Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Implementation of Biotechnological Techniques in Treatment of Groundwater Contaminated with Arsenic
Authors: Sanchari Das, Swayambhik Mukherjee
DOI Link: https://doi.org/10.22214/ijraset.2022.40431
Certificate: View Certificate
Abstract
Over the course of time, there seemed to be an increase in rate of Arsenic (As) contamination in groundwater found around the world. Several organic or/and anthropogenic resources have emerged to be the primary cause in contributing to a negative impact on public health as well as on the environment. A large number of people throughout the world rely on groundwater containing toxic levels of As for their drinking needs. When As is exposed to drinking water in a significant amount for a great period of time, it may lead to several disabling and weakening diseases. the most common one being a dermatological condition known as Arsenicosis. The sources, distribution, migration of As, as well as a worldwide summary of its contamination in drinking water are discussed in this paper. The research also examines the human health hazards associated with As, as well as its absorption process and contribution in the poisonous pathways alongside providing an introduction of modern evidence on treating groundwater with several Biological/Biotechnological techniquesas a substitute or potential replacement of the existing and well known physical-chemical methods used in the purification process.
Introduction
I. INTRODUCTION
Nearly most of the water flows calmly and remain unnoticeable on the surface, however sometimes it makes a glorious display mainly in a large spring, geyser or cave and these prominent features of groundwater were the only knowledge of the prehistoric man. Thus, with course of time people start settling down near the springs and started digging wells to find more water where it was not clearly visible on the surface.[1]. Groundwater can be defined as water existing beneath the Earth’s surface in between the soil pores and rock and rock formation fractures. An aquifer defined as a small segment of rock or an unconsolidated sediment. Water table is formed at an abyss where no spaces are present in between the soil pores, rocks or fractures. The surface of actually responsible for groundwater recharge mainly at seeps and springs forming wetlands or oasis. Extraction wells are constructed and utilized for collecting groundwater for industrial, agricultural and municipal use. A survey also stated that almost 50% or more people of United States, mainly people dwelling in rural areas survive by using groundwater for their drinking purposes.
II. ARSENIC CONTAMINATION IN GROUNDWATER:
In today’s world Arsenic contamination is imposing a serious threat to the health of people consuming Arsenic in groundwater for drinking purposes. A majority of people from the rural areas of India, Bangladesh, Canada, China and several parts of world uses groundwater for drinking and domestic purposes like irrigation are affected by Arsenic poising. The chief natural sources of Arsenic are:
The erosion and weathering of rocks and soil having presence ofArsenic.
- Gold mine operations
- Wood preservative services
- Several anthropogenic sources [2,4]
A number of geochemical and hydrologic factors affect the Arsenic solubility in groundwater. [3]
The World Health Organization (WHO) fixed 10 μg L−1 to be the safe limit of Arsenic in drinking water. A recent survey showed that almost 9 and 42 districts of West Bengal (in India) and Bangladesh respectively have Arsenic level over 50 μg/L in groundwater used for drinking and domestic purposes. In these 9 districts of West Bengal, almost 69 police stations/blocks and 985 villages have been found to be already affected by Arsenic. Since Arsenic contaminated groundwater has been used for agricultural purposes thus increasing the level and longevity of Arsenic in soil finally making the soil unsuitable for irrigation. This situation leads to increase the risk of livestock and human health. [2]
