Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Investigating tRNA-Derived Small RNA Expression Patterns and Bioinformatics Analysis in Epicardial Fat of Atrial Fibrillation Patients
Authors: Moriom Akter Eti, Rabab Khan Rongon
DOI Link: https://doi.org/10.22214/ijraset.2024.61416
Certificate: View Certificate
Abstract
The intricate mechanisms underlying atrial fibrillation (AF) remain elusive. It is proposed that epicardial adipose tissue (EAT) may play a role in arrhythmias by releasing bioactive molecules, including exosomes containing tRNA-derived small RNAs (tsRNAs). Although tsRNAs are known to significantly influence cellular functions, their relationship with AF remains unexplored. To investigate this, we conducted RNA sequencing on EAT samples from 6 AF patients and 6 sinus rhythm control subjects. Our analysis identified 146 upregulated and 126 downregulated tsRNAs in AF. Four tsRNAs (tRF-SeC-TCA-001, tiRNAGly–CCC–003, tRF-Gly-GCC-002, and tRF-Tyr-GTA-007) were validated via quantitative reverse transcription-polymerase chain reaction. Bioinformatic analysis revealed these tsRNAs target genes associated with cell adhesion and various cellular processes mediated by plasma membrane adhesion molecules. KEGG analysis suggested these target genes may contribute to AF pathogenesis through processes such as glycosaminoglycan biosynthesis, AMP-activated protein kinase activity, and the insulin signaling pathway. Our findings unveil altered tsRNA expression profiles in EAT from AF patients, hinting at potential interactions between tsRNAs and mRNA within EAT that could impact AF pathogenesis.
Introduction
I. INTRODUCTION
Atrial fibrillation (AF) is a prevalent arrhythmia associated with heightened risks of severe complications like ischemic stroke, dementia, and heart failure, leading to significant morbidity and mortality. Despite advancements in therapeutic approaches such as catheter ablation, understanding AF's multifaceted pathophysiology remains a challenge due to its diverse origins encompassing structural and electrical remodeling, autonomic nervous dysfunction, and calcium dysregulation. Hence, unraveling AF's mechanism and seeking innovative treatment avenues remain crucial pursuits.
Substantial evidence links obesity to an elevated AF risk, with visceral adipose tissue implicated in atrial remodeling and AF onset and maintenance. Of particular interest is epicardial adipose tissue (EAT), located around the heart within atrioventricular and interventricular grooves. EAT's proximity to the myocardium and its metabolic activity underscore its potential arrhythmogenic role. Moreover, EAT serves as a reservoir for various bioactive molecules, including exosomes carrying small noncoding RNAs (sncRNAs), among which transfer RNAs (tRNAs) play a prominent role in protein synthesis.
Derived from tRNAs via specific cleavage, tRNA-derived small RNAs (tsRNAs) represent a diverse class of molecules, ranging from 18 to 40 nucleotides, initially identified in cancer patients' urine specimens. High-throughput sequencing has expanded our understanding of tsRNAs, revealing their involvement in diverse biological processes such as RNA silencing, ribosome biogenesis, retro transposition, and epigenetic inheritance. Additionally, a significant portion of tsRNAs is detected in extracellular vesicles, suggesting their potential role in immunosuppressive mechanisms and biomarker potential, particularly in large-artery atherosclerotic stroke.
Classified into tRNA-derived fragments (tRFs) and tRNA-derived stress-induced RNAs (tiRNAs) based on cleavage locations, tsRNAs exhibit dynamic functions under various stress conditions, influencing fundamental cellular processes like embryogenesis and cancer pathogenesis. Despite this knowledge, the correlation between tsRNAs from EAT and AF remains unexplored, leaving their interactions and signalling pathways elusive. Our recent study characterized long noncoding RNA and mRNA profiles in EAT of AF patients, prompting further investigation into tsRNAs' potential role in atrial remodelling through miRNA-like mechanisms.
Hence, this study aims to elucidate the tsRNA landscape in EAT of AF patients and unravel the mechanisms underlying tRFs and tiRNAs in AF pathogenesis. The research design is outlined in Figure 1.
