Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
A Clinical Study of Karkashchadaadi Kwath in Medodushti with Special Reference to Dyslipidemia- A Study Protocol
Authors: Dr. Jyoti , Dr. Neelam Kumari, Dr. Neha Lamba
DOI Link: https://doi.org/10.22214/ijraset.2024.61119
Certificate: View Certificate
Abstract
Like other metabolic disorders, Medoroga (Dyslipidemia) is also troublesome. The burden is increasing day by day. It is caused by an abrupt change in lifestyle and dietary habits. In India, approximately 25–30% of urban and 15-20% of rural subjects suffer from dyslipidemia. Overall, raised cholesterol is estimated to cause 2.6 million deaths (4.5% of total) and 29.7 million DALYS, or 2% of total DALYS. It is estimated that abnormal cholesterol levels account for 56% of global IHD cases and 18% of all CVD cases. Statins are the recommended medication for dyslipidemia in contemporary medicine which has many side effects like headaches, nausea, vomiting, bowel upsets, sleep disturbances, muscle tenderness, myopathy, thrombocytopenia, fulminant hepatitis, etc. Keeping in view of the above limitations and side effects, this study has been chosen to fill the gap in providing a safe and effective line of treatment, as Ayurveda provides multiple formulations of Medodushti (dyslipidemia). So, an effort is made for a single-arm, randomised clinical trial to see whether Karkashchadadi Kwath at a dose of 40 ml BD is effective in the management of 40 patients of Medodushti for 60 days. We will have follow-up on the 0th, 15th, 30th, 45th, and 60th days (during treatment) and the 75th day (post treatment). The changes will be analysed on the basis of subjective and objective parameters. Then the results will be analysed using unpaired t test for objectives parameters and non- parametric, Chi square test for subjective parameters or appropriate statistical methods will be used as per need. Trial has been registered under the Clinical Trial Registry of India with CTRI/2023/07/054923
Introduction
I. BACKGROUND
Madhukosha mentions sneha as the factor that causes meda [[i]]. Excessive consumption of sneha dravya causes excessive deposition of Meda in the body. Meda is considered the prime dushya in several disorders, including Medoroga, Prameha, and Sthaulya. The aberrant build-up of Medadhatu, or Medodushti, consists of several Medovikaras, also called Medoroga. Agni derangement and the creation of Aama rasa lead to Medodhatwagnimandya, which in turn causes an incorrect and excessive generation of Medodhatu, the condition known as Medodushti. Meaning of Medodhatu A lipoprotein problem known as dyslipidemia is triggered by either an excess or reduction in lipoproteins during metabolism [[ii]]. All that Medodushti is the predecessor of Medoroga.
Higher plasma total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), or decreased high-density lipoprotein cholesterol (HDL-C) are the hallmarks of dyslipidemia, which is a major risk factor for the development and advancement of atherosclerosis, cardiovascular disease (CVD), and stroke. [[iii],[iv],[v]]
Since CVD continues to be a leading cause of morbidity and death worldwide, primary and secondary risk reduction strategies must include the management of dyslipidaemia. In India, approximately 25-30% of urban and 15-20% of rural population have Dyslipidemia. Although it is more common in men, it affects both genders. 30 to 40 years age group has tendency to high prevalence, but above 60 years it become markedly high. Total cholesterol concentration is greater than 200mg/dL (38.7 percent in men and 23.3 percent in women). Overall, raised cholesterol is estimated to cause 2.6 million deaths (4.5 percent of total) and 29.7 million DALYS (2% of total DALYS). Abnormal cholesterol levels are thought to cause 18% of all CVD and 56% of global IHD. [[vi]]
Statins are recommended as the first-line therapy by International Clinical Practice for dyslipidaemia due to their ability to reduce cardiovascular events. However, side effects like myalgia, rhabdomyolysis, hepatobiliary problems, myositis, cognitive impairment, and early diabetes may occur. [[vii],[viii],[ix],[x]] Long-term use can increase these risks. For patients who are unable to accept the negative effects of these drugs that decrease lipids, managing dyslipidaemia continues to be a significant challenge.