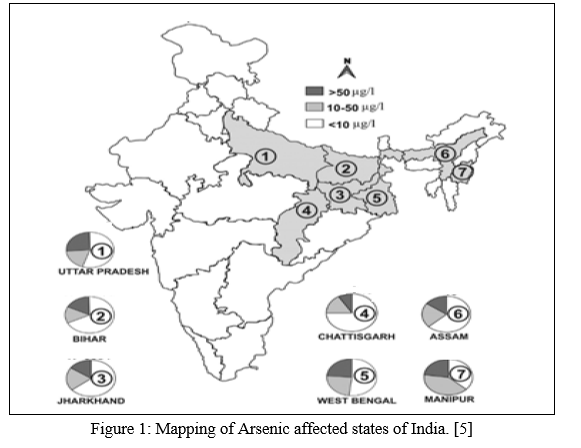
As Arsenic has no specific taste, colour, odour of its own so it is impossible to avoid and detect the presence of Arsenic by a normal individual.[6]
A report estimated that almost 150 million people in today’s world is already affected by As poisoning.[7]
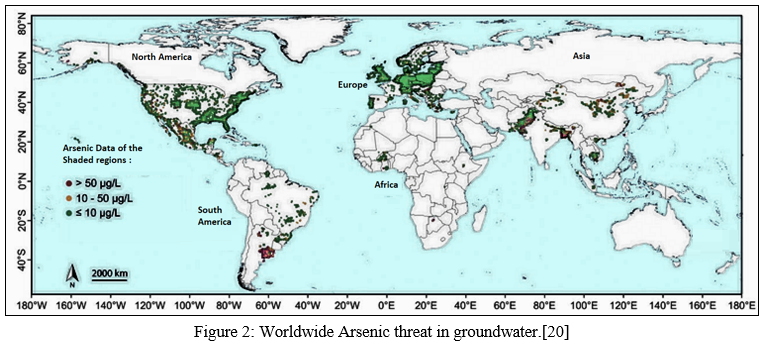
Worldwide large deltas and river valleys or basins are majorly affected by Arsenicosis [8] like Bengal delta [9-11], Paraiba do Sul delta, Brazil [12], Danube River basin, Hungry [13], Mekong delta, Cambodia [14], Hetao river basin, Mongolia [15], Zenne river basin, Belgium [16], Duero Cenozoic Basin, Spain [17] and Tulare Lake, USA [18]. Along term risk is imposed to the ecosystem and human when As gets transferred to the food chain. [19]
III. IMPACT OF ARSENIC CONTAMINATION ON HUMAN HEALTH:
Arsenicosis, a common health problem caused by long term intake of Arsenic, a common carcinogen through food or water.
Arsenicosis involves several disorders like:
- Skin disorders
- Internal cancers like bladder, lung and kidney
- Skin cancers
- Malfunctioning of blood vessels in legs and feet
- Diabetes
- Hypertension
- Reproductive disorders [21-23]
Pentavalent arsenate and trivalent arsenite are more common and toxic than any other organic forms in the terrestrial environment. They react with sulfhydryl groups present in cysteine residues harmfully effect the protein metabolism as their toxicity increases.[24] Arsenicosis victims faces severe consequences in their family life, livelihood and earning capability. Women eventually isolates themselves from the society as her physical appearances detoriates succeeding Arsenicosis.
The pH conditions and redox potentials are the two factors chiefly determining the distribution of the two primary species As(III), As(V) of Arsenic.[25] Since the surface waters get dominated mostly by oxidizing conditions making pentavalent Arsenic as the most predominant species, primarily existing in its oxyanionic forms H2AsO4-, HAsO4 2-with pKa ¼ 2:19; pKb ¼ 6:94 respectively. While the prevailing reducing conditions in groundwaters make the trivalent ArsenicAs (III) as the most predominant and thermodynamically stable species which exist as non-ionic form ofH3AsO3, arsenious acid having pKa ¼ 9:22 It is present in almost all-natural water having vast pH range. It is thus very difficult to get rid of trivalent Arsenic by conventional treatment methods like precipitation, adsorption, etc. as it may show minor reaction with most solid surfaces.[26] The other treatment technologies are coagulation/ filtration, lime softening, adsorption on activated alumina or iron oxides, ion exchange, reverse osmosis which are employed for removing Arsenic from water. [27-29] A preoxidation step is usually employed convert the trivalent form of Arsenic to the pentavalent form
by adding certain chemical reagents like potassium permanganate, hydrogen peroxide, chlorine, ozone or manganese oxides. [27,30,31] On one hand these chemical reagents successfully oxidize the trivalent Arsenic but on the other hand their residuals or by- products may give rise to several secondary problems. Theses chemical reagents can also abruptly increase the operational cost of the entire process. Now a days, these chemical reagents are often replaced with biological iron oxidation, employed for oxidizing trivalent to pentavalent Arsenic. [32,33] Several microorganisms like Leptothrixochracea and Gallionellaferruginea which are originally found in groundwater are employed as catalyst for this iron oxidation reaction. [34] Low level iron oxides containing organic matter in significant amounts are produced as a result from the iron biological oxidation. The intermixing of these resultant iron oxides, existing bacteria and organic matter give rise to solids which have the capability to attract and hold substances by the process of absorption and adsorption.