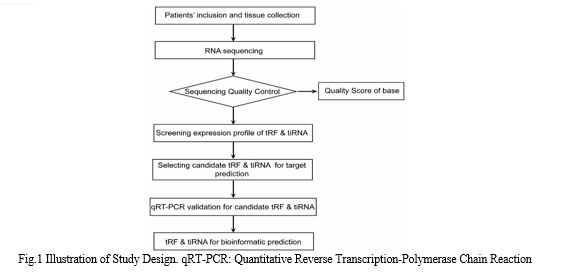
II. MATERIALS AND METHODS
A. Participant Recruitment and Sample Collection
The study adhered to the principles of the 1975 Declaration of Helsinki and obtained approval from the Ethical Committee of Beijing Chaoyang Hospital (2021-ke-246). Written informed consent was obtained from all enrolled patients. Consecutive patients undergoing coronary artery bypass grafting at the Heart Centre Department were recruited. Epicardial adipose tissue (EAT) samples were collected from patients with persistent no valvular atrial fibrillation (AF) (n = 6) and patients in sinus rhythm (SR) (n = 6). Persistent AF was defined as an episode lasting more than 7 days. Biopsy specimens (1–2 cm3) were obtained from the atrioventricular groove before cardiopulmonary bypass initiation, fragmented, rinsed with phosphate-buffered saline (PBS), cryopreserved in liquid nitrogen, and stored at −80°C until RNA extraction.
B. RNA Extraction and Quality Control
Total RNA was extracted from frozen EAT specimens using the QIAGEN miRNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. RNA integrity and concentration were assessed through agarose gel electrophoresis and spectrophotometric analysis using a Nanodrop 1000 instrument (Thermo Scientific, Wilmington, DE).
C. tRF & tiRNA Pretreatment and Library Preparation
Total RNA samples underwent pretreatment to remove RNA modifications hindering library construction, including 3′-aminoacyl (charged) deacylation to 3′-OH, 3′-cP (2′,3″-cyclic phosphate) removal to 3′-OH, 5′-OH phosphorylation to 5′-P, and m1A and m3C demethylation. Pretreated total RNA was used to prepare the sequencing library: RNA was ligated to 3′ and 5′ small RNA adapters, followed by cDNA synthesis and amplification using Illumina’s proprietary RT primers and amplification primers. Amplified fragments (134–160 bp) were purified from the PAGE gel, and libraries were quantified using an Agilent 2100 Bioanalyzer (Invitrogen, USA).
D. Sequencing of tRFs & tiRNAs
Libraries were denatured to produce single-stranded DNA molecules, followed by sequencing for 50 cycles using an Illumina NextSeq 500 system equipped with a NextSeq 500/550 V2 kit (#FC-404-2205, Illumina).
E. Data Analysis of tRFs & tiRNAs
Sequencing quality was assessed using FastQC. The abundance of tRFs and tiRNAs was calculated as counts per million total aligned reads (CPM) using sequencing counts. Differential expression profiles were evaluated with edgeR. Principal component analysis (PCA), Venn diagrams, hierarchical clustering, and scatter/volcano plots were generated in R.
F. Bioinformatic Prediction and Functional Enrichment Analysis
Target prediction for candidate tsRNAs was performed using TargetScan and miRanda, with visualization in Cytoscape. GO enrichment analysis and KEGG pathway analysis were conducted to determine the biological functions of differentially expressed tRFs and tiRNAs.
G. Validation with Quantitative Real-time PCR
Total RNA extraction utilized the QIAGEN miRNeasy Mini Kit (Qiagen, Hilden, Germany), followed by cDNA synthesis using the rtStarTM tRFs & tiRNAs Pretreatment Kit (Arraystar) and the rtStarTM First Strand cDNA Synthesis Kit (Arraystar). Quantitative real-time PCR was performed using the ViiA 7 Real-time PCR system (Applied Biosystems), with normalization to U6 RNA expression levels.
H. Statistical Analysis
Categorical variables were expressed as frequencies and percentages, while numerical variables followed normal distribution were expressed as mean ± SE. Skewed numerical variables were reported as median, 25th, and 75th percentiles. Group comparisons utilized independent-sample t-tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Statistical significance was defined as P < 0.05 using IBM SPSS Statistics 24.0. Sequencing analysis employed the R programming package, with PCA used for dataset dimensionality reduction and expression profile-based sample classification. Differential expression analysis utilized a fold change threshold of >1.5 and significance threshold of P < 0.05.
III. RESULTS
A. Differential Expression Analysis of tRFs & tiRNAs
This study employed high-throughput RNA sequencing to investigate the transcriptome expression profiles of epicardial fat depots in patients with atrial fibrillation (AF) (n = 6) compared to those in sinus rhythm (SR) (n = 6). Baseline characteristics and clinical parameters are detailed in Supplemental Table 1, and the quality score plot for each sample is outlined in Table 1, indicating a high base calling accuracy (Q30) exceeding 99.9% for a majority of bases in each sample. The principal component analysis (PCA) plot depicted distinguishable expression profiles of tRFs and tiRNAs between AF and SR subjects (Fig. 2A).
