Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Hydrogen Production using PEM Electrolyzer
Authors: Kaustubh Patil , Miheer Patil , Prajwal Pustode , Prathamesh Umathe , Athang Rajebhosale , Alkesh Rathod
DOI Link: https://doi.org/10.22214/ijraset.2024.61720
Certificate: View Certificate
Abstract
This paper presents a simulation study of alkaline electrolysis for H2 production using Aspen Plus. The simulation model incorporates reactor, separators, mixer, and heat exchanger units to accurately represent the AWE process. The model is designed to evaluate the performance of an AWE system under different operating conditions and aims to achieve a reference H2 production rate of 0.179663 kg/hr. The simulation results demonstrate that the AWE system can successfully produce H2 at the specified rate. The model provides a detailed analysis of the system\'s behavior and H2 production rate, under varying operating conditions. The findings of this study provide valuable insights into optimizing the design and operation of AWE systems for large-scale H2 production
Introduction
I. ACKNOWLEDGMENTS
This document is prepared by the inspiration received from Professor Satchidanand Satpute, Assistant Professor, Department of Chemical Engineering, BRACT’s Vishwakarma Institute of Technology. Many colleagues at the Chemical Engineering Department have carefully read and improved the document their contributions are gratefully acknowledged.
II. INTRODUCTION
Alkaline electrolysis (AWE) stands as a well-established and efficient technology in the realm of hydrogen (H2) production from water. This process relies on the application of an electric current within an electrolytic cell to split water molecules into hydrogen and oxygen. Notably, AWE has garnered prominence due to its inherent advantages, which include high efficiency, maturity, reliability, scalability, and versatility. These attributes position AWE as a highly promising method for large-scale hydrogen production, contributing significantly to the transition toward sustainable and clean energy sources.
One pivotal aspect of advancing AWE technology lies in the utilization of sophisticated tools for modeling and analysis. Aspen Plus, a comprehensive process simulation software, emerges as a valuable and powerful tool in this context. By developing a detailed AWE model within Aspen Plus, researchers and engineers can glean invaluable insights into the system's performance, unlocking a nuanced understanding of its intricacies.
The advantages of employing Aspen Plus for AWE modeling are multifaceted. Firstly, the software enables a thorough examination of the electrochemical processes inherent in alkaline electrolysis. It facilitates the representation of complex reactions, including water electrolysis into hydrogen and oxygen, providing a detailed account of the thermodynamics and kinetics involved in the process.
Secondly, Aspen Plus allows for the conduct of parametric studies and sensitivity analyses. Researchers can systematically vary input parameters, such as temperature, pressure, and electrolyte concentration, to assess their impact on the efficiency and overall performance of the AWE system. This capability is instrumental in identifying optimal operating conditions and potential areas for improvement.
Moreover, Aspen Plus supports mass balances and reaction kinetics, enabling researchers to scrutinize the distribution of reactants and products within the AWE system. This comprehensive understanding of the system's behavior contributes to the identification of bottlenecks and opportunities for enhanced efficiency.
The software's versatility extends to techno-economic analysis, integrating cost considerations into the simulation. This feature is crucial for assessing the economic feasibility of AWE systems, aiding in decision-making processes related to project development and investment.
Aspen Plus also accommodates dynamic simulation, allowing researchers to observe the AWE system's behavior over time and assess its dynamic stability. Furthermore, the software's process control features contribute to insights into optimal strategies for maintaining stable and efficient AWE operation.
In essence, the synergy between AWE, as a mature and efficient hydrogen production technology, and Aspen Plus, as a robust simulation tool, forms a formidable partnership. The utilization of Aspen Plus in AWE modeling empowers researchers to not only gain a deep understanding of the system but also to fine-tune its design, optimize operational parameters, and contribute to the ongoing advancements in large-scale hydrogen production for a sustainable energy future.
III. PROBLEM STATEMENT
Explore the efficiency and optimization of alkaline electrolysis processes in Aspen Plus for hydrogen production, focusing on parameters such as electrolyte concentration, temperature, and pressure to enhance system performance and contribute to the advancement of sustainable energy technologies.
IV. LITERATURE REVIEW
Here’s the Literature Review of papers we have taken as reference.
[1] Hydrogen, an eco-friendly energy carrier produced through PEM water electrolysis, has great potential for green hydrogen generation. Despite economic challenges, recent research has focused on cost-effective electrocatalysts to enhance efficiency and affordability, benefiting fuel cells and industries. This review highlights advancements in PEM water electrolysis, emphasizing low-cost, high-performance electrocatalysts, and aims to facilitate the commercial viability of green hydrogen production.