These solids are called sorbent showing unique retention properties of metals.[35] Even though Arsenic can be easily eliminated by co-precipitation or direct adsorption on bioactive iron oxides that have already been synthesized, however the iron oxidizing bacteria increases the overall removal efficiency of the trivalent Arsenic oxidation.[36]
IV. RESULTS AND DISCUSSIONS
Our objective is to review the biological/biotechnological processes (notfocusing on the well-known chemical processes) that can be used for effective purification of groundwater contaminated with Arsenic:
A. Purification Processes in General
The purification process for removing Arsenic from groundwater may involve several typical and well known physical–chemical methods which includes adsorption, ion exchange, membrane technology and precipitation. The precipitation agents used for precipitation methods are mostly ferric sulphate, Portland cement, hydrogen peroxide and calcium oxide. With time several methods like membrane separation, adsorption and chemical coagulation–precipitation which removes Arsenic removal from drinking watersuccessfully. But, here, we are only going to focus on the biological/biotechnological methods that can be introduced for the desired purification process.
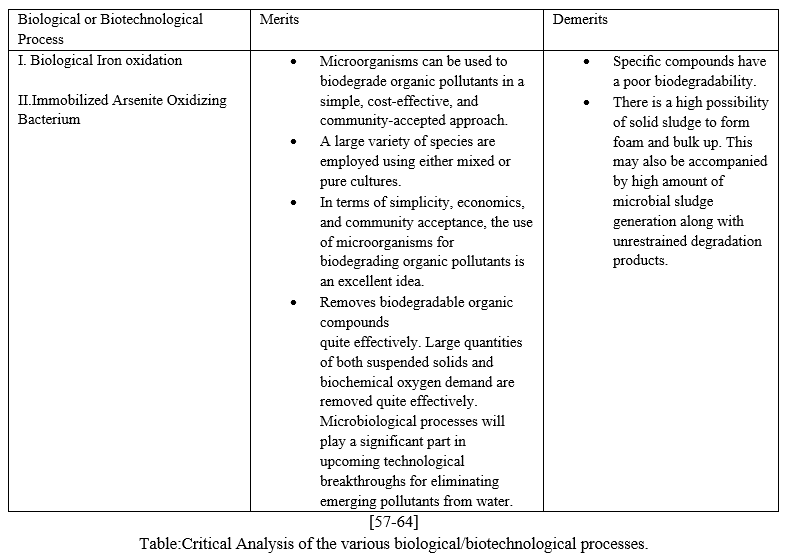
The Biological/Biotechnological methodologies deemed to be effective in the purification process:
- Using Biological Iron Oxidation
Theconditions maintained while removing trivalent Arsenic from the groundwater containing ferrous oxide:
a. pH around 7.2
b. Redox potential: 270–280 Mv
c. Dissolved oxygen concentration of about 2.7 mg/L. [37]
A sample of groundwater was collected and checked for its Arsenic level. If the Arsenic level was found to be too low then more amount of Arsenic is externally added to have a considerable amount which successfully helps in investing the removal process. The stock solution of As (III) was prepared by adding Arsenic oxide (As2O3) as analytical reagent (AnalaR) and 10 ml/ L of HCl acid and then dissolving them in de-ionized water. The final solution is then heated. As(V) was prepared as a stock solution by adding Na2HAsO4 7H2O in distilled water. A unit of fixed-bed up flow filtration is set up for Arsenicelimination using biological iron oxidation. The apparatus involves a Plexiglas column stuffed with polystyrene beads which represents the filtration media. After 3 months the microorganisms employed in the process are deposited and collected in the filtration column.

The schematic diagram above represents the several parts of the biological oxidation and filtration system.
- Continuous flow of Arsenic contaminated groundwater
- Arsenic stock solution
- Peristaltic (feeding) pump
- Influent sampling vessel
- Air injection
- Aeration column
- Filtration column
- Effluent
Traits of Plexiglas column:
- 1 m of active height
- Inner diameter of 68 mm
- Surface area of 0.0036 m2
- Bed volume of 3.6 L
- Total bed porosity of 0.37
- Bead diameter of 3–4 mm.
