Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Review on: Identification of Brain Tumor in MRI Images by the Use of SVM and Fuzzy C-Means Combination
Authors: Harsh Maru, Shyamol Banerjee
DOI Link: https://doi.org/10.22214/ijraset.2024.63406
Certificate: View Certificate
Abstract
This work explores a novel approach to accurately identify brain tumors from magnetic resonance imaging (MRI) images by utilizing the complementary capabilities of fuzzy C-Means (FCM) clustering and support vector machines (SVM). Using the soft clustering approach, MRI images are meticulously segregated in the first stage, known as FCM clustering. By giving individual voxels varying degrees of membership, FCM deftly handles the inherent ambiguities and complexity of medical pictures to produce a nuanced representation of tissue kinds. SVM, a potent supervised learning technique for binary classification applications, comes after segmentation. uses this information in the second phase of classification. Through the creation of a hyperplane that divides tumor and non-tumor regions to the greatest extent possible, support vector machines (SVM) are able to optimize the margin between these classes and improve their ability to generalize. We deliberately integrate FCM clustering with SVM classification in order to take use of the complementary characteristics that each of these techniques possesses. While SVM\'s strong classification skills enable it to fine-tune the identification of tumor regions, FCM\'s sophisticated segmentation captures the spatial distribution of tissues. By working together, we hope to develop a thorough and precise system for detecting brain tumors, which could lead to increased efficiency and precision in diagnosis. Careful parameter tuning is necessary to maximize the performance of the integrated FCM and SVM model, taking into account elements like the kernel type for SVM and the fuzziness coefficient for FCM. This guarantees flexibility to various imaging scenarios and datasets. Furthermore, a crucial factor in determining the model\'s practicality in the real world is its resilience to changes in medical image datasets, such as varying imaging protocols, resolutions, and tumor appearances. Thorough validation is necessary to evaluate the model\'s efficacy and dependability. This validation should involve a variety of datasets with different tumor types and locations. The model\'s ability to precisely detect tumor locations while lowering false positives and negatives is thoroughly assessed using quantitative performance evaluation metrics such as sensitivity, specificity, accuracy, and the Dice coefficient. By comparing the suggested approach\'s performance to tried-and-true methodologies, comparative analyses with current approaches help to clarify the novelty and effectiveness of the suggested strategy. This integrated methodology has a significant potential impact on clinical practice. It may enable timely and targeted treatment, thereby improving patient outcomes in neuro-oncology, by providing a more precise and effective tool for early tumor identification. Subsequent studies may explore the incorporation of more advanced techniques, such deep learning methods, to enhance the effectiveness of brain tumor detection systems. This study advances the field of medical image processing and patient care by providing a significant breakthrough in the rapid and precise diagnosis of brain cancers.
Introduction
I. INTRODUCTION
Research has demonstrated that the synergistic combination of fuzzy C-Means and support vector machines (SVM) improves the diagnostic performance of magnetic resonance imaging (MRI) pictures when used to identify brain cancers. To find metabolic abnormalities, magnetic resonance spectroscopic imaging (MRSI) is utilized in addition to magnetic resonance imaging (MRI), which is the gold standard for diagnosing brain illnesses. This is particularly typical of restricted, heterogeneous lesions such as tumors. Deep neural networks with many parameters encounter challenges because of the limited availability of MRSI data, despite the fact that convolutional neural networks (CNNs) have proven their worth in the field of medical image segmentation. This work explores the use of shallow convolutional neural networks (CNNs) for the classification of mild to moderate recurrent somatosensory integration (MRSI) disorders, exploiting the shared feature of spatial and spectral localization in images and spectra. The proposed CNN-based approach demonstrates a voxel-level brain voxel categorization [1].