[2] PEM water electrolysis is emerging as a promising clean energy solution due to its efficient and pure hydrogen production, adaptability to renewable energy variability, and ongoing advancements in electrocatalyst development. In comparison to other methods, PEM electrolysis offers distinct advantages. This review covers progress in PEM electrolysis, including catalyst improvements and system components, while also addressing current challenges and future prospects in hydrogen production through PEM electrolysis.
[3] We've developed efficient ML models, utilizing polynomial and logistic regression, accurately predicting 11 PEM electrolyzers cell parameters from input variables like hydrogen production rate and cell design. With training on 148 samples and 16 in validation, our models achieved 83.6% classification accuracy and a mean absolute error of 6.825, validated through custom cell fabrication. This pioneering approach offers cost-effective and time-saving solutions for commercial PEM cell design in hydrogen production.
[4] Hydrogen energy is crucial for a sustainable future amid climate change and evolving energy needs. It's obtainable through various methods, including renewables. This paper reviews hydrogen production from solar and wind energy, focusing on different water electrolyzers systems. It offers a comprehensive comparison of electrolyzers types, outlines production techniques, and assesses the economics of green hydrogen production versus other methods. The paper also highlights the challenges associated with these production approaches.
[5] n contrast to Current cases, Future cases project the development of the technology with new materials and capabilities and improved hydrogen production efficiencies, and include longer equipment lifetimes. Generally, capital costs of the systems are further reduced, compared with the Current case.
[6] Hydrogen emerged as a prominent energy carrier last year, offering a promising solution to energy and ecological challenges. The focus on fuel cells, particularly those using proton exchange membrane (PEM) technology that requires pure hydrogen, has spurred the development of electrolyzers. The authors highlight PEM electrolysis as a prospective technology, discussing its advantages and potential applications compared to other electrolyzers types. The summary also touches on significant achievements in PEM electrolysis, including recent advancements made by the authors.
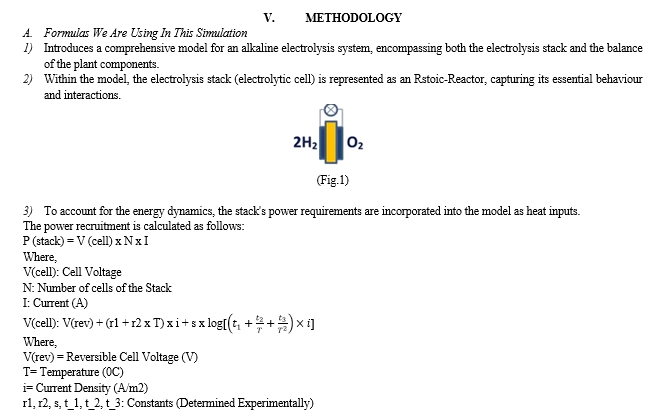
[7] A PEM electrolyzer system for hydrogen production is analyzed using thermodynamic-electrochemical modeling, revealing that Joule heat from irreversibility’s exceeds the energy required for water splitting across various electric current densities. Alternative configurations are proposed to enhance system performance, with corresponding efficiency expressions derived. Efficiency curves demonstrate variations with electric current density, allowing for comparison of different configurations. The optimal operating region for electric current density is identified, and detailed analyses of key parameters provide insights for the optimal design of a practical PEM electrolyzer system for hydrogen production.