????????????????? ????????????? ????????? | ????? ??????? ????? ???????? ??????? ???????? ||
(??. ???. ????? ????, ??????? ????????????? ?? /??)
Karkashchadaadi Kwath, [[xi]] which has Patolapatra (Leaves of Tricosanthes dioecia), Chitrakamool (Root of Plumbago zeylanica), Shatpushpa (Seed of Anethum graveolens), and Hingu (Resin of Ferula narthex) as contents, is mentioned in Bhavparkash Madhyam khand Sthoulya Rogadhikar as medonashak. It possesses the Tikt, Katu Rasa (Taste) Laghu, Rooksha, Teekshan Guna with Katu Vipak and Ushan Veerya which relieve Agnimandya and does Rookshan, Chedan, and Lekhan of Meda Dhatu; which is the major treatment principle of the medohar chikitsa mentioned by Acharya Charak in Sutra Sthaan 21chapter.
Several researches conducted in the past few years have indicated that Hepatoprotective potential has been proven of Patol [[xii],[xiii],[xiv]] and Chitrak [[xv],[xvi]] possesses a range of pharmacological qualities like anthelminthic, antihyperlipidemic, antihyperglycemic antioxidant, antidiabetic, antipyretic, antimicrobial, hepatoprotective, anticancer, antifertility, antiulcer, antifungal, burns, and wound healing. Some pharmacological effects of Shatpushpa (Anethum graveolens) have been reported such as antimicrobial antihyperlipidemic and anti hypercholesterolemic activities. [[xvii],[xviii],[xix]]
Occasionally, oral intake of Hingu may cause adverse reactions such as nausea, vomiting. If used without shodhan (Purification). Bhavparkash had suggested that Hingu can be used after the Purification by roasted it in the ghee to avoid any side effect of drug.
Unfortunately, cost-effectiveness, safety, innovation, and distinctiveness are not being met by the status of clinical research at this time. Numerous studies had demonstrated that their intervention greatly improved lipid profiles; nevertheless, the results may have been impacted by the fact that the randomization, blinding process, sample size calculation, and specific inclusion and exclusion criteria were not disclosed. The purpose of this single arm, randomised clinical trial was to evaluate the safety and lipid-lowering effectiveness of Karkashchadaadi Kwath in the treatment of dyslipidemia, since there haven't been many high-quality randomised controlled trials on this potential at this level.
II. METHODOLOGY/DESIGN
A. Trial Primary Objective
To determine the effect of drug on clinical parameters and biochemical findings associated with Medodushti (Dyslipidemia).
B. Trial Secondary Objectives
- To suggest preventive actions in order to monitor the spread of disease across individuals and communities.
- To standardize the ayurvedic line of treatment, both drug and dose which may have effective role in management of patients of Medodushti (Dyslipidemia).
- To explain the Medodushti (Dyslipidemia) critical review on the basis of classical text of ayurveda and with the help of modern literature.
- To rule out secondary causes that count for lipoprotein disorder.
- To prevent the complication of Dyslipidemia like atherosclerosis, CAD, CVD & PVD.
III. STUDY DESIGN
This is the research plan for a 60-day treatment period randomized single arm clinical trial, which will be conducted between July 2023 and October 2024. Patient recruiting is currently Ongoing. Eligible patients are computerized randomly taken. The protocol was developed according to standard guidelines of Shri Krishna AYUSH University. And after approval from the Institutional Ethical committee and University Research committee.
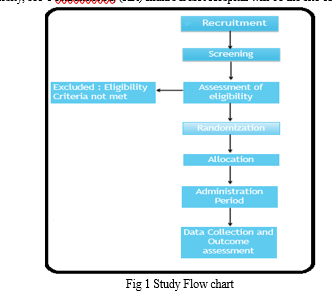
The trial will last for sixty days. To evaluate the effectiveness of treatment, safety, and adverse effects, a total of six visits and one post-follow-up visiting have been planned at initial (visit 1), 15th day (visit 2), 30th day (visit 3), 45th day (visit 4), 60th day (visit 5), and 75th day (visit 6). Based on prior research and clinical experience, a 60-day therapy term was chosen. Furthermore, a thorough collection of potential prognostic criteria pertaining to patient and illness features has been made. Figure 1 shows the flow diagram of the trial.