This is possible when spectroscopic and imaging data are combined. Support vector machines (SVM) and fuzzy C-Means (FCM) clustering are used in tandem to identify brain tumors in magnetic resonance imaging (MRI) pictures. By combining the benefits of FCM's soft clustering for picture segmentation with SVM's robust classification capabilities, this integrated approach improves the accuracy and effectiveness of tumor identification. By combining the benefits of the two classification techniques, this is achieved.The primary application of clustering fuzzy C-Means is the division of magnetic resonance imaging (MRI) images into distinct regions. The partial volume effects and inherent ambiguity found in medical images are taken into consideration by the FCM algorithm. Each voxel is given a degree of membership in order to achieve this. This soft clustering technique provides a detailed image of tissue boundaries, which is crucial for precisely identifying tumor regions [2]. The segmented regions are then further classified into groups associated with cancers and non-tumors using support vector machines. On the other hand, the subsequent classification is carried out. By using a hyperplane to differentiate between classes, the Support Vector Machine (SVM) is a supervised machine learning algorithm that seeks to optimize the margin between them. Support vector machines (SVM) have proven to be an effective tool for handling high-dimensional data and performing binary classification tasks. This makes them ideal for tasks like separating tumor from non-tumor regions in segmented magnetic resonance imaging (MRI) images.FCM and SVM work together to create a workflow that is entirely necessary for development. The FCM-created tissue segments are input features used by the support vector machine classifier. This cooperative method takes advantage of FCM's ability to handle segmentation uncertainty, which enhances SVM's strong classification capabilities. The ultimate result is an improved magnetic resonance imaging (MRI) technology that is more trustworthy and precise in identifying brain tumors.[1]–[5].
There are still some challenges that need to be solved, despite the combined strategy's great potential. The parameters of the FCM and the SVM must be adjusted in order to attain the best results. Furthermore, the system ought to exhibit a strong degree of robustness when it comes to variations in the characteristics of MRI images and the appearance of tumors. The model's reliability and generalizability in real-world circumstances must be ensured by extensive validation using a variety of datasets. The efficacy of the suggested methodology is evaluated using a variety of measures, including as the Dice coefficient, sensitivity, specificity, and accuracy. An understanding of the combined FCM and SVM model's effectiveness can be gained by contrasting it with other currently used approaches or standalone algorithms[6].
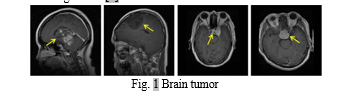
Support Vector Machines (SVM) and fuzzy C-Means (FCM) clustering are used in the intricate process of recognizing brain malignancies from magnetic resonance imaging (MRI) pictures. This integrated technique, meticulously developed to improve the accuracy and efficacy of brain tumor diagnosis, addresses the complexities involved in medical image analysis. Magnetic resonance imaging (MRI) is a key tool in the diagnosis of brain tumors due to its excellent soft tissue contrast. Nonetheless, there are significant obstacles in the way of precisely identifying and defining tumor regions in intricate MRI datasets. The first step is FCM clustering, which is used for image segmentation. The underlying idea behind FCM is its soft clustering algorithm, which gives each voxel a degree of membership according to how likely it is to belong to a particular tissue type. The underlying ambiguity and partial volume effects frequently seen in medical images are accommodated by this nuanced representation, which lays the groundwork for further investigation[7]–[10].
FCM is used to segment MRI images into discrete clusters, each of which represents a different tissue type, in order to detect brain tumors. This segmentation makes it easier to identify abnormal tissue regions linked to tumors by preparing the groundwork for the next classification task. The second aspect of the integrated methodology is realized as the segmented regions that are derived from FCM are fed into the SVM classifier. SVM is an effective supervised machine learning technique that divides data into binary groups, like regions with and without tumors. Support vector machines (SVM) are based on the construction of a hyperplane that divides and maximizes the margin between data points from distinct classes. This feature enhances the algorithm's ability to generalize, a crucial characteristic for accurately classifying complex medical images. A key component of the suggested methodology is the combination of FCM clustering and SVM classification. FCM's capacity to offer a refined segmentation that accurately represents the spatial distribution of tissues harmonizes with SVM's strong classification abilities. Developing a comprehensive and accurate system for brain tumor detection is the aim of merging these techniques.
Parameter tweaking is a key component in optimizing the performance of the composite model. The SVM parameters (such the kernel type and regularization parameter) and FCM parameters (like the fuzziness coefficient) must be carefully adjusted for the best results. The integrated FCM and SVM model can adapt to a variety of datasets and imaging scenarios thanks to this careful parameter tuning. Brain tumor detection involves more than just fine-tuning parameters; it also involves dealing with the unpredictability of medical image datasets and the necessity of rigorous validation. Considerable variation can be found in medical image datasets with regard to imaging protocols, tumor appearances, and resolutions. The suggested approach needs to show that it is resilient to these changes, guaranteeing reliable results on a range of datasets. Thorough validation on a variety of datasets—including ones with different tumor types and locations—is essential to determining how well the model performs in practical situations. Quantitative metrics like sensitivity, specificity, accuracy, and the Dice coefficient are applied in performance evaluation. These metrics provide an extensive assessment of the model's ability to precisely detect tumor locations while lowering false positives and negatives. Comparative analyses with existing methods or individual algorithms contribute to understanding the uniqueness and efficacy of the proposed approach. Benchmarking against established techniques allows for a comprehensive assessment of the integrated FCM and SVM model's performance. The potential impact of the proposed methodology on clinical practice is profound, offering clinicians a more accurate and efficient tool for early tumor identification. This, in turn, facilitates prompt and targeted treatment, ultimately improving patient outcomes in the realm of neuro-oncology. As we look to the future, the proposed methodology paves the way for further advancements in brain tumor detection.