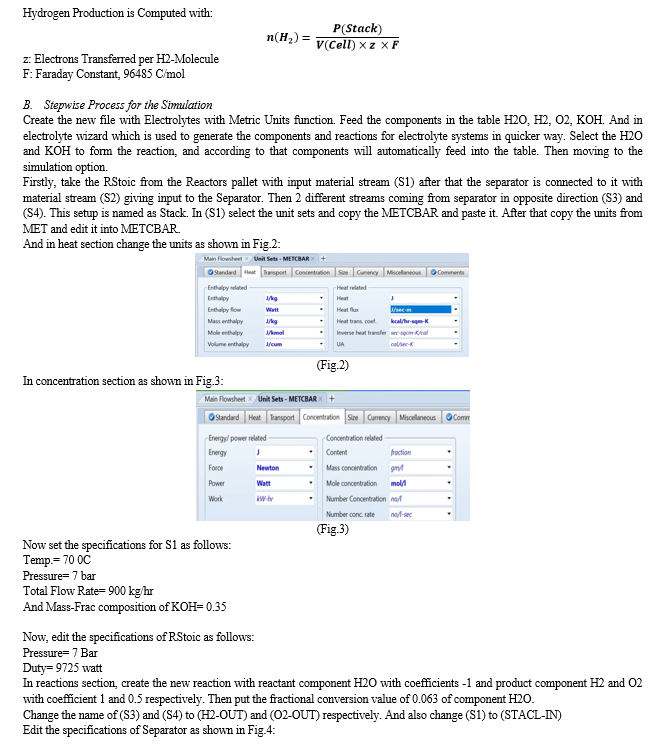
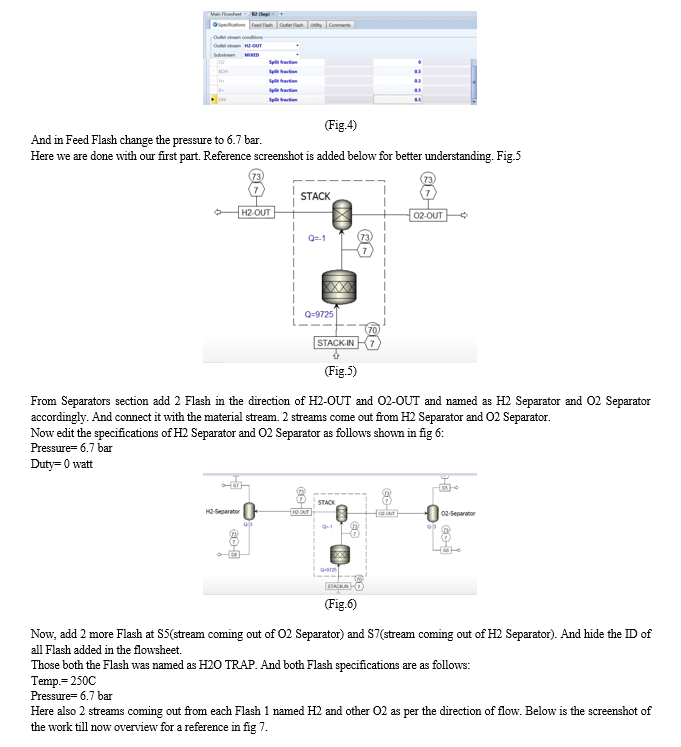
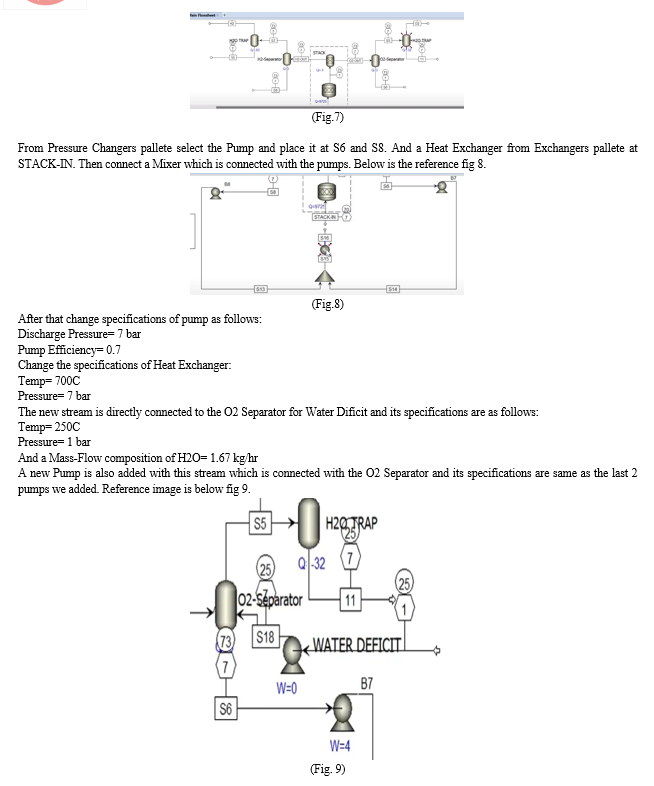
[8] This paper presents a comprehensive model of an alkaline electrolysis plant, encompassing both the stack and balance of plant components. Aspen Plus is employed for simulation, with a custom model for the stack integrated using Aspen Custom Modeler due to the software's lack of electrolysis cell modelling codes. The stack model relies on semi-empirical equations for voltage cell, Faraday efficiency, and gas purity based on current. Other plant components are simulated using standard units in Aspen Plus.
Simulations indicate that optimizing the balance of the plant, with a focus on increasing temperature and reducing pressure, enhances the overall performance of the alkaline electrolysis system for hydrogen production. The proposed model has the potential for future use in techno-economic studies of integrated alkaline electrolysis systems.