In a ferrous iron rich oxidizing environment, Leptothrixochracea and Gallionellaferruginea respectively form sheaths and stalks. The formation of sheaths and stalks provide protective mechanism to these microorganisms as they become unstable in an oxidizing environment which is formed when the reducing capacity of ferrous iron level start increasing. The exhausted bacteria finally reach the filtration media where they start gaining their energy from oxidizing ferrous iron: Gallionella or by ingesting organic matter: Leptothrix [38,39]
The plastic vessel serves as the first sampling point where the two streams of Arsenic stock solution is dissolved in contaminated groundwater stream followed by aeration in a distinct column and then lead to the filtration unit. The concentration of iron in the effluent is increased to prevent the bubbles colliding with the deposited sludge. Several samples are collected from the effluent and then evaluated for iron, As(III) and total As(III and V). The treated water is then released from the topmost point of filtration column.
Re%As = [{Asin - Asout}/(Asin)]100
The above equation is employed to calculate the percentage of Arsenic removal
t =bed height/linear velocity
The above equation is used to calculate the residence time of water present in the filter.
The filter column is cleaned by backwashing after every 3days to prevent filter clogging as the amount of deposited iron increase eventually.
In accordance with Driehaus and Jekel improved method, two approaches of atomic absorption spectrometry (Perkin Elmer 2380) and hydride generation (Perkin Elmer-MHS 10) are employed to calculate the total amount of Arsenic present.[40] The sample is pre-treated with acetic acid followed by hydride generation to specifically calculate the As(III) form as this method involves quick analysis of inorganic Arsenic species when the concentration is lower than 1 mg/L. Spectrophotometric is used for iron speciation (between FeII and FeIII) and determination[41]. Redox potential is measured by using redox) meter (E396 B, Metrohm, with two electrodes (Ag/AgCl and Pt). Dissolved Oxygen Meter (Consort, Z 521) was used for measuring the dissolved oxygen level. The different products of biological precipitation are classified by:
- Z-potential (Rank Brothers Mark II)
- FTIR (Perkin Elmer 1600)
- XRF (Spectro-Xepos)
- XRD (Philips PW 1830)
SEM analysis (SEM, Philips 555M) together with energy dispersive X-ray microanalysis (EDX) is employed for detecting the microorganisms and guaranteeing the formation of stalk and sheath during the process.[34]
B. Using Immobilized Arsenite Oxidizing Bacterium
In former times, Arsenic oxidation was usually carried out by several chemicals like ozone, potassium permanganate, chlorine and hydrogen peroxide. [42-44] However, these chemical processes evolved to be quite expensive and are quite difficult to handle. Eventually these chemical processes were replaced with biological oxidation using Arsenite Oxidizing Bacteria (AOB) to win over these drawbacks. These bacteria are present in numerous ecological specimens like Arsenic-contaminated water, mines, raw sewage geothermal waters, soils and residues. [45-52] It was also discovered that chemoautotrophic and aerobic AOB like Thiomonasarsenivorans, collected from goldmine site, utilize As(III) as the primary source of energy. It is used for As(III) oxidation which further purify the contaminated groundwater making it acceptable for drinking purposes.[53] It was also reported that activated sludge can contain Arsenic-resistant bacterium capable of oxidizing As(III). This bacterium can develop in an aerobic environment without needing any organic carbon source.
