequence downstream of target site. Finally, the Cas-9 protein is activated for DNA cleavage and degrades the complementary nucleic acid targets which are found upstream of PAM, creating a double stranded break (DSB) (Seo et al., 2023).
The gene editing capability of CRISPR Cas-9 can be used as a therapeutic method for HD. One study completed in 2023 used CRISPR interference to suppress the target gene expression without DSB, providing an allele specific treatment that reduces mHTT protein but preserves wtHTT which has important biological function in the brain (Seo et al., 2023). In comparison, normal CRISPR-Cas9 nucleases are not DSB free therefore unable to be allele specific meaning it may reduce both mHTT and wtHTT proteins. CRISPR methods allow permanent, allele specific and efficient changes to deactivate the mHTT gene through a single dose, thereby reducing the number of mutant HUNTINGTIN aggregations and toxicity in the brain (Eisenstein, 2018; Mengstie & Wondimu, 2021).
B. Stem Cell
Stem cell therapies are another prospective method pathway to treat HD. They are able to regenerate the lost neurons by promoting neurogenesis and protect neurons from disease progression using multiple growth factors secreted by stem cells (Ferguson et al., 2022; Im et al., 2009). There are many different types of stem cell therapy, such as mesenchymal stem cells (MSCs), Embryonic stem cells (ESCs), neural stem cells (NSCs) and Induced pluripotent stem cells (iPS cells).
Table 1 shows the different advantages and disadvantages of each method; most notably, a significant limitation of ESCs are its ethical issues regarding the use of human embryos, risks of immunorejection and their ability to cause tumors (teratomas) (Im et al., 2009). In 2006, Takahashi and Yamanaka designed iPS cells which can be obtained by reprogramming animal differentiated cells through introducing four factors: Oct4, Sox2, Klf4 and c-Myc - commonly known as the Yamanka factors (Medvedev et al., 2010). The use of iPS could overcome the problems of immunorejection, as well as modelling HD pathology by forming brain organoids to help us understand the disease progression of HD better as it can reveal particular disease phenotypes that cannot be observed in 2D systems (Conforti et al., 2018; Zhang et al., 2020). However, the risks of teratomas developing and delivery methods for stem cell therapies remain a significant dilemma.
Table 1: Overview of different stem cell therapeutics
|
Stem cell therapy |
Advantages (+) and Disadvantages (-) |
References |
|
Mesenchymal stem cells (MSC) |
+ can differentiate into a neuronal lineage + no teratomas or overgrowths have been found in MSC animal model studies + many sources to obtain + Increases neuroprotection and reduce disease-induced neuroinflammation - Uncertain if MSC implanted in vivo differentiate and function properly - Delivery methods remain invasive |
(Haddad et al., 2016; Liang et al., 2023) |
|
Embryonic stem cells (ESC) |
+ able to generate most cell types - Ethical issues due to destruction of embryo - Prone to immune rejection - Can cause teratomas and cancer development |
(Im et al., 2009) |
|
Neural stem cells (NSC) |
+ differentiation into specific cells such as neurons or glia - can only be obtained from aborted fetuses for cell therapy - current delivery methods remain invasive - risk of immunerejection - ethical problems arise during extraction of stem cell |
(Im et al., 2009) |
|
Induced pluripotent stem cells (iPS cells) |
+ can differentiate into many cell types + can be obtained from many adult cells + eliminate ethical issues + Reduce chance of immunorejection - High risk of teratomas and cancer - maintenance of undifferentiated iPS cells difficult - they have gene expression that does not represent the pure in vivo characteristics accurately
|
(Conner et al., 2023; Zhang et al., 2020) |
C. Antisense Oligonucleotides Therapies
Antisense oligonucleotides (ASO) are short, single stranded synthetic RNA or DNA which are modified to contain nucleotides complementary to the target mRNA (Rinaldi & Wood, 2018). The hybridization of ASO with mRNA can then trigger multiple mechanisms, such as RNA degradation, blocking translation and splice modulation; altering protein expression (Ferguson et al., 2022). ASO is easily customizable for allele specificity, able to be allele specific (only targeting mHTT gene) or allele non-specific (targeting both mHTT and wtHTT). However, the effects of reducing wtHTT needs to be considered, as it plays a critical role during embryogenesis, and more studies need to be done to understand the function of wtHTT in adults. In one study using murine models, the loss of Htt in postnatal mice caused acute pancreatitis while the loss of Htt in adult mice had no effect (Rook & Southwell, 2022).