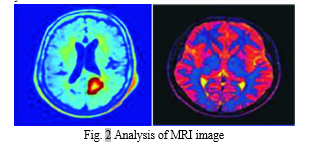
The integration of more sophisticated methods, like deep learning strategies, may be investigated in future research directions in an effort to improve brain tumor detection systems' capabilities. A new era of precision medicine in neuro-oncology may be ushered in by the integration of cutting-edge technologies. The constant advancement of techniques for prompt and accurate diagnosis emphasizes how crucial it is to push the limits of medical image analysis in order to improve patient outcomes and care. In conclusion, Combining fuzzy C-Means clustering with support vector machines is a technically challenging but promising method for finding brain cancers in MRI data. This strategy has important ramifications for the neuro-oncology sector[11], [12].
II. RELATED WORK
Saeedi 2023 et. al Brain MRI scans can distinguish between benign brain tissue and meningiomas, pituitary tumors, and malignant gliomas. If this were the case, early cancer detection would be much simpler for medical professionals. Supplies and Methods The 3,264 MRI scans evaluated for this study included both healthy brain tissue and brain cancers, including meningiomas and gliomas. MRI scans of the brain were the first images we processed and enhanced using a variety of techniques. Using the reserved parameters, we subsequently constructed a convolutional auto-encoder network and a new 2D CNN. Utilizing a 2*2 kernel function at every node, the two-dimensional convolutional neural network (CNN) is a multi-layer network. This network is fairly complicated, with eight convolutional layers and four pooling layers. Each convolutional layer was followed by a batch-normalization layer. The modified auto-encoder network uses the convolutional auto-encoder network's final output encoder layer as the input to a convolutional classification network. In order to classify brain tumors, this study assessed six different machine learning methods. Conclusions: While the recommended 2D CNN had an accuracy of 96.47 percent, the auto-encoder network's accuracy during training was 95.63 percent. 94% and 95%, respectively, are the average recall scores for the auto-encoder and 2D CNN networks. The receiver operating characteristic curves (ROCs) for both networks had an area under the curve of 1. Two appropriate methods for machine learning, K-Nearest Neighbors (KNN) (86% accurate) and Multilayer Perceptron (MLP) (28% accurate) were the least accurate models. A highly significant split in the means between the two approaches presented here and several machine learning techniques was revealed by statistical tests (p.05).
The results show that when analyzing brain cancers, With the best level of classification accuracy, the proposed 2D CNN is used. In comparison to other CNNs and machine learning methods, 2D CNNs performed better in recognizing three types of brain cancers. They also have the fastest execution time without any latency. Since the proposed network is comparable to the auto-encoder network, it may be integrated into clinical systems and utilized by clinicians and radiologists to diagnose brain tumors[3].
Athisayamani 2023 et. al employed to lower the quantity of features from which to choose once they have been retrieved. After that, a softmax classifier and ResNet-152 are used to classify the characteristics. The Figshare dataset is used to implement the suggested strategy in Python. Three measures of the overall performance of the proposed cancer classification system are accuracy, specificity, and sensitivity. The results of the overall evaluation showed that our suggested strategy outperformed its competitors in accuracy by a margin of 98.85%[1].
Liu 2023 et. al have demonstrated remarkable efficacy in an array of computer vision tasks, encompassing semantic segmentation, object recognition, and image categorization. Deep learning-based algorithms have shown encouraging results in brain tumor segmentation. This work presents a comprehensive evaluation of the most recent deep learning-based techniques for brain tumor segmentation, taking into account the notable breakthroughs in cutting-edge technology. Technical subjects like network architecture design, segmentation in unbalanced environments, and multi-modality processes are thoroughly examined in this paper. It looks at and pulls from over 150 academic papers. Regarding future directions for development, there is also intelligent discourse[13].