A study was conducted to know how AOB collected from activated sludge get effected by pH, concentration of initial nitrogen source, water temperature. Several batch experiments were also conducted to investigate the characteristics of collected AOB using the prior stated conditions. AOB were made immobilized using a polyvinyl alcohol gel carrier before the experiments were conducted. Activated sludge was collected from aeration tank and from there arsenite-resistant bacteria (ARB) were separated.[54] In order to separate out the chemoautotrophic ARB from mixed culture, an artificial medium devoid of sodium lactate was used as a carbon source. Finally, based on the different morphology of pure and mixed culture of ARB, the pure form of ARB was collected. The phylogenic analysis of the obtained pure culture AOB was conducted.A bioreactor was used to detect how the continuous biological oxidation of As(III) was influenced by the hydraulic retention time (HRT).[49,55,56]
Conclusion
The increased challenge in the Arsenic contamination have enlarged the number of Arsenicvictims. Few studies have been conducted which provide aid to these Arsenicvictims. These studies mainly focus on the groundwater or drinking water treatment to get rid of any Arsenic impurities if present before they are consumed. Massive physico-chemical treatment plants were established for this treatment process. However, all the physico-chemical treatment process excluding the membrane-based technology showed several weaknesses. The membrane-based technology only evolved to be an effective one resulting in Arsenic-free water. Though both Nanofiltration (NF) and Reverse Osmosis (RO) evolved to successfully remove Arsenic from groundwater but the increased cases of membrane fouling emerged to be their drawback. Soon, the physico-chemical treatment methods were replaced with biological processes.This has made us turn our attention to the biological/biotechnological methods discussed in the paper that can be put into use to obtain the desired results. From our study, we can propose that the process of biological oxidation using the microorganism Leptothrixochracea and Gallionellaferrugineacan be an effective method for removing Arsenic from groundwaters. It can be speculated that during the entire process, iron oxides got continuously accumulated in the filter medium accompanying the exhausted microorganisms which further provide an advantageous environment for Arsenic to get adsorbed and eliminated from the aqueous stream. The As(III) oxidation by these specified microorganisms was observed under suitable experimental conditions which further enhances the process of Arsenic removal. These experimental conditions also helped the successful removal of As(V). Since it does not employ the use of chemical reagents for the process of Arsenic oxidation, the method evolved to be more environment friendly and economical. It even does not involve continuous auditing of the breakthrough point. Since it is a united treatment process of biological oxidation–filtration–sorption, it can be employed for removing a wide range of inorganic contaminants like manganese, iron, Arsenic from groundwater. The phylogenetic analysis helped to compare the rDNA sequence of the AOB isolated with its correlated sequences and also helped to detect their phylogenetic relationship. The optimum ratio of the concentration of nitrogen source to As(III) concentration was detected to be around 0.5. The optimum pH and water temperature for As(III) oxidation was found to be in the range of 6 to 8 and beneath 20? respectively. Thus, the usage of the proposed biological/biotechnological techniques in the purification of groundwater contaminated with Arsenic may actually turn out to be very effective and may open up avenues for further research. • Conflict of Interest: The authors declare no Conflict of Interest.
References