A total of 473 tsRNAs exhibited differential expression, with 146 upregulated and 126 downregulated in AF compared to SR. The distinct tRF and tiRNA expression profiles and their variation between AF and SR are illustrated in Fig. 2B–D. Experimental data detailing the top 20 upregulated and downregulated tsRNAs are provided in Table 2. The Venn diagram in Fig. 3A indicates that 206 tsRNAs were shared between both groups, with 80 uniquely expressed in AF and 8 in SR. Among these, 70 tsRNAs were identified from the tRF database (Fig. 3B). The pie chart depicts the distribution of subtype tRFs and tiRNAs, with notable increases observed in the expression levels of tiRNA-5, tRF-1, tRF-3a, tRF-3b, and tRF-5a in AF compared to SR, while tRF-5c levels exhibited a significant decrease (Fig. 3C and D). Stacked bar charts in Fig. 3E and F illustrate the distribution of tsRNA subtypes across tRNA isodecoders, while frequency distribution with respect to length is shown in Fig. 3G and H, highlighting marked discrepancies in subtype distribution between the two groups.
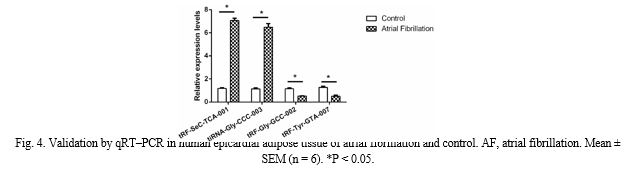
B. Validation via qRT?PCR
To validate the credibility of the high-throughput sequencing data, four prospective tsRNAs (tRF-SeC-TCA-001, tiRNAGly–CCC–003, tRF-Gly-GCC-002, and tRF-Tyr-GTA-007) were randomly selected for qRT-PCR. Consistent with the sequencing results, tRF-SeC-TCA-001 and tiRNA-Gly–CCC–003 exhibited significantly increased expression levels in AF compared to controls, while tRF-Gly-GCC-002 and tRF-Tyr-GTA-007 were downregulated (Fig. 4).
C. Predicted Targets by Bioinformatics Analysis
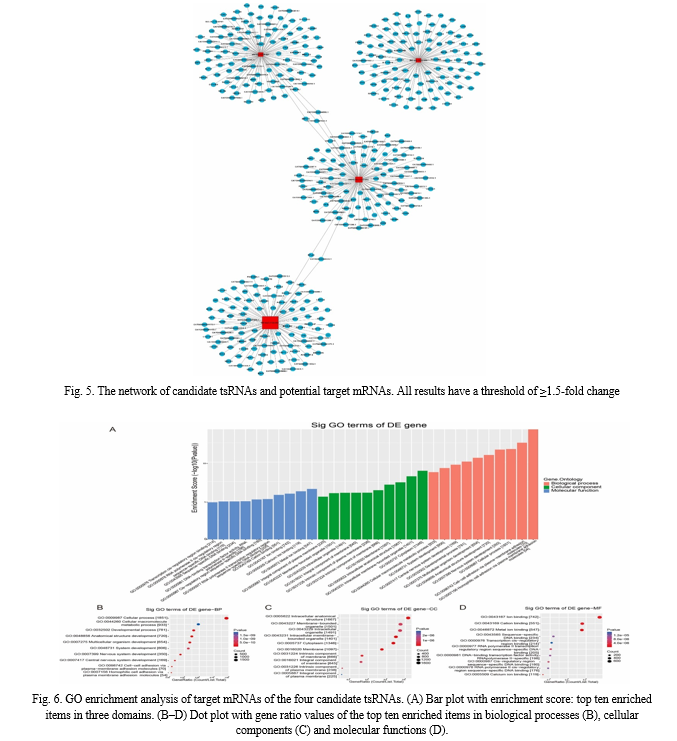
Utilizing bioinformatics tools including TargetScan and miRanda, the four candidate tsRNAs exhibiting differential expression were subjected to target prediction. The tsRNA-target network revealed a total of 2672 target genes associated with these candidate tsRNAs. To visualize the interaction between tsRNAs and mRNA, we constructed a network incorporating the top 100 predicted targets (Fig. 2).