Pitchai 2023 et. al Manually annotating cancer tissue segmentation under the guidance of a human expert is laborious and prone to errors. In the future, getting a proper diagnosis and starting treatment will be simpler and faster with automated segmentation and survival rate prediction models. In this work, we develop an AI-powered MRI prognostic system for brain tumors using RCNN. For this system, a segmentation model is essential. In this study, data collection for the model-training procedure is done using a U-Net encoder. We gather geometric features while the feature extraction procedure is in progress that help determine the size of the tumor. The prognosis of a tumor is obviously influenced by its size, location, and shape. Simulation results and experimental methods are used to validate the performance of the model, and they both show that the suggested strategy improves precision while reducing errors[14].
Khan 2022 et. al a method by which the radiologist is able to focus on the tumor. But evaluating the MRI pictures by hand takes experience and is a laborious process. Thanks to developments in deep learning, machine learning, and computer-aided design (CAD), radiologists can now diagnose brain cancers with greater accuracy. Conventional machine learning methods for this issue necessitate a feature that has been carefully designed for classification purposes. High-quality classification results can be obtained without the need for manual feature extraction by utilizing deep learning techniques. Two deep learning models are suggested by this study to distinguish between several kinds of brain cancer, including pituitary, glioma, and meningioma and binary (normal and pathological). We utilize two publicly accessible datasets, each comprising 3064 and 152 MRI images overall. We begin by using the first dataset to train our models using a 23-layer convolutional neural network (CNN), as there are a lot of MRI images to select from. But our suggested "23-layer CNN" approach suffers from overfitting when dealing with small datasets, like the second one. Transfer learning is used to solve this problem by combining the VGG16 architecture with a reflection of our proposed "23 layers CNN" structure. Lastly, we compare the suggested models to those that are already in the literature. Based on the datasets we used, our models achieved up to 97.8% and 100%, respectively, of the highest classification accuracy of any state-of-the-art model, according to our experimental results. Access to all of the source code, datasets, and suggested models is available at the following URL[15].
TABLE .1 Literature Summary
|
Author/ year |
Title |
Method/ model |
Parameters |
References |
|
|
Sharma/2022 |
Deep Learning Model for Automated Brain Tumor Classification and Forecasting |
CNN |
accuracy of 98% and sensitivity of 94.73% |
[16] |
|
|
Amin/2022 |
A Novel Approach to Brain Tumor Identification Employing Quantum Variation Classifier and Ensemble Transfer Learning |
quantum variation classifier (QVR) |
Accuracy=99.44%, 99.25% |
[17] |
|
|
Srinivas/ 2022 |
Deep Transfer Learning Techniques in MRI Image-Based Performance Analysis of Brain Tumor Classification |
CNN model |
Accuracy=0.96, 0.78 |
[18] |
|
|
Kasinathan/2022 |
Cloud-Based Deep Learning Techniques for Lung Tumor Detection and Stage Classification |
AI, IoT, |
accuracy of 97%-99.1% and an average of 98.6% |
[19] |
|
|
Zhou/2022 |
Brain tumor MRI Segmentation Automatically using Deep Convolutional Network |
deep learning method |
Sensitivity=0.87, 0.89 |
[20] |
|
TABLE 2 RESEARCH GAPS
|
Sr. no. |
Authors |
Year |
Research gap |
|
1. |
Aggarwal [21] |
2023 |
the computational complexities and extended training times of Deep Neural Networks (DNN) in brain tumor segmentation. The proposed solution, an Improved Residual Network (ResNet), aims to enhance precision and expedite the learning process in medical image analysis. |
|
2. |
Dang[22] |
2022 |
improved accuracy in glioma diagnosis, emphasizing the significance of refining segmentation techniques and optimizing deep learning models for enhanced precision, as demonstrated in the study's MRI-based classification approach. |
|
3. |
Shelatkar[23] |
2022 |
pressing need for early detection of brain tumors, despite recent claims about computer-aided diagnosis systems. This study proposes a transfer learning approach with the YOLOv5 deep learning model for efficient detection of malignant brain tumors, emphasizing the importance of advancing techniques to enhance early diagnosis capabilities. |
|
4. |
Kalpana [7] |
2022 |
exploration of an innovative optimization method, Procedure for Lightning Attachment (PLA), along with DenseNet-169 CNN model application for improved growth feature extraction and classification. This study underscores the necessity for enhanced techniques to overcome existing shortcomings in early-stage brain tumor detection. |
|
5. |
Srinivas [24] |
2022 |
The need for effective categorization of brain tumors, which led to a comparison of the performance of CNN models based on transfer learning—VGG-16, ResNet-50, and Inception-v3. The work addresses the need for improved diagnostic tools in brain tumor analysis by highlighting the importance of deep learning in medical diagnosis and concentrating on assessing the precision of the VGG-16 pretrained CNN model. |
III. DETECTION OF BRAIN TUMOR IN MRI IMAGES