[1] Charles R. Fitts, Groundwater Science, Academic Press, 2002. [2] British Geological Survey (BGS), 2000. Executive Summary of the Main Report of Phase I, Groundwater Studies of As Contamination in Bangladesh, by British Geological Survey and Mott MacDonald (UK) for the Government of Bangladesh, Ministry of Local Government, Rural Development and Cooperatives DPHE and DFID (UK), http://www.dainichi-consul.co.jp/english/article/DFID-sum.html. [3] Wang S, Mulligan CN. Arsenic in Canada. Proceedings of the 57th Canadian geotechnical conference and 5th joint-IAH-CNS/CGS conference, Quebec City, Canada Session 1D, environmental geotechnology I; 2004a. p. 1 – 8 October.Luis Rodríguez-Lado,1 * Guifan Sun,2 Michael Berg,1 Qiang Zhang,2 Hanbin Xue,1 Quanmei Zheng,2 C. Annette Johnson1: Science 341, 866 (2013) [4] Luis Rodríguez-Lado,1 * Guifan Sun,2 Michael Berg,1 Qiang Zhang,2 Hanbin Xue,1 Quanmei Zheng,2 C. Annette Johnson1: Science 341, 866 (2013) [5] J. L. Stroud, G. J. Norton, M. R. Islam et al., “The dynamics of arsenic in four paddy fields in the Bengal delta,” Environmental Pollution, vol. 159, no. 4, pp. 947–953, 2011. [6] H.M. Anawar, J. Akai, M. Mihaljevi?, A.M. Sikder, G. Ahmed, S.M. Tareq, M.M. Rahman, Arsenic contamination in groundwater of Bangladesh: perspectives on geochemical, microbial and anthropogenic issues, Water, 3 (2011) 1050-1076.J. L. Stroud, G. J. Norton, M. R. Islam et al., “The dynamics of arsenic in four paddy fields in the Bengal delta,” Environmental Pollution, vol. 159, no. 4, pp. 947–953, 2011. [7] S. Fendorf, H. A. Michael, and A. van Geen, “Spatial and temporal variations of groundwater arsenic in South and Southeast Asia,” Science, vol. 328, no. 5982, pp. 1123–1127, 2010. [8] A. Mukherjee, M. K. Sengupta, M. A. Hossain et al., “Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario,” Journal of Health, Population and Nutrition, vol. 24, no. 2, pp. 142–163, 2006.View at: Google Scholar [9] D. Chakraborti, M. M. Rahman, B. Das et al., “Status of groundwater arsenic contamination in Bangladesh: a 14-year study report,” Water Research, vol. 44, no. 19, pp. 5789–5802, 2010.View at: Publisher Site | Google Scholar [10] D. P. Shukla, C. S. Dubey, N. P. Singh, M. Tajbakhsh, and M. Chaudhry, “Sources and controls of Arsenic contamination in groundwater of Rajnandgaon and Kanker District, Chattisgarh Central India,” Journal of Hydrology, vol. 395, no. 1-2, pp. 49–66, 2010. [11] N. Mirlean, P. Baisch, and D. Diniz, “Arsenic in groundwater of the Paraiba do Sul delta, Brazil: an atmospheric source?” Science of the Total Environment, vol. 482-483, pp. 148–156, 2014. [12] J. Nriagu, P. Bhattacharya, A. Mukherjee, J. Bundschuh, R. Zevenhoven, and R. Loeppert, “Arsenic in soil and groundwater: an overview,” in Arsenic in Soil and Groundwater Environment, P. Bhattacharya, A. Mukherjee, J. Bundschuh, R. Zevenhoven, and R. Loeppert, Eds., pp. 3–60, Elsevier, Amsterdam, The Netherlands, 2007. [13] S. Sthiannopkao, K. W. Kim, S. Sotham, and S. Choup, “Arsenic and manganese in tube well waters of Prey Veng and Kandal Provinces, Cambodia,” Applied Geochemistry, vol. 23, no. 5, pp. 1086–1093, 2008. [14] M. A. Khan and Y.-S. Ho, “Arsenic in drinking water: a review on toxicological effects, mechanism of accumulation and remediation,” Asian Journal of Chemistry, vol. 23, no. 5, pp. 1889–1901, 2011. [15] J. Nriagu, P. Bhattacharya, A. Mukherjee, J. Bundschuh, R. Zevenhoven, and R. Loeppert, “Arsenic in soil and groundwater: an overview,” in Arsenic in Soil and Groundwater Environment, P. Bhattacharya, A. Mukherjee, J. Bundschuh, R. Zevenhoven, and R. Loeppert, Eds., pp. 3–60, Elsevier, Amsterdam, The Netherlands, 2007. [16] J. J. Gómez, J. Lillo, and B. Sahún, “Naturally occurring arsenic in groundwater and identification of the geochemical sources in the Duero Cenozoic Basin, Spain,” Environmental Geology, vol. 50, no. 8, pp. 1151–1170, 2006. [17] W. G. Cutler, R. C. Brewer, A. El-Kadi