As ASO cannot cross the blood brain barrier (BBB), invasive delivery methods such as intrathecal or intraventricular routes remain one of the most substantial obstacles for clinical application of oligonucleotides in CNS disorders (Rinaldi & Wood, 2018).
For example, Intrathecal therapy can cause many complications such as spinal cord injury, nerve injury and infections in addition to the routine risks of surgery and anesthesia meaning serious and devastating complications can arise from this (Staats, 2008) . Intraventricular routes also present serious complications, a major concern being neurovascular injury and intracranial hemorrhage.
Again, infection remains a crucial concern during the surgical insertion of devices such as the Ommaya reservoir, with 32% of 616 patients implemented with Ommaya reservoirs diagnosed with perioperative infections (Mead et al., 2014). These complications related to the common delivery methods for ASO shows the importance of developing a less invasive method to administrate ASO to utilise the potential of ASO in treating HD.

V. THE BLOOD BRAIN BARRIER (BBB) SETS CHALLENGES FOR CNS DRUG DELIVERY
The BBB is a highly selective semipermeable border of epithelial cells regulating the transfer of solutes and chemicals from the blood to the brain (Daneman & Prat, 2015). Its primary function is to protect central neurons and regulate the movement of ions, molecules, and cells between the blood and the CNS (Zlokovic, 2008). The BBB tightly controls the movement of molecules, thus presenting a great challenge for drug delivery to the CNS. In Tom Ireland’s The Good virus, the BBB is described as a remarkable barrier that lets in all the nutrients the brain needs but stops 98% of small molecules and almost all large molecules from passing.
Use of Bacteriophages to cross the Blood Brain Barrier
Bacteriophages are viruses that kill bacteria specifically. They were first discovered by Felix d’Herelle in the 20th century and phages are the most abundant lifeform on earth. Phages have contributed immensely to the development of molecular biology in the 20th century, from the MS2 bacteriophage being the first genome sequenced in 1976, to our understanding of horizontal gene transfer (the mechanism by which bacteria of unrelated species exchange genes) and protein synthesis are all examples of the phages’ amazing works. James Watson, who played a crucial role in the discovery of DNA , obtained his PhD working on phage genetics. Phages continue to offer new insights into the complex world of nature, such as the CRISPR Cas9 system and provides novel solutions to the growing antibiotic resistance crisis.
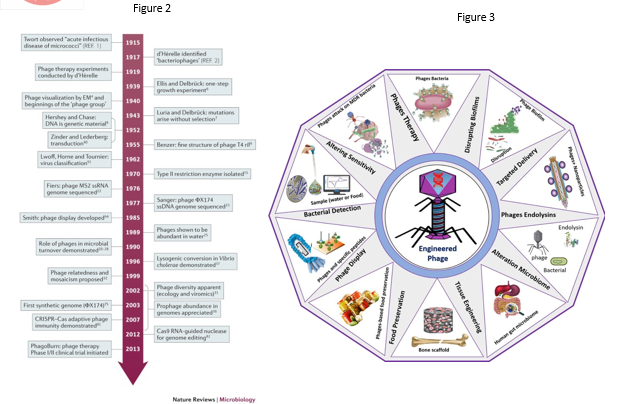
Figure 2- Timeline showing contribution of bacteriophage to modern molecular biology. Image taken from Nature Reviews Microbiology, you may find the image at https://www.nature.com/articles/nrmicro3564
Figure 3- Image showing the numerous applications of engineered bacteriophage. Image taken from Biotechnology Advances, Vol 64 (Hussain et al, 2023), you may find the image at https://www.sciencedirect.com/science/article/abs/pii/S073497502300023X
In several recent studies, researchers have attempted to use phages as a vector to transport drugs past the BBB- with several successes. For example, in one study done by a team of researchers in New York and Detroit MS2 bacteriophage were covered with Angiopep-2 (AP2)- a protein that has been shown to facilitate the transport of large macromolecules and synthetic nanoparticles across the BBB- allowing the phage to pass through the blood brain barrier (Apawu et al., 2018) . The researchers concluded that the phages could be easily filled with desired compounds to aid the diagnosis and treatment of intractable brain conditions. Another way phages can be utilized is by replacing a phage’s normal DNA with complex gene editing molecules which can be used to treat genetic diseases, as well as replacing receptors on phage’s binding sites with molecules that targets particular cells of the body (Ireland, 2023).