TABLE 1
QUALITY SCORE
|
Sample |
Total Read |
Total Base |
BaseQ30 |
Base30 (%) |
|
AF-1 |
10539403 |
537509553 |
501738225 |
93.34 |
|
AF-2 |
9126561 |
465454611 |
432399080 |
92.90 |
|
AF-3 |
9105223 |
464366373 |
433205005 |
93.29 |
|
AF-4 |
11392677 |
581026527 |
542860679 |
93.43 |
|
AF-5 |
9407894 |
479802594 |
448164130 |
93.41 |
|
AF-6 |
7420499 |
378445449 |
353930856 |
93.52 |
|
SR-1 |
8760739 |
446797689 |
416639194 |
93.25 |
|
SR-2 |
10260164 |
523268364 |
490239587 |
93.69 |
|
SR-3 |
8589294 |
438053994 |
406931685 |
92.90 |
|
SR-4 |
8804405 |
449024655 |
419726053 |
93.48 |
|
SR-5 |
8484260 |
432697260 |
404635018 |
93.51 |
|
SR-6 |
9402354 |
479520054 |
447081561 |
93.24 |
AF: Atrial fibrillation; SR: Sinus rhythm.
Total Read: Raw sequencing reads after quality filtering.
Total Base: Number of bases after quality filtering.
BaseQ30: Number of bases of Q score more than 30 after quality filtering.
BaseQ30(%): The proportion of bases (Q ≥ 30) number after quality filtering.
Predicted Target mRNAs: Predicted target mRNAs for each tsRNA are ranked by prediction score, with specific regulatory interactions identified. For example, tRF-Tyr-GTA-007 and tiRNA-Gly–CCC–003 regulate ZNF316, CATG00000044560.1, and CATG00000041525.1 mRNAs, while tiRNA-Gly–CCC–003 and tRF-Sec-TCA-001 regulate RPL24 and CATG00000080524.1.
GO Enrichment Analysis: Gene Ontology (GO) enrichment analysis reveals highly enriched terms in biological processes, cellular components, and molecular functions domains. The top enriched terms include homophilic cell adhesion, cytoplasm, and metal ion binding.
KEGG Pathway Analysis: Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis identifies relevant signaling pathways regulated by candidate tsRNAs, including glycosaminoglycan biosynthesis, the AMP-activated protein kinase (AMPK) signaling pathway, and the insulin signaling pathway. Dot plots depict the gene ratio values of the top ten most significantly enriched terms.
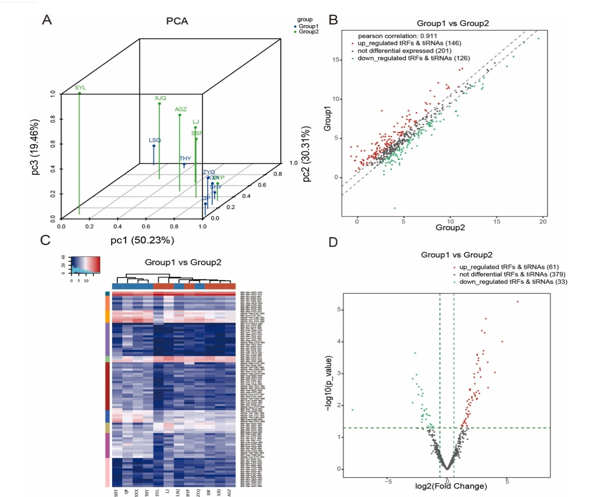
Fig. 2. tsRNA expression level analysis. (A) Principle component analysis. The PCA results show a distinguishable tRF & tiRNA spectrum in the epicardial fat depot between atrial fibrillation and sinus rhythm. (B) Scatter plots of differentially expressed tsRNAs. tRFs & tiRNAs above the top line (red dots, upregulation) or below the bottom line (green dots, downregulation) indicate more than a 1.5-fold change between the two compared groups. Grey dots indicate nondifferentially expressed tRFs & tiRNAs. (C) The hierarchical clustering heatmap for tRFs and tiRNAs in the two groups. (D) The volcano plots of differentially expressed tsRNAs. Red/green circles indicate statistically significant differentially expressed tRFs & tiRNAs with fold changes of no less than 1.5 and p values ≤ 0.05 (red: upregulated; green: downregulated). Grey circles indicate nondifferentially expressed tRFs & tiRNAs.