et al., “Bioaccessible arsenic in soils of former sugar cane plantations, Island of Hawaii,” Science of the Total Environment, vol. 442, pp. 177–188, 2013. [18] R. Tuli, D. Chakrabarty, P. K. Trivedi, and R. D. Tripathi, “Recent advances in arsenic accumulation and metabolism in rice,” Molecular Breeding, vol. 26, no. 2, pp. 307–323, 2010. [19] Tallman DE, Shaikh AU. Redox stability of inorganic arsenic (III) and arsenic(V) in aqueous solution. Anal Chem 1980; 52:199–201 [20] Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev 1989; 89:713–64 [21] https://science.sciencemag.org/content/368/6493/845?rss=1 [22] WHO, Guidelines for Drinking-Water Quality, vol. 4, World Health Organization, 2011. [23] USEPA, “Arsenic in Drinking Water,” 2013, http://water.epa.gov/lawsregs/rulesregs/sdwa/arsenic/index.cfm.View at: Google Scholar [24] S. C. Santra, A. C. Samal, P. Bhattacharya, S. Banerjee, A. Biswas, and J. Majumdar, “Arsenic in foodchain and community health risk: a study in gangetic west Bengal,” Procedia Environmental Sciences, vol. 18, pp. 2–13, 2013, Proceedings of the International Symposium on Environmental Science and Technology (2013 ISEST). [25] A. Rai, P. Tripathi, S. Dwivedi et al., “Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system,” Chemosphere, vol. 82, no. 7, pp. 986–995, 2011 [26] J. Brinkel, M. H. Khan, and A. Kraemer, “A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh,” International Journal of Environmental Research and Public Health, vol. 6, no. 5, pp. 1609–1619, 2009 [27] Clifford DA, Lin CC. Arsenic(III) and arsenic(V) removal from drinking water in San Ysidro, New Mexico. Project summary for USEPA, EPA report EPA/600/S2-91/011, Washington, USA, 1991 [28] Kartinen EO, Martin CJ. An overview of arsenic removal processes. Desalination 1995;103:79–88. [29] Zouboulis AI, Katsoyiannis IA. Removal of arsenates from contaminated water by coagulation-direct filtration. Sep Sci Technol 2002;37(12):2859–73. [30] Driehaus W, Seith R, Jekel M. Oxidation of As(III) with manganese oxides in water treatment. Water Res 1995;29(1):297–305. [31] Kim MJ, NrianguJ. Oxidation of arsenite in groundwater using ozone and oxygen. Sci Tot Environ 2000;247:71–9 [32] Dimitrakos G, Martinez Nieva J, Vayenas D, Lyberatos G. Removal of iron from potable water using a trickling filter. Water Res 1992;31(5):991–6. [33] Mouchet P. From conventional to biological removal of iron and manganese in France. J Am Water Works Assoc 1992;84(4):158–66. [34] Chekalla C, Mevius W, Hanert H. Quantitative removal of iron and manganese by microorganisms in rapid sand filters (in situinvestigations). Water Supply 1985;3:11–123. [35] Ferris FG, Hallberg RO, Lyven B, Pedersen K. Retention of strontium, cesium, lead and uranium by bacterial iron oxides from a subterranean environment. Appl Geochem 2000;15:1035–42. [36] Seith R, Jekel M. Biooxidation of As(III) in fixed bed reactors. Wom Wasser 1997;89:283–96 (in German) [37] Katsoyiannis I, Zouboulis A, Althoff H, Bartel H. As(III) removal from groundwaters using fixed bed upflow bioreactors. Chemosphere 2002;47:325–32. [38] Van Veen WL, Mulder EG, Deinema MH. The Sphaerotilus Leptothrix group of bacteria. Microbiol Rev 1978;42(2):329–56. [39] Hallbeck L, Pedersen K. Culture parameters regulating stalk formation and growth rate of Gallionella ferruginea. J Gen Microbiol 1990;136:1675–80. [40] Driehaus W, Jekel M. Determination of As(III) and total inorganic arsenic by online pretreatment in hydride generation atomic absorption spectrometry. Fresenius J Anal Chem 1992;343:352–6 [41] APHA-AWWA-WPCF. Standard methods for the examination of water, wastewater, 17th ed. APHA-AWWAWPCF: Washington, DC; 1989. [42] Dutre V, Vanecasteele C. Solidification/stabilization of arsenic containing waste: leach tests and behavior of arsenic in the leachate. Waste Manage 1995;15(1):55–62. [43] Healy SM, Wildfang E, Zakharyan RA, Aposhian HV. Diversity of inorganic arsenic biotransformation. Biol Trace Elem Res 1999;68:249–66. [44] Pande, S.P., Deshpande, L.S., Patni, P.M., Lutade, S.L., 1997. Arsenic removal studies in some ground waters of West Bengal, India. Journal of Environmental Science and Health, Part A 32 (7), 1981e1987. [45] Kim, M.