A. Institutional Setting: Site of Study
Shri Krishna AYUSH University, sec-8 Kurukshetra, (HR) India's IASR Hospital will be the site of this study.
IV. RESEARCH QUESTION
To Check the Efficacy of Karkashchadadi Kwatha in Medodushti .
A. Hypothesis
Null Hypothesis (H0): There is no significant efficacy of Karkashchadadi Kwatha in management of Medodushti (Dyslipidemia).
Research Hypothesis (H1): Karkashchadadi Kwatha is significantly effective in management of Medodushti (Dyslipidemia).
B. Trial registry, Recruitment of Participants and Recruitment Techniques
This trial has been registered as a prospective study under CTRI/2023/07/054923 under the Clinical Trial Registry of India, and every participant will be sought out in IASR Hospital, Kurukshetra. Additionally, advertisement, via pamphlets will be posted within the facility Along with brief description of dyslipidaemia and Karkashchadaadi Kwath and investigators' contact details. The researchers can be reached by phone or in person at the OPD by those who are ready to involve in this trial. Individuals who volunteer to participate will be assessed in complying with both the inclusion and exclusion criteria.
Before any procedures linked to the study are started, eligible patients will be required to complete a written informed consent form. No one or any organisation associated with the study will ever be privy to the participants' personal information.
C. Study population, Inclusion criteria and Diagnostic standards
- Diagnosed & confirmed cases of Medodushti (Dyslipidemia) & Medoroga .
- Patients having underweight, normal weight, overweight, and class I obesity according to BMI.
a. Inclusion criteria
- Diagnosed & confirmed cases of Medodushti (Dyslipidemia) & Medoroga on the basis of criteria given by NCEP-ATP-III (Borderline High) [[xx]] i.e. Serum Cholesterol ≥ 200 mg/dl, Serum Triglycerides ≥ 150mg/dl, LDL Cholesterol ≥ 130mg/dl, HDL Cholesterol < 40mg/dl (male) and <50mg/dl (female)
- Age between 20 and 60 years, male or female
- Having freely provided their informed consent.
b. Exclusion criteria
- Individuals with a past medical history of- Unstable angina, Myocardial Infarction, Heart failure or stroke.
- Uncontrolled Hypertension (Diastolic Blood Pressure > 100 mmHg).
- Uncontrolled Diabetes Mellitus.
- Impaired Renal Function (Creatinine ≥ 2 mg/dl).
- ALT >2 times and AST >3 times of upper limit of normal (40 IU).
- Patient on steroid therapy and oral contraceptive therapy.
- Pregnant and lactating patient.
- Patient not fulfilling inclusion criteria.
- Patient not willing for clinical trial.
- Patient below 20yrs of age group and above 60yrs of age group.
D. Randomization
Computer generated randomization and Open Label technique will be applied to this Single arm interventional study.
V. INTERVENTIONS
A. Pathya-Apthya and Lifestyle interventions
As per the Ayurved Classics, The applicants will receive health education regarding nutrition and exercise. In this investigation, the individuals' food and exercise regimens will be considered crucial. A regular meal plan that fits the patient's needs in terms of Pathya ahaar – Laghu and Supachya Ahaar (easily digestible) or calories, protein, and carbs as well as a low-sugar, low-salt, and low-cholesterol diet will be recommended. It is advised that individuals engage in 20–30-minute sessions of moderate-intensity metabolic exercise. Apthya Ahaar, Vihar i.e. Risk factors will be strictly controlled, including alcoholism, smoking, and non-veg diet. It will be asked of the participants to follow a strict diet plan (laghu ahaar) and refrain from high-intensity or brief exercise.