The complex process of recognizing brain cancers from images obtained with magnetic resonance imaging (MRI) is a multifaceted and dynamic endeavor that necessitates a deft combination of numerous advanced techniques within the broad field of medical image analysis. Because of its remarkable contrast with soft tissues, magnetic resonance imaging (MRI) is frequently considered the most advanced neuroimaging modality available. Nonetheless, a major challenge is accurately identifying and demarcating tumor areas in the intricate world of MRI datasets. Brain tumor detection involves a number of steps, such as segmentation, feature extraction, and classification. To complete each of these stages, a specific technique must be used. Partitioning the MRI images to isolate tumor locations from the surrounding normal brain tissue is the first stage in the segmentation process. Several common image processing methods are used in this process, such as region-based methods, fuzzy C-Means clustering algorithms, and edge detection methods. These segmentation techniques aim to identify the spatial boundaries of tumors, which will then form the foundation for additional investigation. Feature extraction is the next step after segmentation, and it entails finding different properties within the segments that have been segmented. These features are employed to distinguish between regions that contain tumors and those that do not. This stage aims to generate a quantitative depiction of the fundamental tissue characteristics by obtaining pertinent data about the tissue, including its dimensions, characteristics of texture, and aspects of intensity. The retrieved characteristics serve as discriminative markers in the next stage of classification.A wide range of machine learning algorithms are used in the process of classifying brain tumors. These algorithms aid in the differentiation of healthy tissues from sick tissues. Using the collected data, Support Vector Machines (SVM), which are well-known for their expertise in binary classification tasks, are essential to the formation of a decision boundary that correctly distinguishes tumor regions. When it comes to learning intricate patterns and representations found within magnetic resonance imaging (MRI) images, Artificial Neural Networks (ANN) and, More recently, Convolutional Neural Networks (CNNs), a type of deep learning model, have shown incredible promise. Deep learning has made a major contribution to the automation of the brain tumor detection procedure, which can automatically learn hierarchical features from data. Thanks to CNNs, which have layers of convolution and pooling, the model can identify complex spatial relationships within the images. This improves accuracy and efficiency over earlier approaches. Because these deep models are trained on large datasets, they can effectively generalize to data that they haven't seen before. This aids in the accurate and automated diagnosis of brain tumors[25]–[28].
Multimodal imaging techniques represent a major advancement in the process of improving the detailed characterization of brain tumors. A comprehensive picture is produced when structural magnetic resonance imaging (MRI) is paired with functional MRI, spectroscopy, or other imaging modalities. This perspective shows more facets of data that are useful in understanding tumor behavior and guiding treatment decisions. As research projects in this area continue to progress, the synergistic integration of different approaches continues to hold great potential. Due to continuing advancements in artificial intelligence, particularly deep learning, it may be possible to use magnetic resonance imaging (MRI) to find brain cancers with even greater efficiency and accuracy. The collaborative efforts of physicians, researchers, and technologists in the intricate field of neuro-oncology pave the way for better treatment approaches and better patient outcomes. The addition of the multimodal approach to brain tumor identification, along with the ongoing advancement of medical imaging technologies, highlights the dedication to deciphering the complexities of neurological disorders and improving the overall landscape of patient care[29].
IV. FUNDAMENTALS OF FUZZY C-MEANS CLUSTERING
A significant development in the process of enhancing the precise characterization of brain tumors is multimodal imaging
techniques. When structural magnetic resonance imaging (MRI) is combined with functional MRI, spectroscopy, or other imaging modalities, a complete picture is generated. This viewpoint highlights additional data points that help explain tumor behavior and inform treatment choices. The synergistic integration of various approaches continues to hold great potential as research projects in this field advance. As artificial intelligence (AI) continues to progress, especially in deep learning, brain tumors may soon be more accurately and effectively identified in MRI scans using magnetic resonance imaging. In the complex area of neuro-oncology, the combined efforts of medical professionals, scientists, and technologists open the door to improved treatment modalities and patient outcomes. The multimodal approach to brain tumor identification and the continuous development of medical imaging technologies demonstrate the commitment to understanding the intricacies of neurological disorders and enhancing patient care in general. As research projects in this area continue to progress, the synergistic integration of different approaches continues to hold great potential. Due to continuing advancements in artificial intelligence, particularly deep learning, it may be possible to detect brain tumors in magnetic resonance imaging (MRI) images with even greater efficiency and accuracy.