This approach in utilizing phages has been used by scientists at the University of Tel-Aviv where modified worm-shaped phages are packed with a cytotoxic drug and bind only to receptors on cancer cell membranes resulting to the release of the drug inside the cancer cell (Bar et al., 2008). These results have shown that the concentration of the drug around the target cells was more than 1000 times greater than if the drugs were released freely into the bloodstream without the phages. Finally, one last use of phages exploits phages’ unique self-assembling properties through the regrowth of human tissue such as the skin, cartilage and neural tissue. In one study carried out by researchers at Zhejiang University in China, phages are injected deep into the brain to repair brain damage following a stroke (Liu et al., 2022). After injecting filamentous phages engineered with stem cells directly into the stroke cavity of rat models, the phage nanofibers stimulated neurogenesis within two weeks for brain regeneration. Overall, the examples provided above shows the abundance of new, innovative applications of phages to combat the challenges and difficulties that current clinical methods and equipments have.

Figure 4- Image showing the application of bacteriophage in new treatment methods and modifying phages with novel functions. Phages can be engineered by one or more of these methods to express desired function. This image was taken from Pharmaceuticals (2021), you may find the image at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8308837/
A. Applications of Bacteriophages in HD
The encouraging therapeutic usage of phages raises the possibility of solving the challenges faced by methods to treat HD. As discussed previously, ASO is a highly encouraging treatment for HD, however it’s usage is currently limited by the difficulties surrounding its administrative methods (intraventricular and intrathecal). The problematic crossing of the BBB may be solved by using a phage-based alternative- using phages as vehicles to transport ASO past the BBB. Phages can also be modified with gene editing capabilities, with CRISPR knockout of mHTT being another promising treatment method for HD. Moreover, phages could be used to counter the effects of HD, which includes mainly of neuron loss in the striatal part of the basal ganglia by promoting neurogenesis The ease of propagation of phages makes them more favorable than other type of viruses, their inability to replicate in human cells makes them particularly useful and their overall safety in humans offers a potential revolutionary treatment for HD (Gibb et al., 2021).
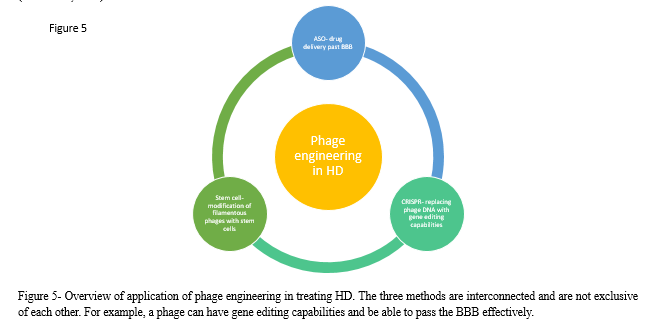
There are still several aspects that need to be addressed regarding the utilisation of phages in humans. Due to the dynamic and rapid evolving nature of phages, it does not meet the rigorous standards of the FDA and currently usage in humans remain prohibited in large parts of the world. Nonetheless, there have been advancements in producing synthetic phages with a group of researchers from Brussels lead by Jean-Paul Pirnay and researchers at the University of Munich creating a completely cell-free system known as “Phactory” able to produce synthetic phages (Ireland, 2023). There are also limitations to the current models used to test phages, such as using murine models which is not representative of complex human response and cannot mimic all the symptoms that humans experience. This is especially important in HD as in human patients dyskinesia (involuntary movement) develops as a symptom of the disease, while in mice it does not (Simmons, 2008). Thus, it is important to use more representative animal models in such experiments such as chimpanzees to accurately model and predict the response in humans. Although this is more costly, it is vital for the development of new therapeutic methods to test their effects before conducting human trials.