B. Pharmacologic Interventions
A total of 40 patients with Medodusti (dyslipidemia) will receive lifestyle counselling and initial data collection, and they will be assigned at random to this trial study. Freshly prepared Karkashchadadi Kwath (Decoction) in a 40-ml dose and twice a day after 1 hour of food intake for 60 days will be given.
Every treatment taken during the study, along with its exact kind (generic version or generic name), its indications, date of administration, and dosage, must be meticulously documented in CRFs by the observer during each visit. The observer must also meticulously record whether the patient takes the medication on time. Throughout the trial, all lipid-lowering therapies—aside from the investigational medications—will be prohibited or limited.
C. Outcomes
Primary outcome
The chief result is the percentage variation in Lipid profile and subjective criteria from baseline at 60th day.
Secondary outcomes
The later results are as follows:
- SGOT, SGPT, TG, body weight, BMI, CBC, Serum creatinine, FBS, Microscopy and urine routine measurements will be made at starting point. (visit 1) and 30th day (visit 3), 60th day (visit 5), and 75th day (visit 6).
- All the subjective parameters mentioned in Madhav Nidaan Medoroganidaan/3 will be evaluated every visit.
D. Safety Assessments
The safety assessments are as follows:
- General physical examination.
- Routine testing for blood and urine
- Adverse events
E. Monitoring of Adverse Reactions
No such toxic effect noted so far in the trial drugs. It is safe to take it in therapeutic uses, also Investigators are required to promptly report adverse outcomes to the State Pharmacovigilance cell and the principle investigator by completing the "Serious Adverse Event form." The medical examiner is required to provide appropriate care based on the medications and symptoms, then thoroughly document and sign at the CRFs. The treating procedures must be disclosed to the principal investigator. Every unfavourable event should be promptly documented and monitored until it is adequately remedied or stabilised.
F. Participant and Assessment Timeline
Following the participant timeline indicated in Table 1, the evaluation indicated in Table 2 will be evaluated using the grading scale indicated in Table 3, which is based on the Madhav Nidaan Medoroganidaan, in that order. [[xxi]]
G. Laboratory Data Collection
Assessments of safety and validity will be conducted using laboratory parameters (see Table 1 for details). as stated during visits and at the start of the clinical study. Participants will get a thorough explanation of the biological specimen collected. The laboratory department of IASR Hospital or any other recognised and approved laboratory will measure lipid profiles, other blood exams, urine routines, and microscopic investigations.
H. Quality Control and Data Management
All investigators and pertinent personnel, such as physicians and researchers, and drug managers, will get uniform training and information about the trial-specific process before the start of the clinical trial. While completing the CRFs, investigators will refer to the participants' previous assessment records and inpatient care records.. The inspectors will routinely review all gathered data to make sure there are no errors or omissions. The investigator must sign and date the adjustments, and the original record of any amendment must be easily readable. The CRFs must then be delivered to the competent authority/ IASR or administration of Shri Krishna AYUSH University. It is necessary to document and preserve this data transfer procedure between investigators, inspectors, and data managers. Before entering data, the data administrator will double-check the information. For future use, all queries and responses will be stored in query tables. CRF data entry will be done on an individual basis. The primary investigator should examine the database with the data manager (supervisors) to get details on the volume of data and its logical content. The initial CRFs must be filled out manually when the patient is present after the authorization of supervisors and will be saved and preserved chronologically, the data and validation as required. According to the Quality Management Standard of the Clinical Trial Registry of India, all original files have to be maintained for the period given. As per protocol all the CRF with all the data will be handed over to the concerned authority of Shri Krishna AYUSH University with the approval of the supervisor after completion of the study.
I. Sample size Selection
The University authority set a capacity of 40 on the trial's sufficient sample size to finish the study on time for thesis work under the supervision of the investigator. This trial was cleared by the Research Committee of Shri Krishna AYUSH University.