The collaborative efforts of physicians, researchers, and technologists in the intricate field of neuro-oncology pave the way for better treatment approaches and better patient outcomes. The addition of the multimodal approach to brain tumor identification, along with the ongoing advancement of medical imaging technologies, highlights the dedication to deciphering the complexities of neurological disorders and improving the overall landscape of patient care.images [30]–[33].
V. ROLE OF SUPPORT VECTOR MACHINES (SVM) IN CLASSIFICATION
The extraordinary ability of Support Vector Machines (SVM) to classify data into binary categories and its effectiveness in distinguishing between regions with and without tumors are two of the most important aspects of the critical function SVM plays in the classification stage of brain tumor detection in MRI images. This is especially true when combining SVM and Fuzzy C-Means (FCM). Support vector machines (SVM) are crucial parts of this complex technology that help improve the accuracy and effectiveness of brain tumor diagnosis. They draw attention to their unique advantages. For the detection stage, the SVM's ability to handle binary classification problems is essential. Because SVM can produce a decision boundary, or hyperplane, that best distinguishes between two distinct classes—tumor regions and normal brain tissue—it is especially useful in the identification of brain tumors. This inherent ability is perfectly matched with the objective of accurately identifying and characterizing diseased areas within the complex terrain of MRI images. One of SVM's most notable advantages is its ability to navigate high-dimensional feature spaces, which is useful for exploiting the complex characteristics gathered in earlier phases, like those discovered during FCM clustering. Support vector machines' (SVM) discriminative capabilities allow for a more thorough analysis of the retrieved characteristics, which when paired with its adaptability to complex datasets, improves tumor classification accuracy.
Support vector machines (SVM) further distinguish themselves in the quest for accurate brain tumor diagnosis by widening the gap between the two groups. As a result ,they can generalize to data that they have never seen before more effectively. Due to the minute distinctions between tissues that are healthy and those that are sick, a discriminating classifier is crucial for medical image analysis. Support vector machines (SVM) are superior to other techniques in precisely classifying brain regions with tumors: they can identify minute patterns and features in the MRI data. Support vector machines (SVM) refine the identification and localization of specific tumor regions, thereby complementing the nuanced segmentation provided by FCM within the collaborative paradigm of the integrated approach with FCM. This synergistic integration makes use of both SVM's discriminating power and FCM's capacity to capture spatial correlations. The end result is a comprehensive methodology for brain tumor diagnosis that seamlessly combines the strengths of both techniques. In the classification stage, A key component in precisely identifying the sites of brain tumors in magnetic resonance imaging (MRI) pictures is support vector machines (SVM). The capacity of the entire brain tumor detection technology to perform well in binary classification, adapt to high-dimensional feature spaces, and discriminate effectively makes it robust and dependable. This demonstrates the synergy that results from the integration of FCM and SVM approaches[34]–[37].
Conclusion
In conclusion, a notable advancement in the field of medical image analysis is the simultaneous use of Support Vector Machines (SVM) and Fuzzy C-Means clustering to detect brain tumors in magnetic resonance imaging (MRI) pictures. Because fuzzy C-Means clustering can give nuanced image segmentation, it is essential to the process of obtaining fine-grained spatial correlations and spectral features required for accurate tumor localization. The various structures and variations that are present in MRI images present a significant challenge that this strategy significantly helps to prevail. The output of the Fuzzy C-Means algorithm is enhanced by adding Support Vector Machines, a dependable classification technique. This approach distinguishes between regions with tumors and regions without tumors with efficiency. Statistical support vector machines (SVM) use the comprehensive data collected using the fuzzy C-means clustering technique to enhance the overall diagnosis accuracy of brain tumors. This cooperative synergy not only resolves the intricacy of MRI images but also offers a feasible path forward for the development of therapeutic applications. The developed methodology has a great deal of potential in clinical settings because it shows increased accuracy. Neurologists and radiologists may believe it to be helpful in the early detection and treatment planning of brain tumor patients. This new method is an illustration of the deliberate attempt to apply modern computer methods for better medical imaging results. It also highlights the ways in which neuroimaging research is developing. The efficaciousness of our amalgamated Fuzzy C-Means and SVM tactic opens the door for additional investigation and refinement in the utilization of intricate techniques to augment diagnostic proficiencies in the challenging domain of brain tumor identification using magnetic resonance imaging. This strengthens the ability to diagnose.