IV. DISCUSSION
As emerging noncoding small RNAs, tsRNAs have recently emerged as key players in gene expression regulation, translation, and epigenetics. Despite increasing evidence implicating alterations in tRFs and tiRNAs in various diseases, particularly their pivotal roles in tumorigenesis, few studies have explored their association with atrial fibrillation (AF) pathogenesis. In this study, we conducted a comprehensive profiling of tRFs and tiRNAs in human epicardial adipose tissue (EAT) associated with AF using small RNA sequencing. Furthermore, bioinformatic analyses unveiled the potential regulatory role of tsRNAs in pathophysiological processes mediated by the AMPK and insulin signaling pathways. This investigation represents the first comprehensive examination of tsRNA expression in human EAT, shedding light on its involvement in AF pathogenesis. Additionally, the functional annotation of predicted targets provides novel insights into potential pathways implicated in AF pathophysiology.
TABLE II
TOP 20 UP- AND DOWN-REGULATED TSRNAS IN EPICARDIAL ADIPOSE TISSUE OF ATRIAL FIBRILLATION
|
tRF_ ID |
Type |
Length |
Regulation |
|
tRF-SeC-TCA-001 |
tRF-5a |
15 |
Up |
|
tRF-Ser-CGA-006 |
tRF-5a |
16 |
Up |
|
tRF-SeC-TCA-007 |
tRF-5a |
14 |
Up |
|
tRF-Trp-TCA-001 |
tRF-3b |
19 |
Up |
|
tiRNA-Gly–CCC–003 |
tiRNA-5 |
33 |
Up |
|
tRF-Leu-AAG-003 |
tRF-5a |
16 |
Up |
|
tiRNA-Lys-CTT-005 |
tiRNA-5 |
34 |
Up |
|
tiRNA-Lys-CTT-001 |
tiRNA-5 |
34 |
Up |
|
tRF-Ser-TGA-032 |
tRF-5a |
15 |
Up |
|
tRF-Leu-TAG-002 |
tRF-5a |
16 |
Up |
|
tRF-Tyr-GTA-014 |
tRF-5a |
14 |
Up |
|
tRF-Gly-TCC-034 |
tRF-1 |
14 |
Up |
|
tiRNA-Lys-CTT-003 |
tiRNA-5 |
34 |
Up |
|
tiRNA-Gln-CTG-003 |
tiRNA-5 |
34 |
Up |
|
tiRNA-Val-TAC-002 |
tiRNA-5 |
34 |
Up |
|
tiRNA-Lys-CTT-002 |
tiRNA-5 |
34 |
Up |
|
tRF-Ser-TGA-011 |
tRF-5a |
16 |
Up |
|
tRF-Leu-CAA-002 |
tRF-5a |
15 |
Up |
|
tRF-Leu-AAG-002 |
tRF-5a |
15 |
Up |
|
tRF-Leu-CAA-003 |
tRF-5a |
16 |
Up |
|
tRF-Cys-GCA-082 |
tRF-1 |
23 |
Down |
|
tRF-Gly-GCC-002 |
tRF-5c |
28 |
Down |
|
tRF-Tyr-GTA-011 |
tRF-3a |
18 |
Down |
|
tRF-Ser-TGA-028 |
tRF-1 |
16 |
Down |
|
tRF-Tyr-GTA-007 |
tRF-3a |
18 |
Down |
|
tRF-Gly–CCC–033 |
tRF-5c |
28 |
Down |
|
tRF-Tyr-GTA-006 |
tRF-3a |
17 |
Down |
|
tRF-Tyr-GTA-010 |
tRF-3a |
18 |
Down |
|
tRF-Gln-TTG-006 |
tRF-5b |
22 |
Down |
|
tRF-Gly-GCC-007 |
tRF-5c |
28 |
Down |
|
tRF-Gly-GCC-008 |
tRF-5c |
29 |
Down |
|
tiRNA-Met-CAT-001 |
tiRNA-5 |
31 |
Down |
|
tRF-Tyr-GTA-005 |
tRF-3a |
17 |
Down |
|
tRF-Gly–CCC–011 |
tRF-5c |
29 |
Down |
|
tRF-Gly-GCC-029 |
tRF-5c |
29 |
Down |
|
tRF-His-GTG-047 |
tRF-1 |
31 |
Down |
|
tRF-Val-CAC-001 |
tRF-3a |
17 |
Down |
|
tRF-Gly–CCC–010 |
tRF-5c |
29 |
Down |
|
tRF-Val-CAC-046 |
tRF-3a |
29 |
Down |
|
tRF-Gly-GCC-010 |
tRF-5c |
28 |
Down |
Due to their prevalence within cells and robust stability, tsRNAs have traditionally been considered to exhibit a relatively high degree of conservation. However, it is increasingly evident that changes in their abundance or nucleotide modification can lead to aberrant translation and a shift in disease status. Accumulating evidence suggests that tsRNAs play significant roles in viral infections, tumorigenesis, neurodegeneration, and metabolic dysfunction.