J. Examining Statistics
A professional statistician will blindly analyse all of the data using statistical methods. The SPSS software 24.0 version or Graph Pad Prism 7.0 version will be used for all data analysis performed statistically. The information gathered on the basis of above observations will be subjected to statistical analysis in terms of mean (x), standard deviation (S.D.) and standard error (S.E.). Necessary - Unpaired t test for objectives parameters and non- parametric, Chi square test for subjective parameters will be applied and further will be subjected to more applicable statistical test as per requirement. The obtained results will be interpreted as:
- Insignificant - P < 0.10
- Significant - P < 0.05
- Highly Significant - P < 0.001
Every adverse event that occurs throughout the trial needs to be reported, together with information about its kind, intensity, and connection to the experimental medication. All adverse events will be classified as adverse events, along with any associated complications.Among them, the terms "certainly related," "probably related," "maybe related," and "suspected related" to the drug will be used conservatively.
K. Protocol Amendments
Although this study has been approved by the Institutional Ethics Committee and Research Committee of Shri Krishna AYUSH University after all the amendments, Furthermore, the major investigator and the Institutional Ethics Committee of Shri Krishna AYUSH University must approve any formal protocol amendments that may be necessary prior to implementation. Changes could have an impact on the study's methodology, the safety or potential benefits for patients, or both.
L. Observation and Examination
Supervisors should conduct routine monitoring to ensure that the data are timely, accurate, comprehensive, and properly documented in the case report forms (CRFs). The project management team (concerned authority of Shri Krishna AYUSH University) will conduct a standard and focused inspection at each stage of the investigation to assess if the study protocol is being followed.
VI. DISCUSSION
Numerous studies support the claims of antidyslipidemic action declared in Bhavparkash Madhyam Khand Sthoulya Rogadhikar regarding the medicinal benefits of Patola, Chitraka, Shatpushpa, and Hingu. As mentioned by Acharya Charak, the main treatment principles of the Medohar Chikitsa are Guru Aptarpan, Agni Vardhan, Rookshan, Chedan, and Lekhan of Meda Dhatu, along with the cleansing of channels. The drugs of Karkashchadaadi Kwath possess these qualities.
Concern over dyslipidemia, a metabolic disease that accounts for 29.7 million DALYS and 2.6 million fatalities in India, is rising. Side effects of modern statins include nausea, headaches, and disturbed sleep. Thus, the purpose of this study is to close the gap in the market by offering a low-cost, secure, and efficient course of treatment for Medodushti (Dyslipidemia). This study protocol compares the results of 60 days treatment period for 40 individuals in a computerised randomised single arm prospective clinical trial at Karkashchadadi Kwatha in Medodushti.
This study is conducted on a small sample size, and the outcome may be affected by the demographic area and Prakriti of the patient; hence, larger sample numbers and more well-planned research are therefore needed and can be performed for Karkashchadadi Kwatha in cases of dyslipidemia (medodushti).
- Trial status: Patients are being recruited for this trial right now. The hiring process is scheduled to take place from July 2023 to October 2024.
- Acknowledgements: The participants' time and interest in the research study are greatly appreciated by the authors.
- Funding: The Shri Krishna AYUSH University in Kurukshetra, Haryana, India, may provide funding for this study.
A. Ethics Approval and Consent to Participate
The clinical trial research regulations and guidelines of Shri Krishna AYUSH University were followed in the planning of this study. The SKAU Ethics Committee has granted ethics approval for this trial protocol under reference number (SKAU/Acad/2022/6749). Patients will have enough time to decide whether or not to participate in the trial. Before recruiting, the researchers and investigators need to get each participant's signed informed consent. Participants will be asked to consent to the use of their files in case that they decide to leave the trial on the consent form.