-J., Nriagu, J., 2000. Oxidation of arsenite in groundwater using ozone and oxygen. Science of the Total Environment 247 (1), 71e79 [46] Dey, M., Williams, K., Coulton, R., 2009. Treatment of arsenic rich waters by the HDS process. Journal of Geochemical Exploration 100 (2e3), 160e162. [47] Sorlini, S., Gialdini, F., 2010. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Research 44 (19), 5653e5659. [48] Philips, S.E., Taylor, M.L., 1976. Oxidation of arsenite to arsenate by Alcaligenes faecalis. Applied and Environmental Microbiology 32 (3), 392e399. [49] Weeger, W., Lie`vremont, D., Perret, M., Lagarde, F., Hubert, J.-C., Leroy, M., Lett, M.-C., 1999. Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. Biometals 12 (2), 141e149 [50] Santini, J.M., Sly, L.I., Schnagl, R.D., Macy, J.M., 2000. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Applied and Environmental Microbiology 66 (1), 92e97 [51] Battaglia-Brunet, F., Dictor, M.-C., Garrido, F., Crouzet, C., Morin, D., Dekeyser, K., Clarens, M., Baranger, P., 2002. An arsenic(III)-oxidizing bacterial population: selection, characterization, and performance in reactors. Journal of Applied Microbiology 93 (4), 656e667. [52] Gihring, T.M., Banfield, J.F., 2001. Arsenite oxidation and arsenate respiration by a new Thermus isolate. FEMS Microbiology Letters 204 (2), 335e340. [53] Salmassi, T.M., Venkateswaren, K., Satomi, M., Newman, D.K., Hering, J.G., 2002. Oxidation of arsenite by Agrobacterium albertimagni, AOL15, sp. nov., isolated from Hot Creek, California. Geomicrobiology Journal 19 (1), 53e66. [54] Kinegam, S., Yingprasertchai, T., Tanasupawat, S., Leepipatpiboon, N., Akaracharanya, A., Kim, K.-W., 2008. Isolation and characterization of arsenite-oxidizing bacteria from arsenic-contaminated soils in Thailand. World Journal of Microbiology and Biotechnology 24 (12), 3091e3096. [55] Garcia-Dominguez, E., Mumford, A., Rhine, E.D., Paschal, A., Young, L.Y., 2008. Novel autotrophic arsenite-oxidizing bacteria isolated from soil and sediments. FEMS Microbiology Ecology 66 (2), 401e410 [56] Battaglia-Brunet, F., Joulian, C., Garrido, F., Dictor, M.C., Morin, D., Coupland, K., Johnson, D.B., Hallberg, K.B., Baranger, P., 2006. Oxidation of arsenite by Thiomonas strains and characterization of Thiomonas arsenivorans sp. nov. Antonie van Leeuwenhoek 89 (1), 99e108 [57] Anawar, H.M., Akai, J., Komaki, K., Terao, H., Yoshioka, T., Ishizuka, T., Safiullah, S., Kato, K., 2003. Geochemical occurrence of arsenic in groundwater of Bangladesh: sources and mobilization processes. Journal of Geochemical Exploration 77 (2e3), 109e131 [58] Mukherjee, A., Fryar, A.E., 2008. Deeper groundwater chemistry and geochemical modeling of the arsenic affected western Bengal basin, West Bengal, India. Applied Geochemistry 23 (4), 863e894. [59] A. Seidel, J.J. Waypa, M. Elimelech, Environ. Eng. Sci. 18 (2) (2001) 105. [60] I.A. Katsoyiannis, A.I. Zouboulis, Water Res. 38 (2004) 17. [61] J. Kim, M.M. Benjamin, Water Res. 38 (2004) 2053. [62] K.N. Ghimire, K. Inoue, H. Yamaguchi, K. Makino, M. Tohru, Water Res. 37 (2003) 4945. [63] J.A. Jay, N.K. Blute, H.F. Hemond, J.L. Durant, Water Res. 38 (2004) 1155. [64] K.V. Hege, M. Verhaege, W. Verstraete, Water Res. 38 (2004) 1550. [65] N. Balasubramanian, K. Madhavan, Chem. Eng. Technol. 24 (5) (2001) 519 [66] Henze M (ed) (2001) Wastewater treatment—biological and chemical processes. Springer, Berlin
Copyright
Copyright © 2022 Sanchari Das, Swayambhik Mukherjee. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET40431
Publish Date : 2022-02-20
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