In the context of viral infections, small noncoding RNAs (sncRNAs) are crucial for modulating host immunity, regulating host-viral interactions, and controlling viral replication. Viruses can enhance their replication and infection
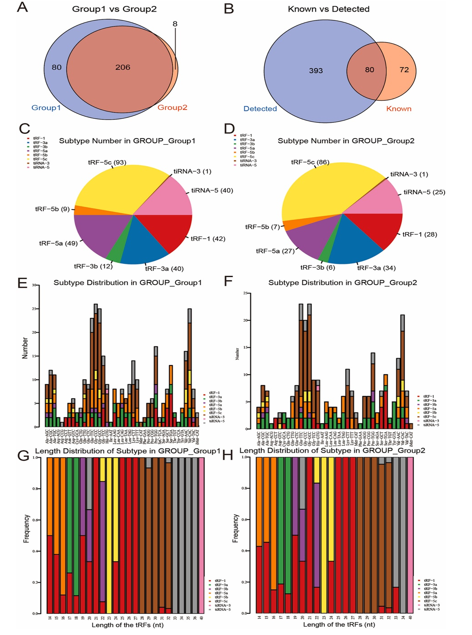
Fig. 3. Analysis of tsRNA subtypes. (A) Venn diagram based on the number of commonly and specifically expressed tRFs and tiRNAs. (B) Venn diagram based on the number of known and detected tRFs and tiRNAs. (C–D) Pie charts of the distribution of subtypes of tRFs & tiRNAs of epicardial adipose tissue in atrial fibrillation (C) and sinus rhythm (D). (E–F) The number of subtypes of tRFs and tiRNAs against tRNA isodecoders in the two groups. (G–H) The frequency of subtype versus length of tRFs and tiRNAs in the two groups.
Efficiency by utilizing host tRFs as guide primers. For instance, respiratory syncytial virus (RSV) induces the production of novel tRF-5GlyCCC and tRF-5LysCTT, contributing to RSV replication and the subsequent cytokine and chemokine cascade effects. Moreover, changes in the sncRNA profile, particularly tRFs, have been observed in samples from individuals infected with SARS-CoV, indicating their involvement in viral pathogenesis.
In tumorigenesis, specific tsRNAs have been implicated in promoting cancer progression and metastasis. For example, tRF-3017A, derived from tRNA-Val-TAC, has been shown to silence tumor suppressor genes and facilitate lymph node metastasis in gastric cancer. Additionally, both tRFs and tiRNAs have emerged as potential biomarkers for evaluating therapeutic efficacy in skin melanoma.
In neurodegenerative disorders, alterations in tRFs have been observed in the hippocampus of Alzheimer's disease patients, suggesting their involvement in disease progression. Furthermore, angiogenin-mediated biogenesis of 5'-tsRNAs in sperm has been linked to paternal inflammation-induced metabolic disorders in offspring.
A. Our Study Uncovered a Significant Finding
The expression levels of tsRNAs within human epicardial adipose tissue (EAT) were substantially altered in the presence of atrial fibrillation (AF) compared to sinus rhythm (SR). The analysis of tsRNA subtypes against tRNA isodecoders and their frequency distribution according to length revealed a diverse landscape, suggesting that tsRNAs may serve as promising candidates for modulating atrial substrates in the context of AF.
TsRNAs, akin to miRNAs, are fragments derived from tRNAs, which bind to complementary sequences in mRNAs, thereby regulating gene expression post-translationally. Employing bioinformatics, we predicted mRNA targets for candidate tsRNAs and established an interactive network depicting their interactions. Target genes, identified via GO enrichment analysis, mainly participate in cell adhesion and cellular processes mediated by plasma membrane adhesion molecules. KEGG pathway analysis revealed glycosaminoglycan biosynthesis, AMPK, and insulin signaling pathways as the top pathways influenced by the tsRNA-mRNA network.
In failing human hearts, there is notable accumulation of perivascular and interstitial chondroitin sulfate glycosaminoglycans (CS-GAGs), particularly in fibrotic regions. CS-GAGs interact with tumor necrosis factor-α, inducing inflammation-related gene activation. Andreas Haryono suggested biphasic effects of CS-GAGs on cardiac function and remodeling in heart failure. AMPK plays a crucial role in maintaining atrial electrophysiological homeostasis, with impaired signaling observed in heart failure associated with atrial fibrillation (AF). Atrium-selective cardiac AMPK deletion in mice highlighted its protective role against electrophysiological reprogramming promoting AF. Insulin resistance in metabolic syndrome is associated with AF occurrence and recurrence post-catheter ablation.