Table 1: Participant/ Patient timeline
|
Research stage |
Starting point |
Duration of treatment |
Following therapy |
|||
|
Item |
0 |
15th day ± 3 days |
30th day ± 3 days |
45th day ± 3 days |
60th day ± 3 days |
75th day ± 3 days |
|
Visit no. |
V1 |
V2 |
V3 |
V4 |
V5 |
V6 |
|
Demographic data Inclusion/exclusion requirements |
√ |
|
|
|
|
|
|
Informed consent |
√ |
|
|
|
|
|
|
Past history of disease and treatment |
√ |
|
|
|
|
|
|
Physical examination |
√ |
√ |
√ |
√ |
√ |
√ |
|
Complications and systemic medications |
√ |
√ |
√ |
√ |
√ |
√ |
|
Outcome assessments Subjective parameters |
√ |
√ |
√ |
√ |
√ |
√ |
|
Objective parameters |
√ |
|
√ |
|
√ |
√ |
|
Blood pressure |
√ |
√ |
√ |
√ |
√ |
√ |
|
Body weight |
√ |
√ |
√ |
√ |
√ |
√ |
|
Safety assessment Blood routine measurements |
√ |
|
|
|
√ |
|
|
Urine routine measurements |
√ |
|
|
|
√ |
|
|
Adverse events |
√ |
√ |
√ |
√ |
√ |
√ |
|
Others Randomization |
√ |
|
|
|
|
|
|
Drug distribution |
√ |
√ |
√ |
√ |
√ |
|
|
Record medications |
√ |
√ |
√ |
√ |
√ |
√ |
|
Study conclusion |
|
|
|
|
√ |
√ |
Table2: Progress Assessment Scale – Subjective and Objective for study
|
S. No. |
Assessment Criteria |
1st day |
15th day |
30th day |
45th day |
60th day |
Post Follow-up |
|
Subjective Criteria |
|
|
|
|
|
|
|
|
|
Kshudra Shwasa (Exertional Dyspnoea) |
|
|
|
|
|
|
|
|
Trishna (Increased Thirst) |
|
|
|
|
|
|
|
|
Atinidra (Increased Sleep) |
|
|
|
|
|
|
|
|
Kranthana (Snoring) |
|
|
|
|
|
|
|
|
Angasada (Fatigue) |
|
|
|
|
|
|
|
|
Atikshuda (Increased appetite) |
|
|
|
|
|
|
|
|
Swedadhikya (Perspiration) |
|
|
|
|
|
|
|
|
Alpapran (General debility) |
|
|
|
|
|
|
|
|
Alpamaithuna (Loss of Libido) |
|
|
|
|
|
|
|
|
Daurgandhya (Bad odour) |
|
|
|
|
|
|
|
|
Moha (Drowsiness) |
|
|
|
|
|
|
|
Objective Parameter |
|
|
|
|
|
|
|
|
|
BMI |
|
|
|
|
|
|
|
|
Serum Cholestrol |
|
|
|
|
|
|
|
|
Serum Triglycerides |
|
|
|
|
|
|
|
|
LDL Cholestrol |
|
|
|
|
|
|
|
|
HDL Cholestrol |
|
|
|
|
|
|
|
|
SGOT |
|
|
|
|
|
|
|
|
SGPT |
|
|
|
|
|
|
|
|
Serum creatinine |
|
|
|
|
|
|
|
|
FBS |
|
|
|
|
|
|
|
|
CBC |
|
|
|
|
|
|
|
|
Urine R & M |
|
|
|
|
|
|
Table-3: Symptom Rating scale for Subjective Criteria
|
SYMPTOM SCALE |
SCORE |
|
Absence of symptoms |
0 |
|
Mild degree of symptoms |
1 |
|
Moderate degree of symptoms |
2 |
|
Severe degree of symptoms |
3 |
|
Agonising symptom |
4 |
References
[1] Madhukosha Teeka of Kanthdatt on Madhav nidan. 1st ed. Varanasi: Chaukhamabha Prakashana; 2019. p. 34. (vol 1). Chapter 34, Verse 1. [2] Jamson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. et al, editors. Harrison`s principle of internal medicine. 20th ed. New York: Mc Grow Hill Education; 2018. p. 2889-02. (vol 2). [3] Munjal YP. API textbook of internal medicine. 9th ed. Mumbai: The Association of Physician of India; 2012. p. 1232-42. (vol 2). [4] Chait A, Eckel RH. Lipids, lipoproteins, and cardiovascular disease: clinical pharmacology now and in the future. J Clin Endocrinol Metab. 