[9] A comprehensive mathematical model for an advanced alkaline electrolyzer has been created, incorporating fundamental thermodynamics, heat transfer theory, and empirical electrochemical relationships. The model, validated through comparisons with measured data from a photovoltaic-hydrogen energy plant in Jülich, accurately predicts cell voltage, hydrogen production, efficiencies, and operating temperature. Designed with a minimal number of parameters, the model is suitable for integrated hydrogen energy system simulations. Compatibility with a transient system simulation program enables integration with standard thermal and electrical renewable energy components, making it valuable for system design, redesign, and control strategy optimization. A year-long simulation of a photovoltaic-hydrogen system demonstrates the model's effectiveness in identifying improved electrolyzer operating strategies
[10] This review explores the historical development and current status of water electrolysis for hydrogen production, a technology dating back two centuries. It covers alkaline water electrolysis, polymer electrolysis membrane (PEM), and high-temperature electrolysis, comparing their efficiencies, thermodynamics, and energy requirements. The low adoption of water electrolysis is attributed to cost inefficiency, high maintenance, low durability, and lower efficiency compared to alternative technologies. The paper analyses the energy requirements, practical cell voltage, efficiency, temperature, pressure effects, and electrode materials in different electrolysis methods, highlighting areas for modification and development to enhance hydrogen production.
[11] Hydrogen, an ideal clean energy source, serves as a storage medium for renewable energy. Water electrolysis, a mainstream method for hydrogen production, enables the conversion of various energy sources into high-purity hydrogen, making it a versatile storage solution. The industrial application of alkaline water electrolysis and PEM electrolysis for hydrogen production is explored, comparing their working principles, process flows, and respective advantages and disadvantages. The article offers valuable insights for researchers, providing a reference for understanding and comparing these water electrolysis technologies in the context of hydrogen production.
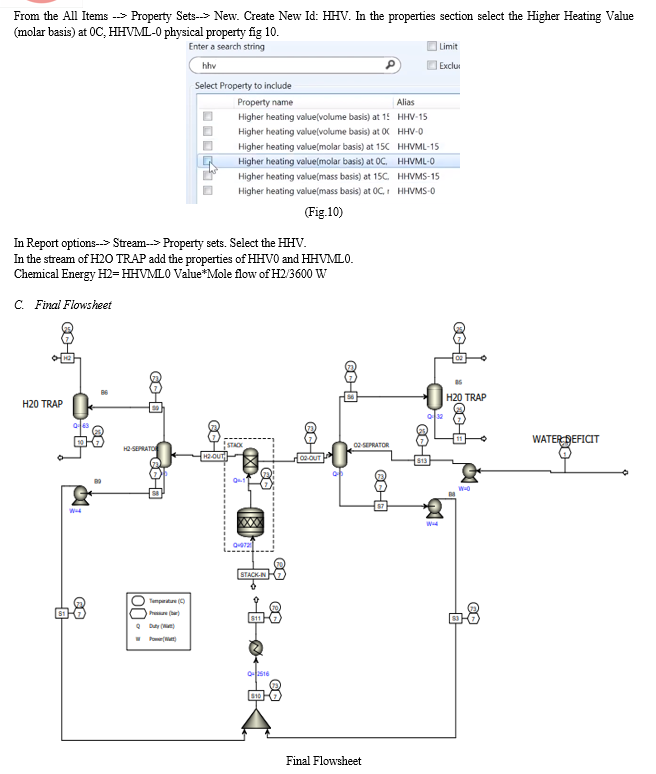
VI. RESULT AND DISCUSSION
A. Result
The alkaline electrolysis plant produces 4.11774 kg/hr of hydrogen per hour. This is a relatively small amount of hydrogen, but it is still a significant achievement. Alkaline electrolysis is a mature and well-established technology, and it is one of the most promising methods for large-scale hydrogen production.
B. Discussion
Alkaline electrolysis is a process that uses electricity to split water into hydrogen and oxygen. The process is carried out in an electrolytic cell, which consists of two electrodes (an anode and a cathode) that are separated by an electrolyte. The electrolyte is typically a solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH).
When an electric current is applied to the cell, water molecules are split into hydrogen ions (H+) and hydroxide ions (OH-). The hydrogen ions migrate to the cathode, where they are reduced to hydrogen gas (H2). The hydroxide ions migrate to the anode, where they are oxidized to oxygen gas (O2).