Investigating epicardial adipose tissue (EAT) in AF pathogenesis, particularly concerning tsRNAs, is crucial. While our study identified differentially expressed tsRNAs in EAT and predicted target genes using bioinformatics, further validation through in vitro and in vivo experiments is needed. In silico methodologies were predominant in our analyses, emphasizing the necessity for animal models and clinical validation. Study limitations, including modest sample size, underscore the need for larger-scale analyses. In summary, our study reveals altered tsRNA expression profiles in EAT from AF patients, predicts potential target genes, and elucidates tsRNA-mRNA interactions potentially contributing to AF pathogenesis. These findings provide insights for future therapeutic strategies targeting atrial remodeling, with further research needed to understand the physiological mechanisms underlying tsRNA modulation of AF progression.
[Data availability statement: The sequencing raw data is available on Gene Expression Omnibus (GEO) (GSE210705, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210705).]

References
[1] M. Conte, L. Petraglia, S. Cabaro, et al., Epicardial adipose tissue and cardiac arrhythmias: focus on atrial fibrillation, Front. Cardiovasc. Med. 92022), https://doi.org/10.3389/fcvm.2022.932262 [2] P.S. Cunha, S. Laranjo, J. Heijman, M.M. Oliveira, The atrium in atrial fibrillation – a clinical review on how to manage atrial fibrotic substrates, Front. Cardiovasc. Med. 92022), https://doi.org/10.3389/fcvm.2022.879984 [3] A. Hruby, F.B. Hu, The epidemiology of obesity: a big picture, Pharmacoeconomics 33 (7) (2015) 673–689, https://doi.org/10.1007/s40273-014-0243-x. [4] S.N. Hatem, A. Redheuil, E. Gandjbakhch, Cardiac adipose tissue and atrial fibrillation: the perils of adiposity, Cardiovasc. Res. 109 (4) (2016) 502–509, https://doi.org/10.1093/cvr/cvw001. [5] L. Zhao, D.L. Harrop, A. Ng, W. Wang, Epicardial adipose tissue is associated with left atrial dysfunction in people without obstructive coronary artery disease or atrial fibrillation, Can. J. Cardiol. 34 (8) (2018) 1019–1025, https://doi.org/10.1016/j.cjca.2018.05.002. [6] E.A. Orellana, E. Siegal, R.I. Gregory, Trna dysregulation and disease, Nat. Rev. Genet. (2022), https://doi.org/10.1038/s41576-022-00501-9. [7] X. Gu, Y. Zhang, X. Qin, S. Ma, Y. Huang, S. Ju, Transfer rna-derived small rna: an emerging small non-coding rna with key roles in cancer, Exp. Hematol. Oncol. 11 (1) (2022), https://doi.org/10.1186/s40164-022-00290-1. [8] E. Borek, B.S. Baliga, C.W. Gehrke, et al., High turnover rate of transfer rna in tumor tissue, Cancer Res. 37 (9) (1977) 3362–3366 [9] J. Du, T. Huang, Z. Zheng, S. Fang, H. Deng, K. Liu, Biological function and clinical application prospect of tsrnas in digestive system biology and pathology, Cell Commun. Signal. 21 (1) (2023) 302, https://doi.org/10.1186/s12964-023-01341-8. [10] N.T. Chiou, R. Kageyama, K.M. Ansel, Selective export into extracellular vesicles and function of trna fragments during t cell activation, Cell Rep. 25 (12) (2018) 3356–3370, https://doi.org/10.1016/j.celrep.2018.11.073 [11] K. Yang, Q. Xiao, K. Wang, et al., Circulating exosomal tsrnas: potential biomarkers for large artery atherosclerotic stroke superior to plasma tsrnas, Clin. Transl. Med. 13 (2) (2023), https://doi.org/10.1002/ctm2.1194. [12] S. Li, Z. Xu, J. Sheng, Trna-derived small rna: a novel regulatory small non-coding rna, Genes 9 (5) (2018), https://doi.org/10.3390/genes9050246. [13] N. Guzzi, C. Bellodi, Novel insights into the emerging roles of trna-derived fragments in mammalian development, RNA Biol. 17 (8) (2020) 1214–1222, https://doi.org/10.1080/15476286.2020.1732694. [14] L. Zhao, Z. Ma, Z. Guo, M. Zheng, K. Li, X. Yang, Analysis of long non-coding rna and mrna profiles in epicardial adipose tissue of patients with atrial fibrillation, Biomed. Pharmacother. 