2016;101: 804–14. [5] Kopin L, Lowenstein CJ. Dyslipidemia. Ann Intern Med. 2017;11:81–96 [6] Khan MA. Dyslipidemia. [Online] 2019. Available from: National Health Portal:2019.[cited 2019 May 05]. [7] Toth PP, Patti AM, Giglio RV, Nikolic D, Castellino G, Rizzo M, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18:157–73. [8] Rosenson RS, Baker SK, Jacobson TA, Kepecky SL, Parker BA. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8: S58–71 [9] Stroes ES, Thompson PD, Corsini A, Vladutiu G, Raal FJ, Ray KK, et al. Statinassociated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment. Etiol Manage Eur Heart J. 2015;36:1012–22. [10] Maki KC, Ridker PM, Brown WV, Grundy SM, Sattar N. An assessment by the Statin Diabetes Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:S17–29 [11] Bhavamishra. Bhavaprakash. 10th ed. Varanasi: Chaukhamba Surbharti Prakashan; 2002. p. 34. (vol 1). Madhyam khand, Chapter 39, Verse 2. [12] Gupta RK, Swain SR, Sahoo J, Gupta A, Chaudhary S. Hepatoprotective Potential of Trichosanthes dioica Roxb in Hepatotoxicity Induced by Simvastatin and its consequences on Biochemical and Haematological Indices. Pharmacognosy Journal. 2018;10(4):720-724. [13] Sharmila Banu G, Kumar G, Rajasekara Pandian M. Cholesterol lowering Activity Of The Aqueous Fruit Extract Of Trichosanthes Dioica Roxb (L.) In Normal And Streptozotocin Diabetic Rats. Journal of Clinical and Diagnostic Research [serial online] 2007 December [cited: 2007 Dec 3]; 1:561- 569. Available from http://www.jcdr.net/back_issues.asp?issn=0973709x&year=2007&month=December&volume=1&issue=6&page=561-569&id=81 [14] Shah BN, Seth AK Pharmacological potential of Trichosanthes dioica – an edible plant. JOURNAL FOR DRUGS AND MEDICINES. Hygeia.J.D.Med.vol.2 (2),2010, 1-7 [15] Chitrak (Plumbago zeylanica) - Properties, Benefits & Dosage. 2019. https://www.planetayurveda.com. [16] Herbal medicinal plant-Chitrak. www.dabur.com/in/en-us/about/science-of-ayurveda/herbal-medicinal-plants/chitrak-plant. Accessed 21 Sept 2020. [17] Nair R, Chanda S. Antibacterial activities of some medicinal plants of the western region of India. Turk J Biol. 2007;31:231–6. [18] Chaurasia SC, Jain PC. Antibacterial activity of essential oils of four medicinal plants. Indian J Hosp Pharm. 1978;15:166–8. [19] Yazdanparast R, Alavi M. Antihyperlipidaemic and antihypercholesterolaemic effects of Anethum graveolens leaves after the removal of furocoumarins. Cytobios. 2001;105:185–91. [20] Expert Panel on Detection, Evaluation and Treatment of High Blood Cholestrol in Adults. Executive summary of the third report of national cholesterol education programme (NCEP)ATP III. JAMA [serial online] 2001 May [cited 2001 May 16]; 285(19):[2497]. Available from: URL:https://jamanetwork.com/journals/jama/article. [21] Madhavkara. Madhav nidana. 1st ed. Varanasi: Chaukhamba Prakashan; 2019. p. 38 (vol 1). Chapter 34, Verse 2.
Copyright
Copyright © 2024 Dr. Jyoti , Dr. Neelam Kumari, Dr. Neha Lamba . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET61119
Publish Date : 2024-04-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