References
[1] S. Athisayamani, R. S. Antonyswamy, V. Sarveshwaran, M. Almeshari, Y. Alzamil, and V. Ravi, “Feature Extraction Using a Residual Deep Convolutional Neural Network (ResNet-152) and Optimized Feature Dimension Reduction for MRI Brain Tumor Classification,” Diagnostics, vol. 13, no. 4, 2023, doi: 10.3390/diagnostics13040668. [2] U. Kosare, L. Bitla, S. Sahare, P. Dongre, S. Jogi, and S. Wasnik, Automatic Brain Tumor Detection and Classification on MRI Images Using Deep Learning Techniques. 2023. doi: 10.1109/I2CT57861.2023.10126412. [3] S. Saeedi, S. Rezayi, H. Keshavarz, and S. R. Niakan Kalhori, “MRI-based brain tumor detection using convolutional deep learning methods and chosen machine learning techniques,” BMC Med. Inform. Decis. Mak., vol. 23, no. 1, pp. 1–17, 2023, doi: 10.1186/s12911-023-02114-6. [4] P. Tiwari et al., “Medical Imaging,” vol. 2022, 2022. [5] Z. Liu, L. Sun, and Q. Zhang, “High Similarity Image Recognition and Classification Algorithm Based on Convolutional Neural Network,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/2836486. [6] Z. Chen, N. Li, C. Liu, and S. Yan, “Deep Convolutional Neural Network-Based Brain Magnetic Resonance Imaging Applied in Glioma Diagnosis and Tumor Region Identification,” Contrast Media Mol. Imaging, vol. 2022, 2022, doi: 10.1155/2022/4938587. [7] R. Kalpana, M. A. Bennet, and A. W. Rahmani, “Metaheuristic Optimization-Driven Novel Deep Learning Approach for Brain Tumor Segmentation,” vol. 2022, 2022. [8] R. Zhou, S. Hu, B. Ma, and B. Ma, “Automatic Segmentation of MRI of Brain Tumor Using Deep Convolutional Network,” vol. 2022, 2022. [9] A. H. Khan et al., “Intelligent Model for Brain Tumor Identification Using Deep Learning,” vol. 2022, 2022. [10] P. Dahiya, A. Kumar, A. Kumar, and B. Nahavandi, “Modified Artificial Bee Colony Algorithm-Based Strategy for Brain Tumor Segmentation,” vol. 2022, 2022. [11] E. U. Haq, H. Jianjun, X. Huarong, K. Li, and L. Weng, “A Hybrid Approach Based on Deep CNN and Machine Learning Classifiers for the Tumor Segmentation and Classification in Brain MRI,” Comput. Math. Methods Med., vol. 2022, 2022, doi: 10.1155/2022/6446680. [12] Z. Liu et al., “Deep Learning Based Brain Tumor Segmentation: A Survey,” 2021. [13] Z. Liu et al., “Deep learning based brain tumor segmentation: a survey,” Complex Intell. Syst., 2022, doi: 10.1007/s40747-022-00815-5. [14] R. Pitchai et al., “Region Convolutional Neural Network for Brain Tumor Segmentation,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/8335255. [15] M. S. I. Khan et al., “Accurate brain tumor detection using deep convolutional neural network,” Comput. Struct. Biotechnol. J., vol. 20, pp. 4733–4745, 2022, doi: 10.1016/j.csbj.2022.08.039. [16] S. Sharma et al., “Deep Learning Model for Automatic Classification and Prediction of Brain Tumor,” J. Sensors, vol. 2022, 2022, doi: 10.1155/2022/3065656. [17] J. Amin, M. A. Anjum, M. Sharif, S. Jabeen, S. Kadry, and P. Moreno Ger, “A New Model for Brain Tumor Detection Using Ensemble Transfer Learning and Quantum Variational Classifier,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/3236305. [18] C. Srinivas et al., “Deep Transfer Learning Approaches in Performance Analysis of Brain Tumor Classification Using MRI Images,” J. Healthc. Eng., vol. 2022, 2022, doi: 10.1155/2022/3264367. [19] G. Kasinathan and S. Jayakumar, “Cloud-Based Lung Tumor Detection and Stage Classification Using Deep Learning Techniques,” Biomed Res. Int., vol. 2022, 2022, doi: 10.1155/2022/4185835. [20] N. B. Bahadure, A. K. Ray, and H. P. Thethi, “Image Analysis for MRI Based Brain Tumor Detection and Feature Extraction Using Biologically Inspired BWT and SVM,” Int. J. Biomed. Imaging, vol. 