Alkaline electrolysis is a relatively efficient process, with an efficiency of up to 70-80%. However, the efficiency of the process can be affected by a number of factors, including the operating temperature, the current density, and the purity of the electrolyte.
Conclusion
Alkaline electrolysis has emerged as a highly promising method for large-scale hydrogen production, offering a mature, efficient, and scalable solution. The technology\'s maturity is evidenced by its long history of use and the wealth of operational data available. With high efficiency in converting electrical energy into hydrogen, alkaline electrolysis holds great potential for addressing the growing global demand for clean and sustainable hydrogen. Its scalability is a significant advantage, allowing for the adaptation of the technology to various production capacities, from small-scale applications to industrial-level hydrogen generation. This scalability aligns with the diverse needs of industries and facilitates the integration of alkaline electrolysis into existing infrastructures. Despite its considerable promise, several challenges impede the widespread adoption of alkaline electrolysis. The cost of electrocatalysts, which play a crucial role in facilitating the electrochemical reactions, is a primary concern. Developing cost-effective catalysts that maintain high efficiency over extended periods is essential for enhancing the economic viability of alkaline electrolysis. Additionally, the stability of electrolytes is another critical challenge. The harsh operating conditions, such as high alkalinity and elevated temperatures, can lead to degradation of electrolyte materials, impacting the overall durability and longevity of the electrolysis system. Addressing these challenges through ongoing research and technological innovations is imperative for unlocking the full potential of alkaline electrolysis and realizing its role in the global transition to a hydrogen-based economy.
References
[1] Shiva Kumar, S., & Himabindu, V. (2019). Hydrogen Production by PEM Water Electrolysis – A Review. Materials Science for Energy Technologies. doi:10.1016/j.mset.2019.03.002 [2] Wang, T., Cao, X. & Jiao, L. PEM water electrolysis for hydrogen production: fundamentals, advances, and prospects. Carb Neutrality 1, 21 (2022). https://doi.org/10.1007/s43979-022-00022-8 [3] Mohamed, A.; Ibrahem, H.; Yang, R.; Kim, K. Optimization of Proton Exchange Membrane Electrolyzer Cell Design Using Machine Learning. Energies 2022, 15, 6657. https://doi.org/10.3390/en15186657 [4] Nasser, M., Megahed, T.F., Ookawara, S. et al. A review of water electrolysis–based systems for hydrogen production using hybrid/solar/wind energy systems. Environ Sci Pollut Res 29, 86994–87018 (2022). https://doi.org/10.1007/s11356-022-23323-y [5] Brian James, Whitney Colella and Jennie Moton et al. (2013) “PEM Electrolysis H2A Production Case Study Documentation”, Prepared for the U.S. Department of Energy, Fuel Cell Technologies Office [6] Grigoriev, S., Porembsky, V., & Fateev, V. (2006). Pure hydrogen production by PEM electrolysis for hydrogen energy. International Journal of Hydrogen Energy, 31(2), 171/175. doi:10.1016/j.ijhydene.2005.04.038 [7] Houcheng Zhang, Shanhe Su, Guoxing Lin, Jincan Chen et al. (2012) “Efficiency Calculation and Configuration Design of a PEM Electrolyzer System for Hydrogen Production,” Department of Physics, Xiamen University, Xiamen 361005, People’s Republic of China [8] Sánchez, M., Amores, E., Abad, D., Rodríguez, L., & Clemente-Jul, C. (2019). Aspen Plus model of an alkaline electrolysis system for hydrogen production. International Journal of Hydrogen Energy. doi:10.1016/j.ijhydene.2019.12.02 [9] Ulleberg, O. (2003). Modeling of advanced alkaline electrolyzers: a system simulation approach. International Journal of Hydrogen Energy, 28(1), 21–33. doi:10.1016/s0360-3199(02)00033-2 [10] Rashid, Mamoon & Al Mesfer, Mohammed & Naseem, Hamid & Danish, Mohd. (2015). Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. International Journal of Engineering and Advanced Technology. ISSN. 2249-8958. [11] Guo, Y., Li, G., Zhou, J., & Liu, Y. (2019). Comparison between hydrogen production by alkaline water electrolysis and hydrogen production by PEM electrolysis. IOP Conference Series: Earth and Environmental Science, 371, 042022. doi:10.1088/1755-1315/371/4/042022
Copyright
Copyright © 2024 Kaustubh Patil , Miheer Patil , Prajwal Pustode , Prathamesh Umathe , Athang Rajebhosale , Alkesh Rathod. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET61720
Publish Date : 2024-05-07
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