1212020) 109634, https://doi.org/10.1016/j.biopha.2019.109634 [15] X. Yu, Y. Xie, S. Zhang, X. Song, B. Xiao, Z. Yan, Trna-derived fragments: mechanisms underlying their regulation of gene expression and potential applications as therapeutic targets in cancers and virus infections, Theranostics 11 (1) (2021) 461–469, https://doi.org/10.7150/thno.51963 [16] J. Zhou, S. Liu, Y. Chen, et al., Identification of two novel functional trna-derived fragments induced in response to respiratory syncytial virus infection, J. Gen. Virol. 98 (7) (2017) 1600–1610, https://doi.org/10.1099/jgv.0.000852 [17] W. Wu, E. Choi, B. Wang, et al., Changes of small non-coding rnas by severe acute respiratory syndrome coronavirus 2 infection, Front. Mol. Biosci. 92022), https://doi.org/10.3389/fmolb.2022.821137 [18] L. Tong, W. Zhang, B. Qu, et al., The trna-derived fragment-3017a promotes metastasis by inhibiting nell2 in human gastric cancer, Front. Oncol. 102021), https://doi.org/10.3389/fonc.2020.570916 [19] L.L. Zheng, W.L. Xu, S. Liu, et al., Trf2cancer: a web server to detect trna-derived small rna fragments (trfs) and their expression in multiple cancers, Nucleic Acids Res. 44 (W1) (2016) W185–W193, https://doi.org/10.1093/nar/gkw414 [20] Y. Fang, Y. Liu, Y. Yan, et al., Differential expression profiles and function predictions for trfs & tirnas in skin injury induced by ultraviolet irradiation, Front. Cell Dev. Biol. 92021), https://doi.org/10.3389/fcell.2021.707572 [21] W. Wu, I. Lee, H. Spratt, X. Fang, X. Bao, Trna-derived fragments in alzheimer’s disease: implications for new disease biomarkers and neuropathological mechanisms, J. Alzheim. Dis. 79 (2) (2021) 793–806, https://doi.org/10.3233/JAD-200917 [22] Y. Zhang, L. Ren, X. Sun, et al., Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsrnas, Nat. Commun. 12 (1) (2021), https://doi.org/10.1038/s41467-021-26909-1. [23] Z. Yang, P. Li, Z. Li, T. Tang, W. Liu, Y. Wang, Altered expression of transfer-rna-derived small rnas in human with rheumatic heart disease, Front. Cardiovasc. Med. 82021), https://doi.org/10.3389/fcvm.2021.716716 [24] R. Zhao, M. Ackers-Johnson, J. Stenzig, et al., Targeting chondroitin sulfate glycosaminoglycans to treat cardiac fibrosis in pathological remodeling, Circulation 137 (23) (2018) 2497–2513, https://doi.org/10.1161/CIRCULATIONAHA.117.030353. [25] A. Haryono, K. Ikeda, D.B. Nugroho, et al., Chgn-2 plays a cardioprotective role in heart failure caused by acute pressure overload, J. Am. Heart Assoc. 11 (7) (2022), https://doi.org/10.1161/JAHA.121.023401. [26] D. Tong, G.G. Schiattarella, N. Jiang, et al., Impaired amp-activated protein kinase signaling in heart failure with preserved ejection fraction–associated atrial fibrillation, Circulation 146 (1) (2022) 73–76, https://doi.org/10.1161/CIRCULATIONAHA.121.058301. [27] K.N. Su, Y. Ma, M. Cacheux, et al., Atrial amp-activated protein kinase is critical for prevention of dysregulation of electrical excitability and atrial fibrillation, JCI Insight 7 (8) (2022), https://doi.org/10.1172/jci.insight.141213. [28] Z. Wang, Y. Wang, Z. Liu, et al., Effect of insulin resistance on recurrence after radiofrequency catheter ablation in patients with atrial fibrillation, Cardiovasc. Drugs Ther. (2022), https://doi.org/10.1007/s10557-022-07317-z. [29] Rabab Khan Rongon, et al., Impact of Quantum Computing in Quantum Supremacy and Parallel Universes. (2023), https://10.13140/RG.2.2.20552.70401 [30] Rabab Khan Rongon, Krishna Das, et al., Obstacle to implement sustainable computing in Bangladesh and approach to being sustainable through green cloud computing methodology. (2023) International Conference on Sustainable Computing (SUSCOM-2022).
Copyright
Copyright © 2024 Moriom Akter Eti, Rabab Khan Rongon. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET61416
Publish Date : 2024-05-01
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