2017, 2017, doi: 10.1155/2017/9749108. [21] M. Aggarwal, A. K. Tiwari, M. P. Sarathi, and A. Bijalwan, “An early detection and segmentation of Brain Tumor using Deep Neural Network,” BMC Med. Inform. Decis. Mak., vol. 8, pp. 1–12, 2023, doi: 10.1186/s12911-023-02174-8. [22] K. Dang, T. Vo, L. Ngo, and H. Ha, “IBRO Neuroscience Reports A deep learning framework integrating MRI image preprocessing methods for brain tumor segmentation and classification,” IBRO Neurosci. Reports, vol. 13, no. November, pp. 523–532, 2022, doi: 10.1016/j.ibneur.2022.10.014. [23] T. Shelatkar, M. Shorfuzzaman, and A. Alsufyani, “Diagnosis of Brain Tumor Using Light Weight Deep Learning Model with Fine-Tuning Approach,” vol. 2022, 2022. [24] C. Srinivas et al., “Brain Tumor Classification Using MRI Images,” vol. 2022, 2022. [25] S. Einy, H. Saygin, H. Hivehch, and Y. Dorostkar Navaei, “Local and Deep Features Based Convolutional Neural Network Frameworks for Brain MRI Anomaly Detection,” Complexity, vol. 2022, 2022, doi: 10.1155/2022/3081748. [26] A. Akilandeswari et al., “Automatic Detection and Segmentation of Colorectal Cancer with Deep Residual Convolutional Neural Network,” Evidence-based Complement. Altern. Med., vol. 2022, 2022, doi: 10.1155/2022/3415603. [27] C. Guo and Z. Li, “Automatic Rock Classification Algorithm Based on Ensemble Residual Network and Merged Region Extraction,” Adv. Multimed., vol. 2022, 2022, doi: 10.1155/2022/3982892. [28] A. S. Ladkat et al., “Deep Neural Network-Based Novel Mathematical Model for 3D Brain Tumor Segmentation,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/4271711. [29] N. Kumar, N. Narayan Das, D. Gupta, K. Gupta, and J. Bindra, “Efficient Automated Disease Diagnosis Using Machine Learning Models,” J. Healthc. Eng., vol. 2021, 2021, doi: 10.1155/2021/9983652. [30] S. Saravanan, V. V. Kumar, V. Sarveshwaran, A. Indirajithu, D. Elangovan, and S. M. Allayear, “Computational and Mathematical Methods in Medicine Glioma Brain Tumor Detection and Classification Using Convolutional Neural Network,” Comput. Math. Methods Med., vol. 2022, 2022, doi: 10.1155/2022/4380901. [31] J. Arun Pandian and K. Kanchanadevi, “An improved deep convolutional neural network for detecting plant leaf diseases,” Concurr. Comput. Pract. Exp., vol. 34, no. 28, 2022, doi: 10.1002/cpe.7357. [32] Z. Qian, L. Xie, and Y. Xu, “3D Automatic Segmentation of Brain Tumor Based on Deep Neural Network and Multimodal MRI Images,” Emerg. Med. Int., vol. 2022, pp. 1–9, 2022, doi: 10.1155/2022/5356069. [33] Z. Zhu, H. Chen, S. Xie, Y. Hu, and J. Chang, “Classification and Reconstruction of Biomedical Signals Based on Convolutional Neural Network,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/6548811. [34] M. Sethi, S. Ahuja, S. Rani, D. Koundal, A. Zaguia, and W. Enbeyle, “An Exploration: Alzheimer’s Disease Classification Based on Convolutional Neural Network,” Biomed Res. Int., vol. 2022, 2022, doi: 10.1155/2022/8739960 [35] M. S. I. Khan et al., “Accurate brain tumor detection using deep convolutional neural network,” Comput. Struct. Biotechnol. J., vol. 20, pp. 4733–4745, 2022, doi: 10.1016/j.csbj.2022.08.039. [36] I. Hassan, T. Ali, G. Ali, and T. Ali, “Neural Network and Deep Transfer Learning Approach with MR Imaging Brain Tumor Classification using Convolutional Neural Network and Deep Transfer Learning Approach with MR Imaging,” 2022. [37] K. U. Kurukshetra, “Building Efficient Neural Networks For Brain Tumor Detection,” vol. 6, no. 11, pp. 222–235, 2022.
Copyright
Copyright © 2024 Harsh Maru, Shyamol Banerjee. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63406
Publish Date : 2024-06-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