Conclusion
The potential therapeutic methods for HD have allowed disease modifying effects, compared to the limited symptomatic treatment of current methods. Current treatment of HD is insufficient; new methods need to have greater specificity, less invasiveness and objective outcome measures when treating HD. CRISPR related therapies are an exciting area of development which offers potentially permanent solutions to HD. As of writing, the world’s first CRISPR-Cas9 treatment has been approved in the UK emphasising the immense potential of such treatment methods in the future. Encouraging success in stem cell therapeutics offers another alternative in treating HD by combating the neurodegenerative effects of mHTT by replacing lost neuronal cells. The use of ASO to reduce mHTT and its allele selective capabilities makes it ideal to treat HD and other neurodegenerative diseases such as Parkinson’s disease. However, all three methods are currently facing the significant challenge of drug delivery and administration, with current administrative routes being invasive and associated with various risks. The BBB is one of the greatest obstacles in cell-based technologies. Therefore, developing a therapeutic method which can both cross the Blood Brain Barrier while being minimally invasive is of crucial importance when considering potential treatments. Bacteriophages’ recent success in crossing the Blood Brain Barrier provides a novel, innovative and prospective approach in the treatment of HD. The use of phages in numerous recent studies confirms its wide-ranging capabilities and potential, benefiting each of the three new therapeutic methods (CRISPR, stem cells, ASOs) in different ways allowing it to be instrumental to the future success of HD treatment. Although further studies are necessary to test the use of phages and meeting the standards of manufacturing in many countries, they are one of the safest methods of treatment delivery. Their ease of propagation allows it to be an accessible method of treatment for all patients with good statistical power allowing experiments with large sample sizes and good statistical power. The use of bacteriophages in HD patients specifically will need to be studied as it has not been done so before, as well as using more representative animal models as to not overlook certain phenotypic characteristics exhibited in humans but not in all animal models and vice versa. Whilst the utilisation of phages to treat HD is still at its infancy, it offers a promising strategy that will meet the needs of a less invasive, allele specific and disease modifying treatment method for HD.
References
[1] Apawu, A. K., Curley, S. M., Dixon, A. R., Hali, M., Sinan, M., Braun, R. D., Castracane, J., Cacace, A. T., Bergkvist, M., & Holt, A. G. (2018). MRI compatible MS2 nanoparticles designed to cross the blood-brain-barrier: providing a path towards tinnitus treatment. Nanomedicine?: Nanotechnology, Biology, and Medicine, 14(7), 1999–2008.https://doi.org/10.1016/J.NANO.2018.04.003 [2] Bar, H., Yacoby, I., & Benhar, I. (2008). Killing cancer cells by targeted drug-carrying phage nanomedicines. BMC Biotechnology, 8(1), 1–14. https://doi.org/10.1186/1472-6750-8-37/FIGURES/5 [3] Conforti, P., Besusso, D., Bocchi, V. D., Faedo, A., Cesana, E., Rossetti, G., Ranzani, V., Svendsen, C. N., Thompson, L. M., Toselli, M., Biella, G., Pagani, M., & Cattaneo, E. (2018). Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes. Proceedings of the National Academy of Sciences of the United States of America, 115(4), E762–E771. https://doi.org/10.1073/PNAS.1715865115/-/DCSUPPLEMENTAL [4] Conner, L. T., Srinageshwar, B., Bakke, J. L., Dunbar, G. L., & Rossignol, J. (2023). Advances in stem cell and other therapies for Huntington’s disease: An update. Brain Research Bulletin, 199, 110673. https://doi.org/10.1016/J.BRAINRESBULL.2023.110673 [5] Daneman, R., & Prat, A. (2015). The Blood–Brain Barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. https://doi.org/10.1101/CSHPERSPECT.A020412 [6] Eisenstein, M. (2018). CRISPR takes on Huntington’s disease outlook /692/698/1688/64 /692/699 /631/208/2489/201 /631/378 n/a. Nature, 557(7707), S42–S43. https://doi.org/10.1038/D41586-018-05177-Y [7] Epping, E. A., & Paulsen, J. S. (2011). Depression in the early stages of Huntington disease. Neurodegenerative Disease Management, 1(5), 407. https://doi.org/10.2217/NMT.11.45 [8] Ferguson, M. W., Kennedy, C. J., Palpagama, T. H., Waldvogel, H. J., Faull, R. L. M., & Kwakowsky, A. (2022). Current and Possible Future Therapeutic Options for Huntington’s Disease. Journal of Central Nervous System Disease, 14, 1–27. https://doi.org/10.1177/11795735221092517 [9] Gibb, B., Hyman, P., & Schneider, C. L. (2021). The Many Applications of Engineered Bacteriophages—An Overview. Pharmaceuticals, 14(7). https://doi.org/10.3390/PH14070634 [10] Haddad, M. S., Wenceslau, C. V., Pompeia, C., & Kerkis, I. (2016). Cell-based technologies for Huntington’s disease. Dementia & Neuropsychologia, 10(4), 287. https://doi.org/10.1590/S1980-5764-2016DN1004006 [11] Hayden, M. R., Leavitt, B. R., Yasothan, U., & Kirkpatrick, P. (2009). Tetrabenazine. Nature Reviews Drug Discovery, 8(1), 17–18. https://doi.org/10.1038/NRD2784 [12] Im, W., Lee, S. T., Chu, K., Kim, M., & Roh, J. K. (2009). Stem Cells Transplantation and Huntington’s Disease. International Journal of Stem Cells, 2(2), 102. https://doi.org/10.15283/IJSC.2009.2.2.102 [13] Ireland, T. (2023). The Good Virus. Hodder & Stoughton. [14] Jankovic, J., & Beach, J. (1997). Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology, 48(2), 358–362. https://doi.org/10.1212/WNL.48.2.358 [15] Li, L.-B., Yu, Z., Teng, X., & Bonini, N. M. (2008). RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature, 453(7198), 1107–1111. https://doi.org/10.1038/nature06909 [16] Liang, X. S., Sun, Z. W., Thomas, A. M., & Li, S. (2023). Mesenchymal Stem Cell Therapy for Huntington Disease: A Meta-Analysis. Stem Cells International, 2023. https://doi.org/10.1155/2023/1109967 [17] Liu, X., Yang, M., Lei, F., Wang, Y., Yang, M., & Mao, C. (2022). Highly Effective Stroke Therapy Enabled by Genetically Engineered Viral Nanofibers. Advanced Materials (Deerfield Beach, Fla.), 34(20). https://doi.org/10.1002/ADMA.202201210 [18] Margolis, R. L., & Ross, C. A. (2003). Diagnosis of Huntington disease. Clinical Chemistry, 49(10), 1726–1732. https://doi.org/10.1373/49.10.1726 [19] Mead, P. A., Safdieh, J. E., Nizza, P., Tuma, S., & Sepkowitz, K. A. (2014). Ommaya reservoir infections: a 16-year retrospective analysis. The Journal of Infection, 68(3), 225–230. https://doi.org/10.1016/J.JINF.2013.11.014 [20] Medvedev, S. P., Shevchenko, A. I., & Zakian, S. M. (2010). Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta Naturae, 2(2), 18. https://doi.org/10.32607/20758251-2010-2-2-18-27 [21] Mengstie, M. A., & Wondimu, B. Z. (2021). Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics?: Targets & Therapy, 15, 353. https://doi.org/10.2147/BTT.S326422 [22] Möller, T. (2010). Neuroinflammation in Huntington’s disease. Journal of Neural Transmission (Vienna, Austria?: 1996), 117(8), 1001–1008. https://doi.org/10.1007/S00702-010-0430-7 [23] Rinaldi, C., & Wood, M. J. A. (2018). Antisense oligonucleotides: The next frontier for treatment of neurological disorders. In Nature Reviews Neurology (Vol. 14, Issue 1, pp. 9–22). Nature Publishing Group. https://doi.org/10.1038/nrneurol.2017.148 [24] Rodriguez-Lebron, E., Denovan-Wright, E. M., Nash, K., Lewin, A. S., & Mandel, R. J. (2005). Intrastriatal rAAV-Mediated Delivery of Anti-huntingtin shRNAs Induces Partial Reversal of Disease Progression in R6/1 Huntington’s Disease Transgenic Mice. Molecular Therapy?: The Journal of the American Society of Gene Therapy, 12(4), 618. https://doi.org/10.1016/J.YMTHE.2005.05.006 [25] Rook, M. E., & Southwell, A. L. (2022). Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. Biodrugs, 36(2), 105. https://doi.org/10.1007/S40259-022-00519-9 [26] Sari, Y. (2011). Huntington’s Disease: From Mutant Huntingtin Protein to Neurotrophic Factor Therapy. International Journal of Biomedical Science?: IJBS, 7(2), 89. https://doi.org/10.59566/ijbs.2011.7089 [27] Scherzinger, E., Sittler, A., Schweiger, K., Heiser, V., Lurz, R., Hasenbank, R., Bates, G. P., Lehrach, H., & Wanker, E. E. (1999). Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: Implications for Huntington’s disease pathology. Proceedings of the National Academy of Sciences of the United States of America, 96(8), 4604. https://doi.org/10.1073/PNAS.96.8.4604 [28] Seo, J. H., Shin, J. H., Lee, J., Kim, D., Hwang, H. Y., Nam, B. G., Lee, J., Kim, H. H., & Cho, S. R. (2023). DNA double-strand break-free CRISPR interference delays Huntington’s disease progression in mice. Communications Biology 2023 6:1, 6(1), 1–12. https://doi.org/10.1038/s42003-023-04829-8 [29] Simmons, D. (2008). The Use of Animal Models in Studying Genetic Disease: Transgenesis and Induced Mutation. Nature Education . [30] Staats, P. S. (2008). Complications of Intrathecal Therapy. Pain Medicine, 9(suppl_1), S102–S107. https://doi.org/10.1111/J.1526-4637.2008.00445.X [31] Trottier, Y., Biancalana, V., & Mandel, J. L. (1994). Instability of CAG repeats in Huntington’s disease: relation to parental transmission and age of onset. Journal of Medical Genetics, 31(5), 377. https://doi.org/10.1136/JMG.31.5.377 [32] Zhang, X., Hu, D., Shang, Y., & Qi, X. (2020). Using induced pluripotent stem cell neuronal models to study neurodegenerative diseases. Biochimica et Biophysica Acta. Molecular Basis of Disease, 1866(4). https://doi.org/10.1016/J.BBADIS.2019.03.004 [33] Zlokovic, B. V. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 57(2), 178–201. https://doi.org/10.1016/J.NEURON.2008.01.003 Image attributions Figure 1a- figure taken from Lioresal Intrathecal webpage, you may find image at https://lioresal.com/lioresal-intrathecal/ , 1b- this figure was release to the public domain by its author, Patrick L. Lynch. You may find image at https://en.wikipedia.org/wiki/Intracerebroventricular_injection Figure 2- . Image taken from Nature Reviews Microbiology, you may find the image at https://www.nature.com/articles/nrmicro 3564 Figure 3- Image taken from Biotechnology Advances, Vol 64 (Hussain et al, 2023), you may find the image at https://www.science direct.com/science/article/abs/pii/S073497502300023X Figure 4- This image was taken from Pharmaceuticals (2021), you may find the image at https://www.ncbi.nlm.nih.gov /pmc/articles/PMC8308837/
Copyright
Copyright © 2024 Justin Zhang. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET58581
Publish Date : 2024-02-24
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

